Abstract
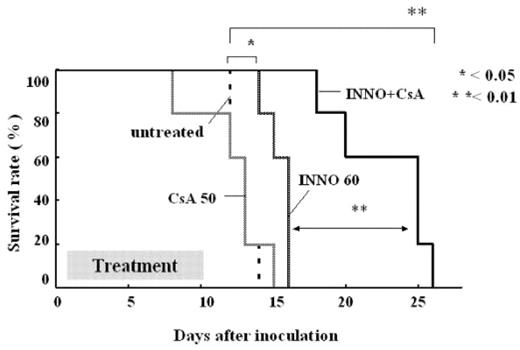
Central nervous system (CNS) is one of the major cites for extramedullary relapse of Ph+ leukemias, which have been treated with imatinib mesylate (IM). The reason for this is that IM is a substrate for P-glycoprotein (P-gp) at the blood brain barrier and effluxed by it. We have already shown in the last annual meeting that INNO-406 had much stronger anti-tumor effects against the murine CNS leukemia model compared with IM, and INNO-406 is also effluxed from the murine CNS by P-gp. In this study, we investigated the combination effect of INNO-406 and P-gp inhibitors, verapamil or cyclosporin-A (CsA). First, we examined the growth-suppressive effect of INNO-406 and the combination with the P-gp inhibitors against the BCR-ABL positive leukemic cell line, K562 and the P-gp-overexpressing K562, K562/D1-9 cell line. K562/D1-9 showed 10 times higher resistant to both IM and INNO-406 compared with K562. Furthermore, both verapamil and CsA synergistically augmented the effect of INNO-406. Next, we investigated the pharmacokinetics of INNO-406 when orally administrated with CsA to mice. Mice were administrated p.o. with 50mg/kg of CsA 2 hours before INNO-406. We found that the concentration of INNO-406 in the CNS elevated by 2 times when combined with CsA, while the plasma concentration was decreased to two thirds of that when singly administrated with INNO-406. It was suggested that the decreased plasma concentration of INNO-406 seen here resulted from the increased uptake into the CNS by CsA inhibiting P-gp at the blood brain barrier. These changes of the drug distribution to the murine tissues may alter the anti-leukemic effect of INNO-406, thus we planned to investigate the combination effect of INNO-406 and CsA in the murine models of both CNS and systemic leukemia. We found that CsA significantly augmented the anti-tumor effect of INNO-406 in the CNS leukemia model. Moreover, in spite of the decreased plasma concentration of INNO-406, the combination with CsA also prolonged the survival phase of the mice in the systemic leukemia model, more significantly than single treatment of INNO-406 (Figure). This may be explained by which CsA increased the intracellular uptake of INNO-406, resulted from the direct inhibition of drug efflux via P-gp expressed in the leukemic cells. Phase I clinical study on INNO-406 is now underway in MD Anderson Cancer Center and in Frankfurt University. From the results of this study, we expected the effective application of INNO-406 in combination with P-gp inhibitor to the patients suffering from refractory Ph+ leukemia as well as CNS relapse.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal