Abstract
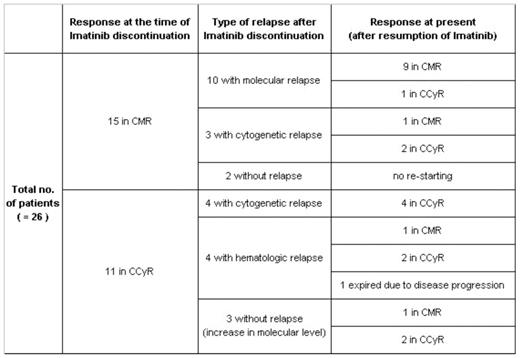
Diagnosis of chronic myeloid leukemia (CML) is based on detection of the BCR-ABL gene or Philadelphia chromosome, and the BCR-ABL tyrosine kinase inhibitor imatinib has been the standard therapy for CML patients. Although imatinib therapy is effective in CML, it is still unclear whether imatinib can be safely discontinued without relapse. This study was designed to investigate the outcome of 26 CML patients after discontinuation of imatinib and to determine whether intermittent imatinib therapy can be employed in CML patients. Between May 2001 and Jun 2007, 555 patients have been treated with imatinib in St Mary’s Hospital of the Catholic University of Korea, and 26 patients discontinued imatinib when they achieved either complete cytogenetic response (CCyR) or complete molecular response (CMR). These 26 patients were diagnosed as Philadelphia positive (Ph+) CML between November 1995 and May 2002, and 22 patients were in chronic phase (CP) and 4 patients were in accelerated phase (AP) at diagnosis. The median age was 35 years (22–56), and 12 patients (46%) were female and 14 (54%) were male. Among 26 patients, 7 received interferon prior to imatinib therapy and 7 underwent SCT. Five patients received both interferon and SCT before imatinib therapy, and the remaining 7 patients received the imatinib as a front line therapy. Imatinib was started at oral dose of 400mg and 600mg daily for patients in CP and AP, respectively, and when they achieved CCyR or CMR, imatinib was discontinued after informed consent of the patient. In case of cytogenetic or molecular relapse, patients in all phases were retreated with imatinib at 400mg daily. Bone marrow (BM) or peripheral blood (PB) samples were obtained at regular intervals from diagnosis for hematologic response (HR), cytogenetic response (CyR) and molecular response (MR) monitorings. Eleven patients discontinued imatinib when they achieved CCyR, and 15 patients discontinued imatinib after achieving CMR. After the median duration of 7 month (4–48) observation without imatinib therapy, hematologic, cytogenetic and molecular relapses occurred in 4, 7 and 10 patients, respectively, and imatinib at oral dose of 400mg daily was reintroduced to all patients except 2 who continued to remain in CMR after imatinib discontinuation. Except 1 patient who expired and 2 patients who are in persistent molecular remission, all of 23 patients are maintaining the best response achieved after imatinib resumption with a median duration of 38 months (16–58).
In conclusion, although imatinib cannot be discontinued completely, intermittent therapy can be considered for the treatment of CML patients. Intermittent imatinib treatment should not be restricted to CP patients who achieve CMR, and AP patients or patients with CCyR also can be considered for intermittent imatinib treatment. We will continue the follow-up of the patients enrolled in this study, and long-term study of intermittent imatinib treatment with expanded pool of patients will enable us to determine the accurate consequences of discontinuation of imatinib and intermittent imatinib treatment.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal