Abstract
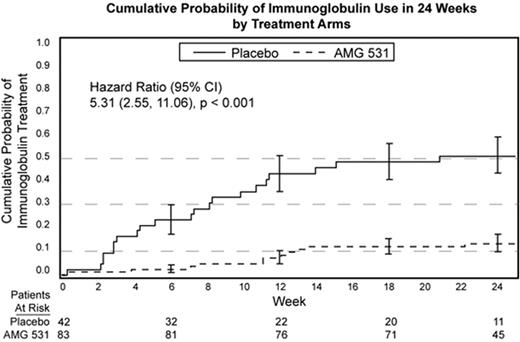
AMG 531 is a novel thrombopoiesis-stimulating peptibody that is being studied for its ability to increase platelet production by stimulating the thrombopoietin receptor. We report pooled data on acute use of immunoglobulin (IVIG or Anti D) in both splenectomized and nonsplenectomized patients from 2 randomized, double blind, placebo-controlled Phase 3 studies designed to evaluate the efficacy and safety of AMG 531 in adult patients with chronic ITP. Patients were randomized to receive either AMG 531 or placebo at a 2:1 ratio, and concomitant medications were continued. Of the 125 patients enrolled, 63 were splenectomized (placebo, 21; AMG 531, 42), and 62 were nonsplenectomized (placebo, 21; AMG 531, 41) with a median age of 52 years (range 21 to 88) and a mean baseline platelet count of 16.5x109/L. Immunoglobulin was allowed as rescue medication when medically necessary at the discretion of the physician. For both trial arms, we calculated the percentage of patients requiring immunoglobulin intervention in each month of treatment, and the age and sex-adjusted 24-week cumulative probability of immunoglobulin use. No difference was observed between splenectomized and nonsplenectomized patients; therefore pooled results are shown. Fisher’s exact tests and Log-Rank tests were used to compare the between-arm differences. We also performed subgroup analyses of immunoglobulin use in overall responders (defined as either platelet count ≥50x109/L for ≥6 weeks during the last 8 weeks of the 24-week treatment period in the absence of rescue medications at any point in the study, or ≥4 weekly platelet counts ≥50x109/L in the absence of rescue medications in the previous 8 weeks), and placebo patients. In the 24-week study period, there were 19 immunoglobulin administrations among 83 AMG 531 patients, and 68 immunoglobulin administrations among 42 placebo patients. The cumulative probability of incurring immunoglobulin use in 24 weeks was 0.51 (SE: 0.08) for the placebo arm and 0.13 (SE: 0.04) for the AMG 531 arm (see Figure), with a hazard ratio of 5.31 (95% CI: 2.55–11.06, p<0.001). Per-cycle immunoglobulin use ranged from 19% to 29% in the placebo arm, and from 0% to 6% in the AMG 531 arm (p≤0.01 in all cycles). Subgroup analysis showed that compared to placebo patients, overall responders of AMG 531 had even greater reduction in immunoglobulin use in each cycle (0 to 4%, p<0.006). AMG 531 treatment was associated with significantly reduced immunoglobulin use in both splenectomized and nonsplenectomized patients with chronic ITP. Moreover, placebo patients were 5 times more likely to receive immunoglobulin therapy compared to AMG 531-treated patients.
Cumulative Probability of Immunoglobulin Use in 24 Weeks by Treatment Arms
Cumulative Probability of Immunoglobulin Use in 24 Weeks by Treatment Arms
Author notes
Disclosure: Employment: SG, RS, JN: Amgen. Consultancy: T Gernsheimer: Amgen, GlaxoSmithKline; AN: GlaxoSmithKline, Protalex. Ownership Interests:; SG, RS, JN: Amgen. Research Funding: TGernsheimer, AN: Amgen, GlaxoSmithKline; VP, JWasser, TGuthrie, JdeWolf, SH: Amgen. Honoraria Information: TGernsheimer: Amgen, GlaxoSmithKline; AN: Amgen, Baxter, GlaxoSmithKline, Membership Information: VP: Novartis, Schering-Plough; TG: Amgen, GlaxoSmithKline; AN: Amgen, Baxter, GlaxoSmithKline, Protalex.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal