Abstract
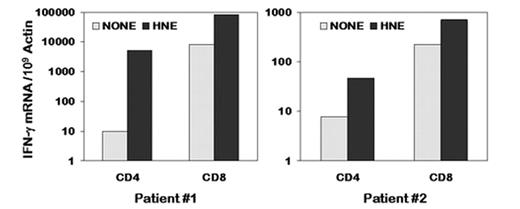
The detection of naturally occurring T cells responding to proteins overexpressed in myeloid leukemia suggests that vaccines could boost the immune response to leukemia and achieve disease control. Human neutrophil elastase (HNE) is a myeloid-restricted protein highly expressed in myeloid leukemia cells. HNE protein can induce both CD4+ and CD8+ T cell responses in healthy individuals including HLA-A*0201 negative individuals. To develop HNE protein vaccine, and to identify major histocompatibility complex (MHC) class II restricted or HLA-A*0201 negative MHC class I restricted epitopes in HNE, we stimulated peripherial blood mononuclear cells (PBMC) from 6 myeloid leukemia pre-transplant patients with purified full length HNE protein. HNE protein induced a response in CD4+ and CD8+ T cells from 4 patients with acute myeloid leukemia (AML), as measured by IFN-γ protein expression in flow cytometric assay. However, no response was detected in CD4+ and CD8+ T cells from 2 pre-transplant patients with chronic myeloid leukemia (CML). Responses of CD4+ and CD8+ T cells to HNE protein stimulation were enhanced by HNE expressing antigen-presenting T cells (T-APC), as measured by flow cytometric assay. HNE protein induced significant IFN-γ expression in CD4+ and CD8+ T cells at 540X and 10X above background in an (HLA-A11, 31) AML patient (patient #1 in the figure), and at 6X and 3.5X above background in an (HLA-A*0205, 03*) AML patient (patient #2 in the figure), as measured by quantitative Real-Time RT-PCR assay (QRT-PCR). HNE protein also induced significant IL-2 production in CD4+ and CD8+ T cells at 7.7X and 6.1X above background in patient #1. Finally, we showed by QRT-PCR that the T cell responses to HNE protein stimulation were associated with the levels of HNE gene transcripts in the PBMC. Thus we found that full-length HNE protein stimulated both CD4+ and CD8+ T cell responses in HLA-A*0201 negative patients with AML. These results provide the rationale for using the full-length HNE protein to vaccinate patients with myeloid leukemias pre- or post-stem cell transplantation to improve graft-versus-leukemia effects.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal