Abstract
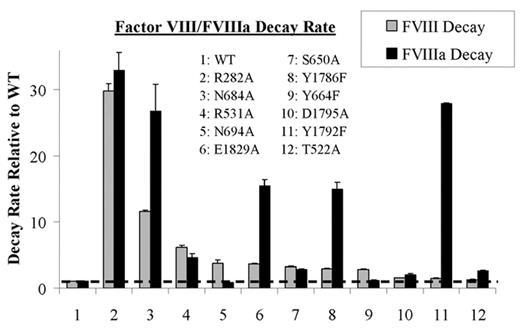
Factor VIII circulates as a heterodimer composed of a heavy chain and light chain. Thrombin converts factor VIII into the active cofactor, factor VIIIa, by cleaving heavy chain into A1 and A2 subunits. While the A1 subunit maintains a stable interaction with the light chain-derived A3C1C2 subunit, the A2 subunit is weakly associated in the trimer and this low affinity interaction accounts for the instability of factor VIIIa activity. In examining the ceruloplasmin-based factor VIII A domain model, potential hydrogen bond pairings based upon spatial separations of <2.8Å were found between side chains of the A2 domain residues and residues in the A1 or A3 domain represented by residues D27, R282, E287, D302, S313, H317, Y476, T522, R531, N538, E540, S650, Y664, N684, N694, S695, D696, Y1786, S1791, Y1792, D1795, E1829, and S1949. Since hydrogen bonds at an interactive site contribute to structural stability, we performed a scanning mutagenesis study where these residues were individually replaced with Ala, except Tyr residues were replaced with Phe, in order to examine the contribution of each site to the stability of the factor VIII/VIIIa forms. Factor VIII activity decay was followed by incubating factor VIII (5 nM) at 55°C, and at indicated times removing an aliquot, activating with thrombin and measuring residual activity by a factor Xa generation assay. Factor VIIIa activity decay was measured by mixing factor VIII (5 nM) with factor IXa (40 nM), activating factor VIII with thrombin, and following factor Xase activity at 23°C by factor Xa generation. Non-linear least squares regression using a single exponential decay equation of activity versus time was performed to obtain rates for factor VIII/VIIIa activity decay. Eleven out of 23 factor VIII mutants showed increases in either or both decay rates by >2-fold compared to wild type (WT) (Figure). Of these mutants, R282A showed the largest increase in both factor VIII and VIIIa decay rates (∼30-fold compared to WT). Interestingly, 5 mutants (T522A, D1795A, Y1792F, Y1786F, and E1892A) showed >2-fold increased rates in factor VIIIa decay compared with the rates for factor VIII decay, whereas 2 mutants (N694A and Y664F) showed >2-fold increased rates in factor VIII decay compared with rates for factor VIIIa decay. These results suggest that several residues at the A1-A2 and A2-A3 domain interfaces contribute to stabilizing the protein through hydrogen bonding and that mutation at these sites result in loss of stability as determined by enhanced rates of activity decay. Furthermore, these results permit discrimination between stabilizing hydrogen bonding in the procofactor from active cofactor, where bonding in the latter appears to make a more significant contribution to stability. This observation is consistent with an altered conformation involving new inter-subunit interactions for the A2 domain following factor VIII activation.
Factor VIII/FVIIIa Decay Rate
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal