Abstract
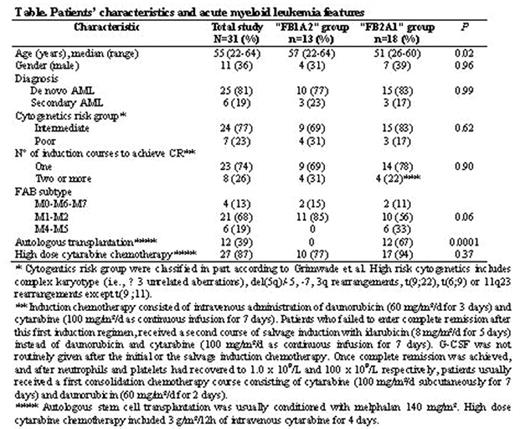
In the setting of AML, RIC regimens have emerged as an attractive modality to decrease toxicity. However, toxicity might represent only one aspect of the problem, since AML encompasses a group of chemosensitive diseases, raising concerns that significant reduction of the intensity of the preparative regimen, while increasing immunosuppression, may have a negative impact on long-term outcome. This report describes the comparative results of 31 AML patients in CR1 receiving RIC allo-SCT from an HLA-identical sibling in 2 institutions (Nantes, n=13; and Marseille, n=18) using 2 different “global” treatment approaches (Table below). After achievement of CR1, the “Nantes” approach included administration of one or two courses of consolidation with high-dose cytarabine (HDC), followed immediately by allo-SCT conditioned with a genuine nonmyeloablative, but highly immunosuppressive RIC regimen including fludarabine, low dose busulfan (4 mg/Kg), ATG (5 mg/Kg) and both CsA and corticosteroids for GVHD prophylaxis (“FB1A2” group). The “Marseille” program aimed to deliver after CR1, in addition to HDC, an autologous SCT followed by allo-SCT conditioned with fludarabine, an intermediate dose of busulfan (8 mg/Kg), low dose ATG (2.5 mg/Kg) and CsA alone for GVHD prophylaxis (“FB2A1” group). In the “FB2A1” group, 12 patients (67%) could actually receive the planned auto-SCT. With a median follow-up of 47 months, leukemia-free survival (LFS) in the whole study population was 56% at 5 years. However, the KM estimate of LFS was significantly higher in the “FB2A1” group as compared to the “FB1A2” group (P=0.01; 72% vs. 31% at 5 years). Overall, 8 patients (26%; 95%CI, 11–41%) had relapsed at a median of 320 (range, 241–707) days after diagnosis, and the significant difference between the 2 groups in terms of LFS was likely due to a higher risk of leukemia relapse in the “FB1A2” group (6/13, vs. 2/18; P=0.07). Five patients died from toxicity, for an overall incidence of TRM of 16% (95%CI, 6–34%), with this being comparable between the 2 groups (2/13 vs. 3/18; P=NS). Such comparable TRM despite a more “intensive” approach, translated towards a higher overall survival in the “FB2A1” group as compared to the “FB1A2” group (72% vs. 42% at 5 years; P=0.07). After controlling for relevant factors, in the multivariate analysis, actual performance of auto-SCT prior to RIC allo-SCT (P=0.04; RR=4.9; 95%CI, 1.1–22.4), was significantly predictive of an improved LFS. We conclude that RIC allo-SCT from an HLA-matched related donor represents a valid option for AML patients not eligible for standard allo-SCT. However, in order to achieve optimal results, a comprehensive treatment “package” including some form of high dose therapy prior to RIC allo-SCT and/or semi-intensive cytoreduction/myeloablation incorporated within the RIC regimen is likely necessary to allow sufficient time for the GVL effect.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal