Abstract
Background: [18F] FDG-PET after only 2–3 cycles of chemotherapy is highly prognostic in newly diagnosed aggressive non-Hodgkin’s lymphoma (NHL), with reported relapse rates of 71–100% after standard therapy if midtreatment PET remains positive. We hypothesized that early treatment intensification, based on individualized risk assessment by PET, would improve outcome.
Methods: A phase II, single center trial was activated in 2/2004 for patients (pts) with newly diagnosed aggressive NHL. PET-CT was performed between days 11 and 20 of cycle 2 or 3 of CHOP + rituximab (R). Pts whose PET scan was considered positive on semiquantitative visual interpretation, irrespective of stage or international prognostic index (IPI), received 2 cycles of (R)ESHAP or (R)ICE, followed by autologous stem cell transplantation (SCT) with busulfan-cyclophosphamide conditioning. Biopsies of FDG positive areas were not performed. Pts with negative midtreatment PET completed standard therapy. Tumor FDG uptake was graded on a 5-point scale: 0, no tumor activity (cold); 1+, minimal; 2+, equivocal (equal to mediastinal blood pool); 3+, moderately greater than blood pool; 4+, strong. Scores of 3+ or 4+ were considered positive.
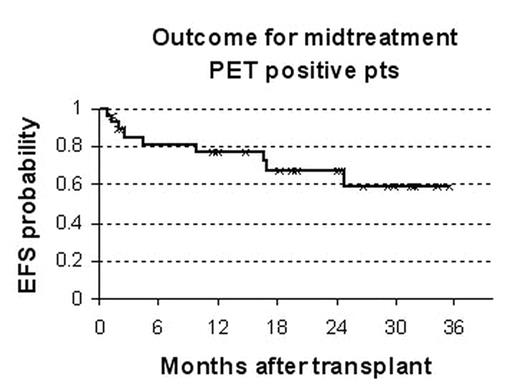
Results: As of 8/16/2007, 58 evaluable pts have been accrued, 55 with large B-cell lymphoma. Median age at diagnosis was 51 (20–78); 39 pts had stage III or IV disease. Thirty-two pts (55%) had positive midtreatment PET (17 with score of 3+, 15 with score of 4+). Twenty-six pts (45%) had negative midtreatment PET (10 with score of 0, 3 with score of 1+, 13 with score of 2+). PET was positive in 23 of 38 pts (61%) with IPI 0–2 and in 9 of 18 pts (50%) with IPI 3-5; 2 pts had undetermined IPI. Twenty-eight of 32 pts with positive midtreatment PET have completed SCT; 3 were ineligible because of early disease progression; 1 withdrew consent. At a median follow-up of 20.4 months, their actuarial 2-year event-free survival (EFS) after SCT is 67% (figure). Overall, 19 of 28 transplant recipients are alive and without evidence of relapse or secondary cancers: 6 relapsed at a median of 3.6 months (< 1–17 months) after SCT; 1 died of venoocclusive disease (1.4 months); 1 developed MDS (24.8 months); 1 had an unrelated death (9.9 months). On intent-to-treat analysis, the actuarial 2-year EFS from the time of midtreatment imaging is 60% if PET positive (including pts not eligible for transplant), 86% if PET negative. Of the PET negative pts, 2 have relapsed and 1 developed AML.
Conclusion: These data confirm the strong prognostic value of negative midtreatment PET scans, with excellent outcomes in such pts treated with (R)CHOP alone. Early treatment intensification for poor-risk pts, as identified by midtreatment PET, results in a significantly better outcome than that achieved historically.
Outcome for midtreatment PET positive pts
Author notes
Disclosure:Consultancy: LJS has received consulting fees from Genentech/Biogen Idec; RLW has received consulting fees from Nihon Mediphysics. Research Funding: YLK and LJS have received research funding from Genentech/Biogen Idec; RLW has received research funding from GE Healthcare. Honoraria Information: LJS has received honoraria from Genentech/Biogen Idec. RLW has received honoraria from GE Healthcare. Membership Information: LJS has served as a speaker for Genentech/Biogen Idec.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal