Abstract
INTRODUCTION: The majority of patients (pts) with MDS become red blood cell (RBC) transfusion dependent with escalating risk for transfusional hemosiderosis and its adverse effect on morbidity and mortality. The US03 trial is designed to evaluate long-term efficacy and safety of the oral iron chelator, deferasirox (DFX), in pts with lower risk MDS. In this ongoing study 53 pts have completed 12 months’ (mos) treatment.
METHODS: US03 is a Phase II, open-label, 3-year trial in pts with Low- or Int-1 IPSS risk MDS and transfusional iron overload (SF ≥1000 μg/L and >20 units RBC transfusions [tx]), with serum creatinine (SCr) ≤2-fold the upper limit of normal (ULN). Initial DFX dose was 20 mg/kg/day and could be increased to 40 mg/kg/day based on tolerability and response. SF was monitored monthly; LPI, the reactive species of non-transferrin-bound iron, was assessed quarterly.
BASELINE FEATURES: 176 pts were enrolled at 45 centers. Mean age was 70 years (range, 21−90), including 102 men, 71 women; and IPSS risk groups of Low 46 pts (27%), Int-1 123 (71%), other 4 (2%). Mean baseline iron status: SF 3398 μg/L (range, 863−36,280); LPI 0.4 μmol/L (0.0−3.6); mean lifetime txs prior to study, 63; years of prior tx 3.5 (0−34). MDS therapy at study entry included chemotherapy, 22 pts; growth factors, 46. Estimated creatinine clearance: normal (>80 mL/min), 77 pts; mild (51−80 mL/min), 68; moderate, (30–50 mL/min) 25; severe (<30 mL/min) 2. Over 12 mos the mean dose was 21 mg/kg/day, and the mean tx rate was 4.1 units/mo. Forty percent of pts had elevated LPI at baseline (≥0.5 μmol/L).
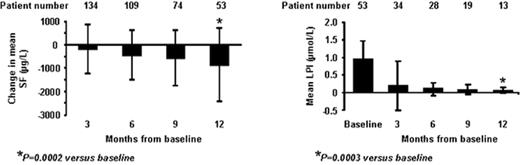
RESULTS: Mean SF±SD (μg/L) values: baseline 3398±3088; 3 mos 3065±1743; 6 mos 2775±1355; 9 mos 2759±1562; 12 mos 2603±1336. Sustained suppression of mean LPI to the normal range was achieved after 3 mos of treatment. (Figure shows changes in SF and LPI). Hematologic improvement by IWG criteria: 5 pts (6%); erythroid response: 3 (major 1; minor 2); platelet 2 (major); neutrophil 1 (major).
SAFETY: Of 165 pts, only 10 (6%) discontinued secondary to suspected adverse events (AEs), and 7 (4%) serious AEs. There were 11 deaths (7%), all unrelated to DFX. Of 140 pts with normal baseline SCr, 35 (25%) increased >ULN (2.2 mg/dL max SCr). SCr increased >33% above baseline in 11 pts (8%) abnormal at baseline. New onset of thrombocytopenia and neutropenia were 52/165 (32%) and 22/165 (13%), respectively.
CONCLUSIONS: DFX therapy decreased mean SF over 1 year in this heavily iron-replete MDS population. Trough LPI normalized in 100% of pts over 12 mos, indicating 24-hour sustained suppression. DFX had a manageable safety profile in this population; new cytopenias were consistent with hematologic progression of MDS. Recent reviews show a 30% increase in hazard ratio for every 500 ng/mL increase in SF >1000 ng/mL; NCCN guidelines recommend consideration of iron chelation in iron-overloaded MDS pts. Ongoing assessments evaluating cardiac, hepatic and endocrine function will evaluate the impact of iron reduction on morbidity and mortality in MDS.
Author notes
Disclosure:Employment: J Esposito, J Virkus and C Paley are Novartis employees. Consultancy: A List - Celgene, Pharmion, Kanisa, SBIO; A Raza - Advisory board (Novartis). Research Funding: A List - Celgene and Novartis; D Steensma - to Mayo Clinic for clinical and ancillary studies (Novartis, MGI Pharma, OBIUS, Amgen); A Raza and E Besa - Clinical trials of deferasirox (Novartis). Honoraria Information: A List - Celgene, Pharmion, MGI Pharma; A Raza - Novartis. Membership Information: A List - Celgene, Pharmion, Kanisa, SBIO; A Raza - Speakers’ Bureau and Advisory Board (Novartis); E Besa - Speakers’ Bureau (Novartis).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal