Abstract
Background: 90Y ibritumomab tiuxetan (Zevalin®) has been shown to be an effective therapy for patients with both follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). We have previously reported the feasibility of adding high-dose 90Y ibritumomab tiuxetan to high-dose VP-16 and CY followed by AHSCT without additional toxicity. Herein, we reported longer follow-up results of this high-dose regimen.
Methods: Patients undergo dosimetry study (day-21) with 5 mCi 111In-ibritumomab tiuxetan following 250 mg/m2of rituximab, followed by 90Y ibritumomab tiuxetan (day-14) to deliver a target dose of 1000 cGy to highest normal organ and then VP-16 (day-4), and CY (day-2). Bone marrow biopsy is done on day-7 to estimate the radiation dose. Stem cells are re-infused when the radiation dose to re-infused stem cells is estimated to be <5 cGy.
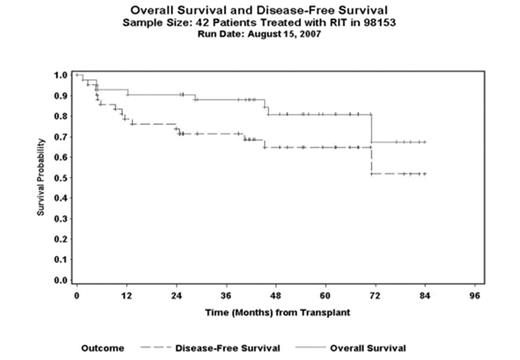
Results: Between 5/00 and 7/05, 53 patients were enrolled; 11 had an imaging study but did not proceed to treatment due to dosimetry ineligibility (7), progressive disease (2), reaction to 111In-ibritumomab tiuxetan (1) and reaction to rituximab (1). 42 patients (25 M: 17F), median age 51 yrs (range 25–59.6) with FL (n=13), DLBCL (n=20), mantle cell (MCL) (n=8) and transformed lymphoma (n=1) were treated. Disease status at AHSCT: 1stCR/PR=11, ≥2ndCR=13, REL=9 and IF=9. Twenty-three (55%) had prior BM involvement. Median number of prior chemo regimens was 2 (range 1–6). All but two had received rituximab. As part of phase I/II trial, 6 received VP-16 40mg/kg and the rest received 60 mg/kg. The median 90Y ibritumomab tiuxetan dose delivered was 70.8 mCi (range 37–105). The treatment was well tolerated with mucositis and neutropenic fever being the most common acute toxicities. All but one patient engrafted. The median time to reach ANC>500/μl and platelet>20,000/μl was 10 days (range 8–17) and 12.5days (range 8–123), respectively. The transplant-related mortality (TRM) at day 100 was 2%. There were 8 deaths due to relapse (3), second malignancies (2), graft failure (1), alcohol induced liver failure (1) and sudden death (1). Secondary malignancies occurred in 3 (7%) heavily pre-treated FL: pancreatic cancer at 3.8 yrs, acute myeloid leukemia at 5 years and one with abnormal chromosome but without morphologic evidence of MDS at 1 year. At a median follow-up of 55 months (range, 25–84) for the surviving patients, the 4-year estimated overall survival (OS) and disease-free survival (DFS) is 81% (95% CI, 67–89%), and 65% (95% CI, 54–74%), respectively (figure.1). The 4-year estimated DFS for FL, DLBCL and MCL is 71% (95% CI, 51–85%), 67% (95% CI, 49–79%) and 47% (95% CI, 29–63%), respectively.
Conclusion: Our long-term results suggest that the combination of high-dose 90Y ibritumomab tiuxetan and high-dose VP-16 and CY is an effective high-dose regimen, especially for FL and DLBCL. Short term toxicities appear comparable to other conventional high-dose regimens. Further prospective studies are ongoing to determine the curability and long term toxicities of this preparative regimen.
Overall Survival and Disease-Free Survival Sample Size: 42 patients Treated with RIT in 98153 Run Date: August 15, 2007
Overall Survival and Disease-Free Survival Sample Size: 42 patients Treated with RIT in 98153 Run Date: August 15, 2007
Author notes
Disclosure:Research Funding: Research funding for the study from Biogen Idec. Off Label Use: Using Zevalin in the autologous stem cell transplant setting.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal