Abstract
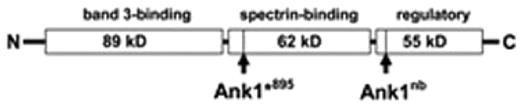
As part of a concerted program to generate new models of human disease we have generated multiple heritable mutant mouse strains using ethylnitrosourea (ENU) random mutagenesis combined with a dominant blood screen. Here we describe a mutant strain with dominantly-inherited red blood cell (RBC) low mean corpuscular volume. Preliminary phenotypic analysis of affected mice demonstrated spherical RBC morphology, increased osmotic fragility, mild reticulocytosis and a reduction in circulating lifetime - features characteristic of human hereditary spherocytosis. Gene mapping revealed the causative point mutation to be in the mouse ankyrin-1 gene (Ank1) locus at exon 26 - a G to T transversion causing substitution of a glutamate codon with a premature stop (Ank1*895). The resulting gene product is a truncated ankyrin-1 protein (terminating at amino acid 894) consisting primarily of the N-terminal band 3-binding domain without a functional ZU5 domain (spectrin-binding) or regulatory/death domain (Fig.1). The Ank1*895 allele results in a hypomorph such that stably-expressed protein isolated from RBC ghost preparations is undetectable by Coomasie stain and only barely detectable by Western. Our ENU-generated mutation is distinct from the spontaneous mutation identified in the normoblastosis (nb) mouse (Peters et al. 1991. J. Cell Biol; Birkenmeier et al. 2003. Hematol J.) which yields a functional protein product with intact band 3- and spectrin-binding domains (Fig.1).
Schematic representation of mouse ankyrin-1 peptide showing sites of truncation products encoded by the ENU-generated nonsense mutation Ank1*895 (Glu → stop) and the normoblastosis (nb) mouse (Ank1nb). While the phenotype of the heterozygous (Ank1*895/+) mutant line on the C3H background is mild, intercross breeding of mutant mice did not yield pups homozygous for the mutant allele - suggesting an embryonic lethal phenotype. Surprisingly, when the C3H-Ank1*895 line was bred with the SvImJ/129 strain we were able to obtain viable homozygous Ank1*895/*895 offspring from intercross of the Ank1*895/+ 129xC3H hybrid mutant line. Homozygous Ank1*895 mice were obtained at low frequency and displayed a severe phenotype with remarkable splenomegaly. In this study we have generated a novel mouse model of hereditary spherocytosis and examined the compensatory mechanisms that permit the survival of homozygous Ank1*895 mice from embryo to adults. In addition, we determined the stability of Ank1*895 protein in homozygous mice and its effect on the assembly of RBC membrane structural complexes in the absence of full-length ankyrin-1. MRH and SM are fellows of the CIHR/HSFC Strategic Training Program in Transfusion Science at the UBC Centre for Blood Research (CBR). KMM is a Michael Smith Foundation for Health Research Scholar and CBR member. This study was supported by a group operating grant from the CIHR (FRN 74611) and fellowships from the Heart & Stroke/Richard Lewar Centre of Excellence.
Schematic representation of mouse ankyrin-1 peptide showing sites of truncation products encoded by the ENU-generated nonsense mutation Ank1*895 (Glu → stop) and the normoblastosis (nb) mouse (Ank1nb). While the phenotype of the heterozygous (Ank1*895/+) mutant line on the C3H background is mild, intercross breeding of mutant mice did not yield pups homozygous for the mutant allele - suggesting an embryonic lethal phenotype. Surprisingly, when the C3H-Ank1*895 line was bred with the SvImJ/129 strain we were able to obtain viable homozygous Ank1*895/*895 offspring from intercross of the Ank1*895/+ 129xC3H hybrid mutant line. Homozygous Ank1*895 mice were obtained at low frequency and displayed a severe phenotype with remarkable splenomegaly. In this study we have generated a novel mouse model of hereditary spherocytosis and examined the compensatory mechanisms that permit the survival of homozygous Ank1*895 mice from embryo to adults. In addition, we determined the stability of Ank1*895 protein in homozygous mice and its effect on the assembly of RBC membrane structural complexes in the absence of full-length ankyrin-1. MRH and SM are fellows of the CIHR/HSFC Strategic Training Program in Transfusion Science at the UBC Centre for Blood Research (CBR). KMM is a Michael Smith Foundation for Health Research Scholar and CBR member. This study was supported by a group operating grant from the CIHR (FRN 74611) and fellowships from the Heart & Stroke/Richard Lewar Centre of Excellence.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal