Abstract
Background: Within the concept of reduced-intensity stem cell transplantation (RIST), there is a wide range of differences in regimens utilized, in terms of toxicities and antileukemia effects, and only a little information is available on the clinical impact of chimerism status in patients conditioned with a busulfan-containing regimen. To examine this point, we reviewed the pattern of lineage-specific chimerism to correlate with subsequent clinical outcomes.
Patients and Methods: We retrospectively reviewed the data of 117 patients (median age, 52 years: range, 29–68) who had various hematological malignancies and underwent busulfan-containing RIST with related blood stem cells (n=81), related marrow (n=4) or unrelated marrow (n=32), between January 2000 and December 2006. The conditioning regimen consisted of busulfan (8 mg/kg) and fludarabine (180 mg/m2, n=64) or cladribine (0.66 mg/kg, n=53), with or without 2–4 Gy TBI (n=26) or anti-thymocyte globulin (5–10 mg/kg: n=31). Prophylaxis for GVHD consisted of cyclosporin or tacrolimus, with or without methotrexate. Chimerism was evaluated with peripheral blood samples taken on days 30, 60 and 90 after transplantation by PCR-based amplification of polymorphic short tandem repeat regions.
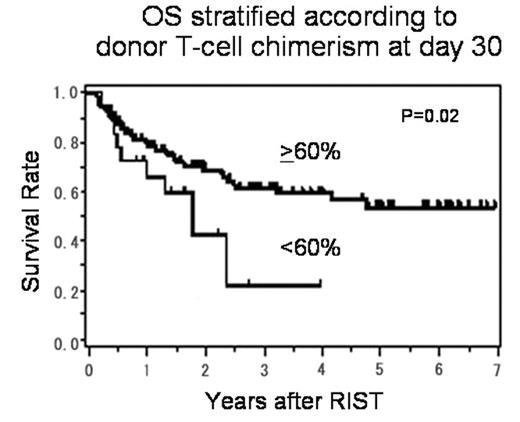
Results: The median follow-up of surviving patients was 857 days (50–2535). Percent donor-chimerism was significantly higher in granulocytes than T-cell fraction throughout the entire course, and the mean values were, respectively, 96% vs 83%, 98% vs 89% and 98% vs 91% at days 30, 60 and 90 after RIST. At day 30, the numbers of patients with T-cell chimerism >90%, 60–90% and <60% were 67 (58%), 32 (27%) and 18 (15%), respectively. The mean percentage of donor T-cell chimerism on day 30 was 18% (0–63%) in 5 patients who experienced graft failure (GF), which was significantly lower than that (86%; 15–100%) in the rest of the patients (p<0.01). No correlation was found between the kinetics of T-cell chimerism and the occurrence of acute or chronic GVHD. A multivariate analysis showed that low donor T-cell chimerism of <60% at day 30 was significantly associated with an increased risk of treatment failure (TF) at day 100, which included GF, progressive disease, relapse and non-relapse mortality (HR: 3.3 [95% CI, 1.4–7.8] p<0.01), but not with 1-year TF. The stem cell source and the addition of TBI or ATG were not associated with the degree of T-cell chimerism, overall survival (OS) or TF. In a Cox proportional hazard model, low donor T-cell chimerism of <60% at day 30 was associated with poor OS (HR: 2.2 [95% CI, 1.1–4.4] p=0.02) (Figure) and TF (HR: 2.0 [95% CI, 1.1–3.8] p=0.02).
Conclusion: We found that 42% of the patients retained mixed donor T-cell chimerism (≤90% donor), whereas 92% achieved complete chimerism in granulocyte fraction. Low donor T-cell chimerism of <60% at day 30 may predict a poor outcome, and a prospective study to examine the value of early intervention based on chimerism data is warranted.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal