Abstract
A large percentage of cancer cases present without knowledge of the causative genetic events. Tyrosine kinases are frequently implicated in the pathogenesis of cancer, but identification of specific tyrosine kinases as cancer targets has been a slow process. Tyrosine kinases are thought to play a causative role in acute myeloid leukemia (AML), based in part on the high percentage of cases with phosphorylation of STAT5—a marker for activity of tyrosine kinase signaling. However, known abnormalities in tyrosine kinases in AML are restricted to internal tandem duplications of FLT3 or genetic aberrations in FLT3, c-KIT, and a few other genes. Thus, the specific tyrosine kinases that form the basis for targeted intervention have remained unclear in the majority of AML patients. Here, we report a novel assay by which cells from AML patients are functionally screened with RNAi to elucidate tyrosine kinase targets amenable to therapeutic intervention.
Methods: To determine targets necessary for viability of malignant cells, we screened cell lines as well as primary cells from AML patients by electroporating siRNAs individually targeting each member of the tyrosine kinase family. Four days later, we determined the cell viability and tabulated sensitivity of the cells to any individual tyrosine kinase. Where possible, results were confirmed by treating samples with small-molecule inhibitors with activity against the genes identified by the assay. In addition, the mechanism of oncogenesis was investigated for each positive result.
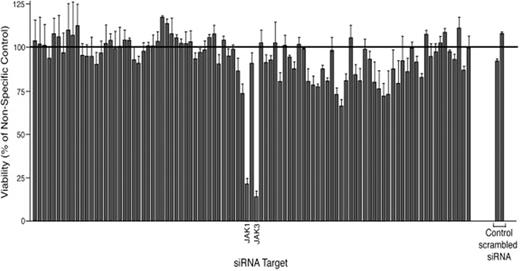
Results: We demonstrate that siRNA screening can identify tyrosine kinase targets containing activating mutations in JAK3 (A572V) in CMK cells (Figure 1) and c-KIT (V560G) in HMC1.1 cells. In addition, this assay identifies targets that do not contain mutations, such as JAK1 (Figure 1) and the focal adhesion kinases, yet are still crucial to the survival of the cells. We have also used this assay to determine sensitivity of numerous primary AML samples to inhibition of individual tyrosine kinases. Candidate targets found in primary samples include FLT1, PDGFR, JAK1/3, JAK2, CSF1R, ROR1, and EPHA5. Studies using small-molecule kinase inhibitors have confirmed sensitivity of specific samples to inhibition of target genes identified by the assay. Finally, the mechanism of oncogenesis and its relation to the gene target has been established in select samples with genetic abnormalities ranging from point mutations and insertional mutations to evidence of chromosomal rearrangements.
Conclusions: We demonstrate that RNAi functional screening can determine sensitivity to individual tyrosine kinases, both in cell lines and in primary samples. For the first time, this technique offers the potential to match specific therapies for targeted intervention with individual patients based on a functional assay.
CMK cells were transfected with an siRNA library individually targeting each member of the tyrosine kinase family, N-RAS, K-RAS, and non-specific controls. Cell viability was determined by an MTS assay 4 days later. Each bar represents an individual kinase with values shown as percent mean ± s.e.m (n = 3) (normalized to non-specific controls).
CMK cells were transfected with an siRNA library individually targeting each member of the tyrosine kinase family, N-RAS, K-RAS, and non-specific controls. Cell viability was determined by an MTS assay 4 days later. Each bar represents an individual kinase with values shown as percent mean ± s.e.m (n = 3) (normalized to non-specific controls).
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal