Abstract
Chronic graft-versus-host disease (cGVHD) is a common cause of morbidity and mortality in allogeneic bone marrow transplantation (alloBMT). However, effective strategies for the treatment of cGVHD have not been established. In this study, we examined the therapeutic utility of modified dendritic cells (DCs) with a greater capacity to regulate immune responses than previously known tolerogenic DCs, regulatory DCs (DCregs), in the major histocompatibility complex-compatible, and multiple minor histocompatibility antigen-incompatible model of cGVHD in alloBMT. Treatment of the recipient mice after alloBMT with the recipient-type DCregs led to greater suppression of the incidence and severity of cutaneous cGVHD than rapamycin, whereas treatment with the recipient-type mature DCs promoted the pathogenesis. Analysis of the recipient mice suggested that the protective effect of the recipient-type DCregs involved the peripheral generation of alloreactive CD4+CD25+Foxp3+regulatory T (TR) cells from donor-derived CD4+CD25−Foxp3− T cells. Thus, immunotherapy with DCregs is a promising strategy for the treatment of cGVHD in alloBMT mediated through the induction of a dominant tolerance involving CD4+CD25+Foxp3+ TR cells.
Introduction
Allogeneic bone marrow (BM) transplantation (alloBMT) is an effective treatment for leukemia as well as genetic disorders.1-3 However, its success is dependent on the prompt identification of a suitable donor and on the avoidance of opportunistic infections and severe graft-versus-host disease (GVHD).1-3 GVHD is due mainly to the activation of alloreactive T cells in the donor BM inoculum. Most therapeutic approaches designed to reduce GVHD have focused on the development of immunosuppressive agents and the ex vivo removal of the unfractionated donor T-cell population from the marrow graft.1-3 Although these strategies have reportedly reduced GVHD, there has been a reciprocal increase in the rate of graft rejection, more severe immunosuppression, and lethal infections as well as severe side effects.1-3
GVHD can be divided into acute and chronic forms that are likely to have different requirements for initiation and pathogenesis.3-5 Acute GVHD (aGVHD) and chronic GVHD (cGVHD) are traditionally diagnosed primarily by time of onset, with cGVHD occurring after day 100 after transplantation.3-5 However, cGVHD has distinct clinicopathologic features and is often diagnosed based on these features regardless of time of onset.3-5 aGVHD typically presents with inflamed skin, gastrointestinal disease, and/or hepatic disease, whereas cGVHD is characterized by cutaneous fibrosis, the involvement of exocrine glands, myositis, and hepatic disease.3-5 Previous studies have suggested that donor-derived CD8+ T cells and residual host antigen (Ag)-presenting cells (APCs) are required for aGVHD, whereas either donor or host APCs play a crucial role in cGVHD mediated by donor-derived CD4+ T cells in murine alloBMT.4,5 Relative to aGVHD, much less is understood about an effective therapy for cGVHD because of the limited number of murine models that resemble human cGVHD.
Dendritic cells (DCs), the most potent APCs, are defined by their dendritic morphology and consist of heterogeneous subsets with a myeloid-or lymphoid lineage as well as maturity in both lymphoid and peripheral tissues.6 Immature DCs (iDCs) sense the presence of invading pathogens via various pattern-recognition receptors and process the pathogens intracellularly in inflamed tissues, developing into mature DCs (mDCs) with the up-regulation of major histocompatibility complex (MHC) and costimulatory molecules in inflammatory microenvironments.6 Subsequently, mDCs home into secondary lymphoid tissues where they present the processed Ags to naive T cells to generate effector T cells.6 DCs thereby play a crucial role in the link between innate and adaptive immunity.6
The induction of tolerance is critical for the maintenance of immune homeostasis.7,8 In the thymus, central tolerance is a well-established mechanism that involves clonal deletion of self-reactive T cells.7,8 In the periphery, the mechanisms involved include clonal deletion and anergy as well as active suppression by regulatory T (TR) cells, such as thymic-derived, naturally occurring CD4+CD25+Foxp3+ TR (nTR) cells and inducible interleukin-10 (IL-10)-producing Tr1 cells, which can develop from peripheral naive CD4+ T cells, depending on environmental conditions.8,9 Accumulating indirect evidence suggests that iDCs are involved in the induction of peripheral tolerance under steady-state conditions in vivo.7,8,10 On the other hand, the modification of iDCs with certain immunosuppressive molecules generates tolerogenic DCs, which show the defective expression of MHC and costimulatory molecules and then lead to the reduction of T-cell stimulatory capacity.11-13 In addition, these tolerogenic DCs not only produce anergic T cells and IL-10–producing Tr1 cells but also possibly could expand the number of CD4+CD25+Foxp3+ nTR cells.11-13 However, the involvement of DC subsets in the peripheral generation of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells remains unclear.14
We have previously established modified DCs with a capacity to produce anergic T cells as well as CD4+CD25+ TR cells that is greater than previously known tolerogenic DCs, which we therefore designated as regulatory DCs (DCregs).15,16 In this study, we examined the ability of DCregs to control cutaneous cGVHD in MHC-compatible and multiple minor histocompatibility Ag (miHAg)-incompatible alloBMT.
Materials and methods
Mice
BALB/c mice (H-2d) were purchased from the Charles River Laboratories (Raleigh, NC). B10.D2 mice (H-2d) were purchased from The Jackson Laboratory (Bar Harbor, ME). Ovalbumin (OVA)-specific T cell receptor (TCR) (KJ1–26 clonotype) transgenic Rag2+/+DO11.10 BALB/c mice and Rag2−/−DO11.10 BALB/c mice have been described.17 All mice were used between 5 and 8 weeks of age and maintained in specific pathogen-free conditions and in accordance with guidelines of the Institutional Animal Care Committee of the RIKEN Institute.
Preparation of DCs
The DCs were prepared as described previously15,16 with some modifications. In brief, mDCs were prepared by culturing BM cells obtained from BALB/c mice with murine granulocyte macrophage-colony-stimulating factor (GM-CSF, 20 ng/mL; Wako Pure Chemical Industries, Osaka, Japan) for 8 days followed by stimulation with lipopolysaccharide (LPS; 1 μg/mL; Sigma-Aldrich, St Louis, MO) for 24 hours. DCregs were generated from BM cells obtained from BALB/c mice cultured with murine GM-CSF (20 ng/mL), murine IL-10 (20 ng/mL; Wako Pure Chemical Industries) and human transforming growth factor-β1 (TGF-β1, 20 ng/mL; Wako Pure Chemical Industries) for 8 days followed by stimulation with LPS (1 μg/mL) for 24 hours. Subsequently, DCregs were depleted of CD40+CD80+CD86+cells (approximately 20% of total cell preparations) with biotinylated monoclonal antibodies (mAbs) to CD40, CD80, and CD86 (all from BD Bioscience, San Jose, CA) plus IMag streptavidin Particles Plus-DM (BD Biosciences). In some experiments, mDCs or DCregs were pulsed with OVA323-339 peptide (10 μM) for 4 hours to generate OVA323-339 peptide-pulsed mDCs or DCregs (OVA323-339 peptide/mDCs or OVA323-339 peptide/DCregs, respectively).
Plasmacytoid DCs (pDCs) were purified from spleen mononuclear cells by AutoMACS with anti-mPDCA-1 microbeads (both from Miltenyi Biotec, Bergisch Gladbach, Germany). In another experiment, DCs (5 × 105) were left unstimulated or stimulated with CpG oligodeoxynucleotides (ODN, 0.1 μM; Hokkaido System Science, Sapporo, Japan) for 24 hours, and the culture supernatants were assayed for IL-12p40, IL-10, and interferon-α (IFN-α) using enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Camarillo, CA).
Flow cytometry
Cells were stained with fluorescein-conjugated mAbs to mouse CD4, CD8α, CD11b, CD11c, CD40, CD45RB, CD54, CD80, CD86, H-2Kd, I-A/I-E, 33D1, B220, Gr-1, isotype-matched control mAb (BD Biosciences), Foxp3 (eBioscience, San Diego, CA), CD25, mPDCA-1 (Miltenyi Biotec), CD205 (SeroTec, Raleigh, NC), and KJ1–26 (Caltag Laboratories, Burlingame, CA). For analysis of the intracellular expression of cytokines, cells were stimulated with immobilized anti-CD3 mAb and soluble anti-CD28 mAb (both 10 μg/mL from BD Biosciences) for 5 hours in the presence of Golgi Block (BD Biosciences). Intracellular staining was performed using a Cytofix/Cytoperm kit, and mAbs to IL-2, IL-4, IL-10, and IFN-γ (all from BD Biosciences) according to the manufacturer's instructions.16 Fluorescence staining was analyzed with a FACSCalibur flow cytometer and CELLQuest Software (both from BD Immunocytometry Systems, San Jose, CA).
Isolation of CD4+ T cells and their subsets
CD4+ T cells were negatively selected from spleen mononuclear cells obtained from B10.D2 mice with mouse CD4 T lymphocyte Enrichment Set-DM (BD Biosciences). Subsequently, CD4+ T cells were sorted into CD4+CD25− T cells and CD4+CD25+ T cells with high purity (> 98%) by AutoMACS with a CD4+CD25+Regulatory T cells Isolation Kit (Miltenyi Biotec) followed by FACSVantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) with fluorescein-conjugated mAbs to CD4 and CD25. Likewise, Rag2+/+KJ1–26+ T cells and Rag2−/−KJ1–26+ T cells were prepared from Rag2+/+DO11.10 BALB/c mice and Rag2−/−BALB/c DO11.10 mice, and then KJ1–26+ T cells were sorted into CD25− T cells and CD25+ T cells.
In vitro priming of CD4+ T cells
CD4+CD25− T cells (5 × 106) obtained from B10.D2 mice were cultured with the irradiated (15 Gy from a cesium 137 source; Gammacell 40 Exactor, MDS Nordion, Ottawa, Ontario, Canada) mDCs or DCregs (5 × 105) obtained from BALB/c mice in six-well flat plates (BD Biosciences) for 7 days. In another experiment, 106 CD4+CD25− T cells and 105 DCregs were cocultured in 24-well flat plates (BD Biosciences) or placed separately in 24-well Transwell cell culture chambers (Millicell; Millipore, Billerica, MA) in the presence or absence of neutralizing anti-IL-10 mAb (10 μg/mL; clone JES5–2A5; BD Biosciences), neutralizing anti-TGF-β mAb (10 μg/mL, clone 1D11), or preimmune rat IgG (10 μg/mL) used as a control Ig for 7 days. After incubation, CD4+ T cells were negatively selected with biotinylated anti-I-A/I-E mAb (BD Bioscience) plus IMag streptavidin Particles Plus-DM. These preparations typically contained less than 0.1% of I-A/I-E+DCs as indicated by the fluorescence-activated cell sorting analysis and were used in subsequent experiments.
Ag presentation assay
CD4+CD25− T cells (2 × 105) obtained from B10.D2 mice or the mice that received transplants were cultured with the irradiated mDCs or DCregs (2 × 104) obtained from BALB/c mice for 3 days in 96-well flat-bottomed plates (Becton Dickinson). Likewise, Rag2−/−KJ1–26+ T cells (5 × 104) were cultured with the irradiated unpulsed or OVA323-339 peptide-pulsed mDCs or DCregs (5 × 103) for 3 days. In another experiment, 105 KJ1–26+ T cells obtained from the mice that had received adoptive transfers as described in “In vivo generation of CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells” were cultured with 105 irradiated syngeneic hemolyzed splenocytes used as APCs in the presence of OVA323-339 peptide (1 μM). The culture was also performed in the presence or absence of murine IL-2 (103 U/mL; Wako Pure Chemical Industries) or mAbs to CD3 and CD28 (10 μg/mL). [3H]thymidine (GE Healthcare, Chalfont St. Giles, UK) incorporation was measured on day 3 for the last 18 hours.
Models for cGVHD and the evaluation
The induction and the evaluation of cGVHD were performed according to previous reports4,5 with some modifications. In brief, recipient BALB/c mice (10 per group) received a single dose (800 cGy) of lethal total body irradiation (TBI). Three hours after the TBI, all recipients received T-cell–depleted BM cells (107/mouse) and CD4+CD25− T cells (2 × 106/mouse)obtained from B10.D2 mice through a tail vein. The day of transplantation was designated day 0. Subsequently, the mice that received transplants received repetitive intravenous injections of mDCs or DCregs (2 × 106) obtained from BALB/c mice on days 2, 9, and 16 or days 18, 25, and 32 after transplantation. Rapamycin (RAPA; Sigma-Aldrich) suspended in carboxymethylcellulose (Sigma-Aldrich) was intraperitoneally administered at a dose of 1.5 mg/kg beginning on the day of the transplantation and continuing daily for 16 days. For in vivo-blockade experiments, the recipient mice were intraperitoneally injected with neutralizing anti-CD25 mAb (clone PC61) or control Ig (each 250 μg/mouse) on days 0, 3, 6, 9, 12, and 15. For the adoptive transfer experiments, CD4+CD25+ T cells (2 × 106/mouse) obtained from B10.D2 mice or the mice that received transplants were simultaneously intravenously injected with T-cell–depleted BM cells and CD4+CD25− T cells into the irradiated recipient BALB/c mice. Recipients were monitored once every day from the day of transplantation to the indicated days after transplantation (in Figure 3) to determine the incidence and severity of cutaneous cGVHD as well as mobility, diarrhea, and weight loss. The following scoring system for cutaneous cGVHD was used: 0, healthy appearance; 1, skin lesions with alopecia less than 1 cm2 in area; 2, skin lesions with alopecia 1 to 2 cm2 in area; and 3, skin lesions with alopecia more than 2 cm2 in area. Incidence was expressed as the percentage of mice that showed clinical manifestations. In another experiment, recipients were killed on day 30 after allogeneic transplantation to obtain spleen and serum. The titers of serum TNF-α, IL-12p40, IFN-γ, and IL-4 were measured with ELISA kits (all from BioSource).
In vivo generation of CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells
Rag2−/−KJ1–26+ T cells (107/mouse) were adoptively transferred with or without OVA323–339 peptide/mDCs or OVA323-339 peptide/DCregs (2 × 106/mouse) into BALB/c mice. After 2, 4, 6, 8, and 10 days, CD4+ T cells were purified from the recipient mice as described above, and the expression of CD25 and Foxp3 among gated KJ1–26+ T cells was analyzed by flow cytometry. In another experiment, KJ1–26+ T cells, KJ1–26+CD25− T cells, and KJ1–26+CD25+ T cells were isolated from CD4+ T cells 8 days after the adoptive transfer and used for subsequent experiments.
Analysis of TR cell function
CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated mDCs (5 × 103) obtained from BALB/c mice in the presence of CD4+CD25+ T cells (6.25 × 103–5 × 104) obtained from B10.D2 mice or the mice that received transplants in 96-well round-bottomed plates (BD Biosciences). Likewise, Rag2−/−KJ1–26+ T cells, KJ1–26+CD25− T cells, and/or KJ1–26+CD25+ T cells (5 × 104) obtained from Rag2+/+DO11.10 BALB/c mice or the mice that had received adoptive transfers were cultured with the irradiated syngeneic APCs (5 × 104) in the presence of anti-CD3 mAb (10 μg/mL) or OVA323-339 peptide (1 μM). The proliferation was evaluated on day 3 based on [3H]thymidine incorporation.
Statistical analysis
Data are expressed as mean values (± SD). All analyses for statistically significant differences were performed with the Student paired t test or the log-rank test. *P values less than .01 were considered significant.
Results
DCregs generate CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells in the allogeneic culture
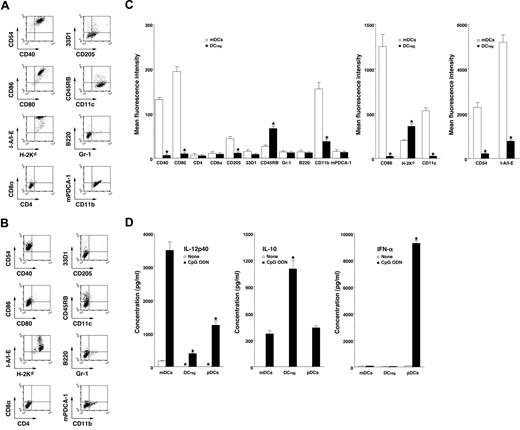
mDCs expressed high levels of CD11c, CD40, CD80, CD86, and MHC molecules (Figure 1A,C) whereas DCregs had relatively high levels of MHC molecules and extremely low levels of CD11c, CD40, CD80, and CD86 (Figure 1B,C). In addition, DCregs showed a higher level of CD45RB and lower expressions of CD11b, CD54, and CD205 than mDCs (Figure 1A-C). mDCs and DCregs showed little or no expression of CD4, CD8α, and 33D1 or of Gr-1, B220, and mPDCA-1 as cell surface markers of pDCs18 (Figure 1A-C). On the other hand, DCregs produced a greater amount of IL-10 and less IL-12p40 compared with mDCs and pDCs (Figure 1D). pDCs strongly produced IFN-α, whereas neither mDCs nor DCregs produced IFN-α (Figure 1D) or TGF-β1 (< 100 pg/mL; data not shown). Together, these results indicate that DCregs are myeloid DC subsets distinct from conventional and plasmacytoid DC subsets.
Characteristic profile of DCregs. (A-C) The expression of cell surface molecules on mDCs (A,C) and DCregs (B,C) was analyzed by flow cytometry, and data are represented by a dot plot (A,B) and mean fluorescence intensity (C). The results are representative of 4 experiments with similar results. The error bars indicate SD. *P less than .01 compared with mDCs. (D) DCs (5 × 105) were stimulated or not stimulated with CpG ODN (0.1 μM) for 24 hours, and the culture supernatants were analyzed for cytokine production. The results are representative of 4 experiments with similar results. The error bars indicate SD. *P less than .01 compared with mDCs.
Characteristic profile of DCregs. (A-C) The expression of cell surface molecules on mDCs (A,C) and DCregs (B,C) was analyzed by flow cytometry, and data are represented by a dot plot (A,B) and mean fluorescence intensity (C). The results are representative of 4 experiments with similar results. The error bars indicate SD. *P less than .01 compared with mDCs. (D) DCs (5 × 105) were stimulated or not stimulated with CpG ODN (0.1 μM) for 24 hours, and the culture supernatants were analyzed for cytokine production. The results are representative of 4 experiments with similar results. The error bars indicate SD. *P less than .01 compared with mDCs.
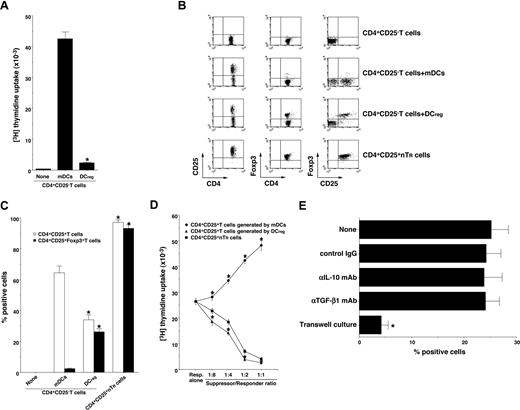
Foxp3 is reportedly a key transcription factor in the development and function of CD4+CD25+ nTR cells.9,14,17 We and others have reported previously that DCregs and other tolerogenic DCs expanded the number of CD4+CD25+Foxp3+ TR cells in vivo and in vitro.10,11,15,16 To address whether DCregs could generate CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells, CD4+CD25−Foxp3− T cells were cultured with allogeneic DCs, and the resulting cells were analyzed. DCregs showed a significant lower activation of allogenic CD4+CD25− T cells compared with mDCs (Figure 2A). Although mDCs generated numerous CD4+CD25+ T cells, they contained less than 3% Foxp3+ T cells (Figure 2B,C). In contrast, DCregs generated approximately 35% CD4+CD25+ T cells, and they predominantly expressed Foxp3 (Figure 2B,C). In addition, CD4+CD25+ T cells generated by DCregs showed a more potent suppressive effect on the allogeneic activation of CD4+CD25− T cells by mDCs than did CD4+CD25+ nTR cells, whereas CD4+CD25+ T cells generated by mDCs enhanced the response (Figure 2D). We also observed that anti–IL-10 mAb or anti-TGF-β mAb had little or no effect on the generation of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells by DCregs, whereas the separation of CD4+CD25−Foxp3− T cells and DCregs significantly abolished the generation of CD4+CD25+Foxp3+ TR cells (Figure 2E). These results indicate that DCregs preferentially could generate CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells in the allogeneic culture.
DCregs generate alloreactive CD4+CD25+Foxp3+ TR cells from CD4+CD25− Foxp3− T cells in vitro. (A) CD4+CD25− T cells (2 × 105) obtained from B10.D2 mice were cultured with or without the irradiated DCs (2 × 104) obtained from BALB/c mice for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with mDCs. Results of 4 replicated experiments were pooled. (B-D) CD4+CD25− T cells (5 × 106) obtained from B10.D2 mice were cultured with the irradiated DCs (5 × 105) obtained from BALB/c mice for 7 days, and CD4+ T cells were collected. (B,C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (B) and are expressed as% positive cells (C). The error bars indicate SD. *P < .01 compared with the CD4+ T cells primed with allogeneic mDCs by Student paired t test. Results of 4 replicated experiments were pooled. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated mDCs (5 × 103) obtained from BALB/c mice in the presence of CD4+CD25+ T cells (6.25 × 103-5 × 104) obtained from B10.D2 mice or each culture for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with CD4+CD25+ nTR cells by Student paired t test. Results of 4 replicated experiments were pooled. (E) CD4+CD25− T cells (106) obtained from B10.D2 mice and the irradiated DCregs (105) obtained from BALB/c mice were cocultured or placed separately in the presence or absence of anti–IL-10 mAb, anti–TGF-β mAb or control Ig for 7 days, and CD4+ T cells were collected. The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are expressed as percentage of cells positive for both CD25 and Foxp3. The error bars indicate SD. *P < .01 compared with the primed CD4+ T cells primed with allogeneic DCregs by Student paired t test. Results of 3 replicated experiments were pooled.
DCregs generate alloreactive CD4+CD25+Foxp3+ TR cells from CD4+CD25− Foxp3− T cells in vitro. (A) CD4+CD25− T cells (2 × 105) obtained from B10.D2 mice were cultured with or without the irradiated DCs (2 × 104) obtained from BALB/c mice for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with mDCs. Results of 4 replicated experiments were pooled. (B-D) CD4+CD25− T cells (5 × 106) obtained from B10.D2 mice were cultured with the irradiated DCs (5 × 105) obtained from BALB/c mice for 7 days, and CD4+ T cells were collected. (B,C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (B) and are expressed as% positive cells (C). The error bars indicate SD. *P < .01 compared with the CD4+ T cells primed with allogeneic mDCs by Student paired t test. Results of 4 replicated experiments were pooled. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated mDCs (5 × 103) obtained from BALB/c mice in the presence of CD4+CD25+ T cells (6.25 × 103-5 × 104) obtained from B10.D2 mice or each culture for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with CD4+CD25+ nTR cells by Student paired t test. Results of 4 replicated experiments were pooled. (E) CD4+CD25− T cells (106) obtained from B10.D2 mice and the irradiated DCregs (105) obtained from BALB/c mice were cocultured or placed separately in the presence or absence of anti–IL-10 mAb, anti–TGF-β mAb or control Ig for 7 days, and CD4+ T cells were collected. The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are expressed as percentage of cells positive for both CD25 and Foxp3. The error bars indicate SD. *P < .01 compared with the primed CD4+ T cells primed with allogeneic DCregs by Student paired t test. Results of 3 replicated experiments were pooled.
DCregs protect the recipients of allogeneic BMT from cutaneous cGVHD
B10.D2 mice and BALB/c mice are MHC-compatible (H-2d) and miHAg-incompatible strains, and a murine model of cGVHD established by transplanting T-cell–depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice into irradiated recipient BALB/c mice shares critical characteristics with human cGVHD, including skin fibrosis as a result of increased collagen deposition, follicular dropout, loss of subdermal fat, and dermal mononuclear infiltrates.4,5 Approximately 98% of spleen mononuclear cells had disappeared by day 5 after TBI, whereas less than 5% of recipient residual CD4+CD25+ T cells were still detected in spleen mononuclear cells.4 On the other hand, spleen CD4+ T cells were apparently detected in the recipient mice 5 days after allogeneic transplantation,15 indicating that a large proportion of the CD4+ T cells observed in the irradiated recipients of allogeneic transplantation were mainly of donor origin.
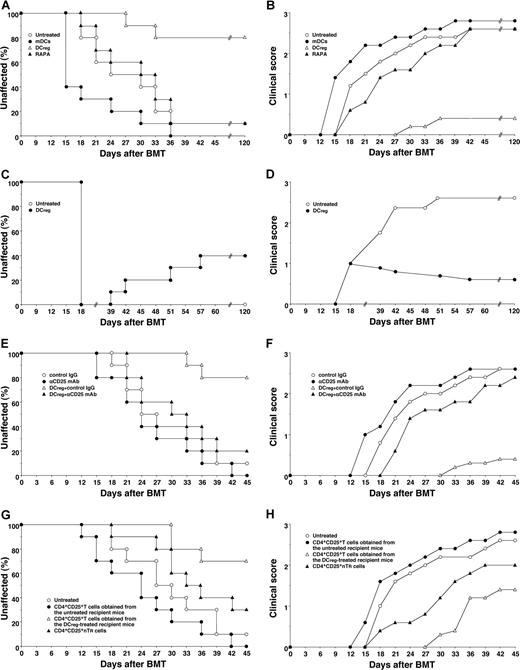
To clarify the effect of DCs on cGVHD in allogeneic BMT, the irradiated recipient BALB/c mice received transplants of T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice. Thereafter the recipient mice were treated with the recipient-type DCs on days 2, 9, and 16 after transplantation, and the incidence and the severity of cutaneous cGVHD were monitored. Almost all recipient mice showed clinical symptoms, having skin lesions with alopecia, lower mobility, diarrhea and weight loss until 45 days after allogeneic transplantation (Figure 3). The traditional treatment with RAPA had little suppressive effect on the incidence or severity of cutaneous cGVHD (Figure 3A,B). Treatment with the recipient-type mDCs promoted the incidence and severity of cutaneous cGVHD (P < .01, Figure 3A,B). In contrast, treatment with the recipient-type DCregs suppressed the incidence and severity of cutaneous cGVHD compared with the untreated recipient mice (P < .01, Figure 3A,B), and these protected mice had little or no signs of cutaneous cGVHD over the course of 120 days.
DCregs protect mice from the incidence and severity of cutaneous cGVHD. (A,B) The irradiated recipient BALB/c mice (10 per group) received transplants of T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice and were then treated with or without the recipient-type mDCs, DCregs, or RAPA after the transplantation. The incidence (A) and severity (B) of cutaneous cGVHD were monitored for 120 days after the transplantation. (C,D) The irradiated recipient BALB/c mice (10 per group) received T-cell-depleted BM cell and CD4+CD25− T cell transplants obtained from B10.D2 mice, and were then treated with or without the recipient-type DCregs after the disease onset. The incidence (C) and severity (D) of cutaneous cGVHD were monitored for 120 days after the transplantation. (E,F) The irradiated recipient BALB/c mice (10 per group) received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type DCregs in combination with control Ig or anti-CD25 mAb. The incidence (E) and severity (F) of cutaneous cGVHD were monitored for 45 days after the transplantation. (G,H) CD4+CD25+ T cells (obtained from B10.D2 mice or the mice who received transplants), T-cell-depleted BM cells, and CD4+CD25− T cells (obtained from B10.D2 mice) were transplanted into the irradiated recipient BALB/c mice (10 per group). The incidence (G) and severity (H) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice by the log-rank test. Two replicate experiments with similar results were pooled.
DCregs protect mice from the incidence and severity of cutaneous cGVHD. (A,B) The irradiated recipient BALB/c mice (10 per group) received transplants of T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice and were then treated with or without the recipient-type mDCs, DCregs, or RAPA after the transplantation. The incidence (A) and severity (B) of cutaneous cGVHD were monitored for 120 days after the transplantation. (C,D) The irradiated recipient BALB/c mice (10 per group) received T-cell-depleted BM cell and CD4+CD25− T cell transplants obtained from B10.D2 mice, and were then treated with or without the recipient-type DCregs after the disease onset. The incidence (C) and severity (D) of cutaneous cGVHD were monitored for 120 days after the transplantation. (E,F) The irradiated recipient BALB/c mice (10 per group) received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type DCregs in combination with control Ig or anti-CD25 mAb. The incidence (E) and severity (F) of cutaneous cGVHD were monitored for 45 days after the transplantation. (G,H) CD4+CD25+ T cells (obtained from B10.D2 mice or the mice who received transplants), T-cell-depleted BM cells, and CD4+CD25− T cells (obtained from B10.D2 mice) were transplanted into the irradiated recipient BALB/c mice (10 per group). The incidence (G) and severity (H) of cutaneous cGVHD were monitored for 45 days after the transplantation. *P < .01 compared with the untreated recipient mice by the log-rank test. Two replicate experiments with similar results were pooled.
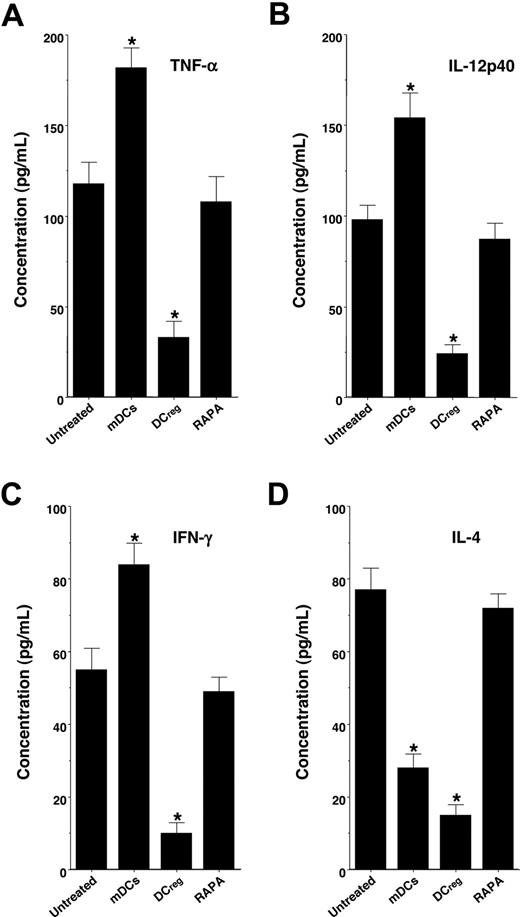
We also examined serum levels of TNF-α, IL-12p40, IFN-γ, and IL-4 in the recipient mice (Figure 4). Treatment with the recipient-type mDCs markedly enhanced serum production of TNF-α, IL-12p40, and IFN-γ, whereas serum production of IL-4 was inhibited, possibly due to the IL-12-mediated induction of T type-1 helper (TH1) cells compared with levels in the untreated recipient mice. In contrast, treatment with the recipient-type DCregs significantly inhibited serum levels of TNF-α, IL-12p40, IFN-γ, and IL-4, whereas RAPA had little or no effect on serum production of these cytokines.
DCregs suppress cytokine production in the transplanted recipient mice. The irradiated recipient BALB/c mice received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type mDCs, DCregs, or RAPA after the transplantation as described in Figure 3A,B. Subsequently, serum was collected from each recipient mouse 30 days after the transplantation. Serum production of TNF-α (A), IL-12p40 (B), IFN-γ (C), and IL-4 (D) was measured by ELISA. The error bars are SD. *P < .01 compared with the untreated group by Student paired t test. Results of 3 replicated experiments were pooled.
DCregs suppress cytokine production in the transplanted recipient mice. The irradiated recipient BALB/c mice received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type mDCs, DCregs, or RAPA after the transplantation as described in Figure 3A,B. Subsequently, serum was collected from each recipient mouse 30 days after the transplantation. Serum production of TNF-α (A), IL-12p40 (B), IFN-γ (C), and IL-4 (D) was measured by ELISA. The error bars are SD. *P < .01 compared with the untreated group by Student paired t test. Results of 3 replicated experiments were pooled.
To determine the therapeutic effect of DCregs on cutaneous cGVHD, the transplant recipient mice received repetitive intravenous injections of the recipient-type DCregs on days 18, 25, and 32 after transplantation, and the incidence and severity of cutaneous cGVHD were monitored (Figure 3C,D). Treatment with the recipient-type DCregs, even after disease onset, not only suppressed the severity but also reduced the incidence of cutaneous cGVHD.
Involvement of in vivo-generated alloreactive CD4+CD25+Foxp3+ TR cells in the protective effect of DCregs on cGVHD
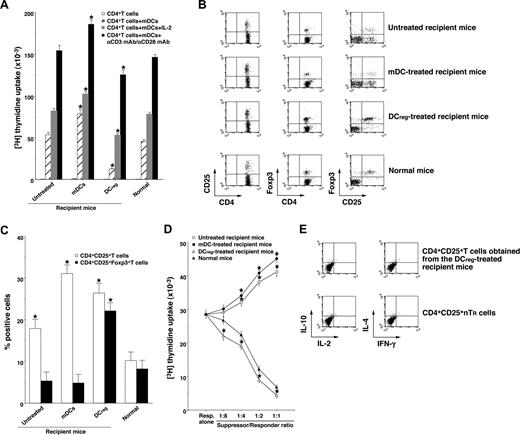
To clarify the mechanism responsible for the DCreg-mediated regulation of cutaneous cGVHD, we analyzed donor-derived CD4+ T cells obtained from the transplant recipient mice. Donor-derived CD4+ T cells obtained from the mDC-treated recipient mice showed a more vigorous response to recipient-type mDCs than those obtained from the untreated recipient mice (Figure 5A). In contrast, CD4+ T cells obtained from the DCreg-treated recipient mice were hyporesponsive to recipient-type mDCs, and additional stimulation with IL-2 or anti-CD3 mAb plus anti-CD28 mAb partly restored the response (Figure 5A). Therefore, the recipient-type DCregs could generate anergic alloreactive donor-derived CD4+ T cells in an Ag-specific manner in the transplanted recipient mice.
DCregs induce donor-derived alloreactive CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells in the mice that received transplants. The irradiated recipient BALB/c mice received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type mDCs, DCregs after the transplantation as described in Figure 3A,B. Subsequently, donor-derived CD4+ T cells were collected from each transplant recipient 30 days after the transplantation. (A) Donor-derived CD4+ T cells (2 × 105) were cultured with the irradiated mDCs (2 × 104) obtained from BALB/c mice in the presence or absence of IL-2 (103 U/mL) or mAbs to CD3 and CD28 (each 10 μg/mL) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with normal mice by Student paired t test. Three replicate experiments with similar results were pooled. (B,C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (B) and are expressed as percentage positive cells (C). The error bars indicates SD. *P < .01 compared with normal mice by Student paired t test. Three replicate experiments with similar results were pooled. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated allogeneic mDCs (5 × 103) obtained from BALB/c mice in the presence of CD4+CD25+ T cells (6.25 × 103-5 × 104) obtained from B10.D2 mice or from each group of the transplanted mice for 3 days, and the proliferative response was measured. The error bars are SD. *P < .01 compared with CD4+CD25+ nTR cells by Student paired t test. Results of three replicated experiments were pooled. (E) The production of IL-2, IL-4, IL-10, and IFN-γ by freshly isolated CD4+CD25+ T cells from B10.D2 mice and the DCreg-treated recipient mice was analyzed by flow cytometry, and data are represented by a dot plot. Results of 3 replicated experiments were pooled.
DCregs induce donor-derived alloreactive CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells in the mice that received transplants. The irradiated recipient BALB/c mice received T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice, and were then treated with or without the recipient-type mDCs, DCregs after the transplantation as described in Figure 3A,B. Subsequently, donor-derived CD4+ T cells were collected from each transplant recipient 30 days after the transplantation. (A) Donor-derived CD4+ T cells (2 × 105) were cultured with the irradiated mDCs (2 × 104) obtained from BALB/c mice in the presence or absence of IL-2 (103 U/mL) or mAbs to CD3 and CD28 (each 10 μg/mL) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with normal mice by Student paired t test. Three replicate experiments with similar results were pooled. (B,C) The expression of CD25 and Foxp3 on CD4+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (B) and are expressed as percentage positive cells (C). The error bars indicates SD. *P < .01 compared with normal mice by Student paired t test. Three replicate experiments with similar results were pooled. (D) CD4+CD25− T cells obtained from B10.D2 mice (5 × 104) were cultured with the irradiated allogeneic mDCs (5 × 103) obtained from BALB/c mice in the presence of CD4+CD25+ T cells (6.25 × 103-5 × 104) obtained from B10.D2 mice or from each group of the transplanted mice for 3 days, and the proliferative response was measured. The error bars are SD. *P < .01 compared with CD4+CD25+ nTR cells by Student paired t test. Results of three replicated experiments were pooled. (E) The production of IL-2, IL-4, IL-10, and IFN-γ by freshly isolated CD4+CD25+ T cells from B10.D2 mice and the DCreg-treated recipient mice was analyzed by flow cytometry, and data are represented by a dot plot. Results of 3 replicated experiments were pooled.
We also examined the generation of donor-derived CD4+CD25+Foxp3+ T cells in the transplant recipient mice. Flow cytometric analysis showed that CD4+CD25+ T cells were increased, whereas CD4+CD25+Foxp3+ T cells were slightly decreased in number in both the untreated and the mDC-treated recipient mice compared with normal mice (Figure 5B,C). On the other hand, the DCreg-treated recipient mice had a larger proportion of CD4+CD25+Foxp3+ T cells than normal mice (Figure 5B,C). In addition, CD4+CD25+ T cells obtained from the DCreg-treated recipient mice showed greater suppression of the allogeneic activation of CD4+CD25− T cells by recipient-type mDCs than CD4+CD25+ nTR cells, whereas CD4+CD25+ T cells obtained from the untreated recipient mice and the mDC-treated recipient mice enhanced these responses (Figure 5D). Consistent with a previous report,17 freshly isolated CD4+CD25+ T cells from the DCreg-treated recipient mice as well as CD4+CD25+ nTR cells produced little IL-2, IL-4, IL-10, and IFN-γ (Figure 5E). These results indicate that the recipient-type DCregs could generate alloreactive CD4+CD25+Foxp3+ T cells from donor-derived CD4+CD25−Foxp3− T cells in the recipient mice.
To clarify the role of CD4+CD25+ TR cells in the DCreg-mediated suppression of cutaneous cGVHD, in vivo blockade experiments with anti-CD25 mAb were performed. Depletion of CD4+CD25+ T cells in the transplant recipient mice with anti-CD25 mAb slightly enhanced the incidence and severity of cutaneous cGVHD (Figure 3E,F). On the other hand, treatment with anti-CD25 mAb significantly impaired the protective effect of DCreg on the incidence and severity of cutaneous cGVHD (P < .01, Figure 3E,F). In addition, the adoptive transfer of CD4+CD25+ T cells obtained from the DCreg-treated recipient mice with T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice into the mice that received transplants more potently reduced the incidence and severity of cutaneous cGVHD than CD4+CD25+ nTR cells, whereas the adoptive transfer of CD4+CD25+ T cells obtained from the untreated recipient mice with T-cell-depleted BM cells and CD4+CD25− T cells obtained from B10.D2 mice enhanced the pathogenesis (P < .01, Figure 3G,H). Therefore, donor-derived alloreactive CD4+CD25+Foxp3+ T cells contributed to the protective effect of DCregs on cutaneous cGVHD.
DCregs induce Ag-specific peripheral generation of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells
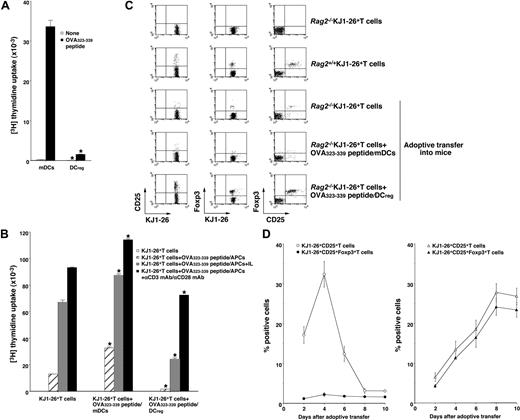
Although certain types of DCs exert in vivo regulatory effects on immune responses possibly mediated through the expansion of CD4+CD25+Foxp3+ nTR cell number and/or the generation of IL-10-producing Tr1 cells,11-13,19,20 the role of the DC subsets in the Ag-specific peripheral conversion of CD4+CD25−Foxp3− T cells into CD4+CD25+Foxp3+ TR cells has not been established. The above study indicates that the injection of recipient-type DCregs into the recipient mice after the transplantation with T-cell-depleted BM cells and CD4+CD25− T cells generated donor-derived CD4+CD25+Foxp3+ T cells (Figure 5B,C). However, it remains unclear whether the production of alloreactive donor-derived CD4+CD25+Foxp3+ T cells by DCregs is due to the expansion of thymic-derived CD4+CD25+Foxp3+ nTR cells differentiated from the transplanted BM cells or de novo conversion of CD4+CD25+Foxp3+ TR cells from the transplanted CD4+CD25−Foxp3− T cells in an Ag-specific manner. We therefore used Rag2−/−OVA-specific TCR (KJ1–26 clonotype) transgenic mice (DO11.10 mice), which lack CD25+Foxp3+ TR cells (Rag2−/−KJ1-26+ T cells).17 OVA323-339 peptide/mDCs induced a vigorous Ag-specific proliferative response of Rag2−/−KJ1–26+ T cells, whereas OVA323-339 peptide/DCregs showed defective Ag-specific activation among Rag2−/−KJ1–26+ T cells (Figure 6A).
Antigenic peptide-pulsed DCregs induce Ag-specific peripheral generation of CD4+CD25+ Foxp3+ TR cells from CD4+CD25−Foxp3− T cells. (A) Rag2−/−KJ1–26+ T cells (5 × 104) were cultured with the irradiated syngeneic DCs (5 × 103) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with mDCs by Student paired t test. Results of 4 replicated experiments were pooled. (B-D) Rag2−/−KJ1–26+ T cells were adoptively transferred with or without OVA323–339 peptide/mDCs or OVA323-339 peptide/DCregs into BALB/c mice. On day 8 (B,C) or the indicated days (D) after the adoptive transfer, KJ1–26+ T cells were isolated from the recipient mice. (B) KJ1–26+ T cells (105) obtained from each group of the mice that received an adoptive transfer were cultured with or without the irradiated syngeneic APCs (105) in the presence of OVA323-339 peptide (1 μM), IL-2 (103 U/mL), and/or mAbs to CD3 and CD28 (each 10 μg/mL) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with KJ1–26+ T cells by Student paired t test. Results of 3 replicated experiments were pooled. (C,D) The expression of CD25 and Foxp3 on KJ1–26+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (C) and expressed as percentage of positive cells (D) of the OVA323-339 peptide/mDC-adoptive transfer group (left panel) and OVA323-339 peptide/DCregs-adoptive transfer group (right panel). The error bars indicate SD. Results of 3 replicated experiments were pooled.
Antigenic peptide-pulsed DCregs induce Ag-specific peripheral generation of CD4+CD25+ Foxp3+ TR cells from CD4+CD25−Foxp3− T cells. (A) Rag2−/−KJ1–26+ T cells (5 × 104) were cultured with the irradiated syngeneic DCs (5 × 103) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with mDCs by Student paired t test. Results of 4 replicated experiments were pooled. (B-D) Rag2−/−KJ1–26+ T cells were adoptively transferred with or without OVA323–339 peptide/mDCs or OVA323-339 peptide/DCregs into BALB/c mice. On day 8 (B,C) or the indicated days (D) after the adoptive transfer, KJ1–26+ T cells were isolated from the recipient mice. (B) KJ1–26+ T cells (105) obtained from each group of the mice that received an adoptive transfer were cultured with or without the irradiated syngeneic APCs (105) in the presence of OVA323-339 peptide (1 μM), IL-2 (103 U/mL), and/or mAbs to CD3 and CD28 (each 10 μg/mL) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with KJ1–26+ T cells by Student paired t test. Results of 3 replicated experiments were pooled. (C,D) The expression of CD25 and Foxp3 on KJ1–26+ T cells was analyzed by flow cytometry, and data are represented by a dot plot (C) and expressed as percentage of positive cells (D) of the OVA323-339 peptide/mDC-adoptive transfer group (left panel) and OVA323-339 peptide/DCregs-adoptive transfer group (right panel). The error bars indicate SD. Results of 3 replicated experiments were pooled.
To address whether DCregs could induce Ag-specific peripheral generation of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells in vivo, Rag2−/−KJ1–26+ T cells together with or without OVA323-339 peptide/mDCs or OVA323-339 peptide/DCregs were adoptively transferred into naive BALB/c mice (OVA323-339 peptide/mDCs- or OVA323-339 peptide/DCreg-adoptive transfer group, respectively), and the resulting cells were analyzed. KJ1–26+ T cells obtained from the OVA323-339 peptide/mDC-adoptive transfer group enhanced the proliferative response to antigenic stimulation compared with KJ1–26+ T cells obtained from mice that received adoptive transfers of Rag2−/−KJ1–26+ T cells alone (Figure 6B). In contrast, KJ1–26+ T cells obtained from the OVA323-339 peptide/DCreg-adoptive transfer group showed less of a proliferative response, which was partly rescued by IL-2 or amti-CD3 mAb plus anti-CD28 mAb (Figure 6B). These results indicate that antigenic peptide-pulsed DCregs could generate Ag-specific anergic CD4+ T cells at the clonal level in vivo.
We further analyzed the characteristic features of the in vivo-generated KJ1–26+ T cells obtained from each mouse that received an adoptive transfer. Flow cytometric analysis showed that approximately 5% of KJ1–26+ T cells obtained from Rag2+/+DO11.10 mice (Rag2+/+KJ1–26+ T cells) expressed both CD25 and Foxp3, whereas Rag2−/−KJ1–26+ T cells expressed neither CD25 nor Foxp317 (Figure 6C). KJ1–26+ T cells obtained from mice receiving adoptive transfers of Rag2−/−KJ1–26+ T cells alone (Figure 6C) as well as the unpulsed mDC- or DCreg-adoptive transfer group (data not shown) had little expression of CD25 and Foxp3 on day 8 after the adoptive transfer. The expression of CD25 on KJ1–26+ T cells was increased until 4 days after the adoptive transfer and decreased thereafter, whereas the expression of Foxp3 on KJ1–26+CD25+ T cells was not changed at low levels in the OVA323-339 peptide/mDC-adoptive transfer group (Figure 6C,D, left panel). In contrast, KJ1–26+ T cells obtained from the OVA323-339 peptide/DCreg-adoptive transfer group showed a significant percentage of cells positive for both CD25 and Foxp3 (Figure 6C) as well as GITR and CD152 (data not shown) on day 8 after the adoptive transfer. In addition, the generation of KJ1–26+CD25+Foxp3+ T cells by OVA323-339 peptide/DCregs occurred in a time-dependent manner (Figure 6D right panel).
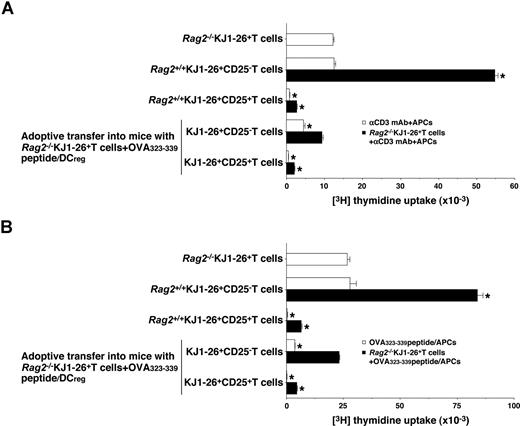
We further examined the function of in vivo-generated KJ1–26+CD25− T cells and KJ1–26+CD25+ T cells isolated from the OVA323-339 peptide/DCreg-adoptive transfer group. Rag2+/+KJ1–26+CD25− T cells showed a proliferative response to polyclonal (Figure 7A) and antigenic (Figure 7B) stimulation, whereas KJ1–26+CD25− T cells isolated from the OVA323-339 peptide/DCreg-adoptive transfer group were hyporesponsive to these treatments (Figure 7A,B). On the other hand, KJ1–26+CD25+ T cells isolated from both Rag2+/+DO11.10 mice and the OVA323-339 peptide/DCreg-adoptive transfer group not only entered an anergic state in response to polyclonal and antigenic stimulation but also exerted a regulatory function against the response of Rag2−/−KJ1–26+ T cells to these stimulations (Figure 7A,B). These results indicate that antigenic peptide-pulsed DCregs could induce the Ag-specific peripheral generation of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells.
Regulatory function of CD4+CD25+ Foxp3+ TR cells generated in vivo by antigenic peptide-pulsed DCregs.Rag2−/−KJ1–26+ T cells were adoptively transferred with OVA323-339 peptide/DCregs into BALB/c mice. After 8 days, KJ1–26+CD25− T cells and KJ1–26+CD25+ T cells were isolated from the recipient mice. Subsequently, Rag2−/−KJ1–26+ T cells, KJ1–26+CD25− T cells, or KJ1–26+CD25+ T cells (5 × 104) obtained from Rag2+/+DO11.10 BALB/c mice and the mice that received adoptive transfers were cultured with the irradiated syngeneic APCs (5 × 104) in the presence of anti-CD3 mAb (10 μg/mL) (A) or OVA323-339 peptide (1 μM) (B) for 3 days, and the proliferative response was measured. In another experiment, Rag2−/−KJ1–26+ T cells (5 × 104) were cultured with the irradiated syngeneic APCs (5 × 104) in combination with anti-CD3 mAb (10 μg/mL) (A) or OVA323-339 peptide (1 μM) (B) in the presence or absence of KJ1–26+CD25− T cells and KJ1–26+CD25+ T cells (5 × 104) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with Rag2−/−KJ1–26+ T cells by Student paired t test. Results of 3 replicated experiments were pooled.
Regulatory function of CD4+CD25+ Foxp3+ TR cells generated in vivo by antigenic peptide-pulsed DCregs.Rag2−/−KJ1–26+ T cells were adoptively transferred with OVA323-339 peptide/DCregs into BALB/c mice. After 8 days, KJ1–26+CD25− T cells and KJ1–26+CD25+ T cells were isolated from the recipient mice. Subsequently, Rag2−/−KJ1–26+ T cells, KJ1–26+CD25− T cells, or KJ1–26+CD25+ T cells (5 × 104) obtained from Rag2+/+DO11.10 BALB/c mice and the mice that received adoptive transfers were cultured with the irradiated syngeneic APCs (5 × 104) in the presence of anti-CD3 mAb (10 μg/mL) (A) or OVA323-339 peptide (1 μM) (B) for 3 days, and the proliferative response was measured. In another experiment, Rag2−/−KJ1–26+ T cells (5 × 104) were cultured with the irradiated syngeneic APCs (5 × 104) in combination with anti-CD3 mAb (10 μg/mL) (A) or OVA323-339 peptide (1 μM) (B) in the presence or absence of KJ1–26+CD25− T cells and KJ1–26+CD25+ T cells (5 × 104) for 3 days, and the proliferative response was measured. The error bars indicate SD. *P < .01 compared with Rag2−/−KJ1–26+ T cells by Student paired t test. Results of 3 replicated experiments were pooled.
Discussion
cGVHD is an increasingly frequent complication of alloBMT.3-5 Despite a better understanding of the inervention of aGVHD, how cGVHD is regulated remains unclear. Here we report that the recipient-type DCregs had a therapeutic effect in a MHC-compatible and miHAg-incompatible model of cutaneous cGVHD possibly mediated through the generation of alloreactive CD4+CD25+Foxp3+ TR cells from donor-derived CD4+CD25−Foxp3− T cells.
Previous papers reported that mDCs efficiently increased the number of allogeneic CD4+CD25+Foxp3+ nTR cells, whereas splenic DCs caused naive CD4+CD25− T cells to differentiate into CD4+CD25+Foxp3+ TR cells in the presence of TGF-β1 in vitro.21,22 We showed that allogeneic mDCs failed to generate CD4+CD25+Foxp3+ TR cells from naive CD4+CD25−Foxp3− T cells, although they produced CD4+CD25+Foxp3− T cells in the allogeneic culture. In addition, the recipient-type mDCs not only potently generated donor-derived CD4+CD25+Foxp3− T cells in the mice receiving allogeneic T-cell-depleted BM cells and CD4+CD25− T cells but also enhanced the incidence and severity of cGVHD. Therefore, the recipient-type mDCs could generate alloreactive CD4+CD25+Foxp3− activated T cells rather than CD4+CD25+Foxp3+ TR cells from donor-derived naive CD4+CD25−Foxp3− T cells under inflammatory conditions, and these regulatory functions may lead to promotion of the pathogenesis of cutaneous cGVHD.
cGVHD has distinct clinicopathologic features and different requirements for initiation and development from aGVHD, and an effective means of treating cGVHD remains to be established.4,5 Indeed, the traditional treatment with RAPA failed to suppress the incidence and severity of cutaneous cGVHD, although this treatment was reportedly effective in preventing aGVHD-induced lethality.23 A series of studies has reported that iDCs had a preventive effect in several murine models of the immunopathogenic diseases.11 However, the clinical application of iDCs may not be suitable for the treatment of immunopathogenic diseases, because they probably mature under inflammatory conditions.8 Indeed, our preliminary experiments showed that treatment with recipient-type iDCs as well as semi-mDCs after allogeneic transplantation did not suppress the incidence or severity of cutaneous cGVHD (data not shown). On the other hand, treatment with recipient-type DCregs significantly protected the mice receiving allogeneic transplants from cutaneous cGVHD. We also observed that DCregs derived from donor mice or other strains had little or no inhibitory effect (data not shown). Therefore, the immunotherapeutic strategy using recipient-type DCregs might have preventive and therapeutic potential for the treatment of cGVHD.
Although CD4+CD25+ nTR cells reportedly protected against the lethality of aGVHD,24 the role of CD4+CD25+ nTR cells in the pathogenesis of cGHVD is less defined. We showed that in vivo depletion of CD4+CD25+ T cells in the mice that received transplants by anti-CD25 mAb slightly promoted the incidence and severity of cutaneous cGVHD. In addition, the adoptive transfer of CD4+CD25+ nTR cells obtained from naive donor mice modestly ameliorated the pathogenesis of cutaneous cGVHD. Therefore, these results suggest that surviving recipient residual CD4+CD25+ nTR cells after TBI4 and CD4+CD25+ nTR cells in the donor BM inoculum potentially regulate the pathogenesis of cGVHD.
We showed that DCregs efficiently induced CD4+CD25+Foxp3+ T cells from naive CD4+CD25−Foxp3− T cells in vitro, and these CD4+CD25+Foxp3+ T cells exerted a more potent regulatory function against the allogeneic activation of CD4+CD25− T cells than CD4+CD25+ nTR cells. In addition, mAbs to IL-10 and TGF-β had no effect on the generation of CD4+CD25+Foxp3+ T cells by DCregs, whereas the separation of CD4+CD25−Foxp3− T cells and DCregs virtually abolished this generation. Therefore, DCregs could generate alloAg-specific CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells dependent on cell contact and independent of IL-10 and TGF-β1 in vitro.
The recipient-type DCregs enhanced the production of alloreactive CD4+CD25+Foxp3+ TR cells in the mice receiving allogeneic T-cell-depleted BM cells and CD4+CD25− T cells. In vivo-blockade experiments with anti-CD25 mAb significantly impaired the effect of the recipient-type DCregs on the incidence and severity of cutaneous cGVHD. In addition, the adoptive transfer of CD4+CD25+ T cells obtained from the DCreg-treated recipient mice exhibited a more potent suppressive effect on the pathogenesis of cutaneous cGVHD than that with CD4+CD25+ nTR cells. Collectively, the protective effect of the recipient-type DCregs on the pathogenesis of cGVHD could involve the generation of alloreactive CD4+CD25+Foxp3+ T cells from donor-derived CD4+CD25−Foxp3− T cells.
Although a series of studies has shown that tolerogenic DCs not only produced IL-10-producing Tr1 cells but also expanded the number of CD4+CD25+Foxp3+ nTR cells, there is no direct evidence about the involvement of DC subsets in the development of CD4+CD25+Foxp3+ TR cells from CD4+CD25−Foxp3− T cells in an Ag-specific manner.10-14 Our results using an adoptive transfer system clearly showed that antigenic peptide-pulsed DCregs directly generated CD4+CD25+Foxp3+ T cells from CD4+CD25−Foxp3− T cells in an Ag-specific manner. In addition, these in vivo-generated CD4+CD25+ T cells and CD4+CD25+ nTR cells showed almost the same expression of both CD25 and Foxp3 as well as regulatory activity toward the activation of CD4+CD25− T cells, indicating that CD4+CD25+ T cells generated by antigenic peptide-pulsed DCregs are identical to CD4+CD25+ nTR cells. All together, our results suggest that the recipient-type DCregs reduced the incidence and severity of cutaneous cGVHD induced by MHC-compatible and miHAg-incompatible BMT mediated through the generation of alloreactive CD4+CD25+Foxp3+ TR cells from donor-derived naive CD4+CD25−Foxp3− T cells in an Ag-specific manner. Further investigation of the molecular mechanism responsible for the Ag-specific peripheral generation of CD4+CD25+Foxp3+ TR cells from naive CD4+CD25−Foxp3− T cells by DCregs is being conducted in our laboratories.
In summary, the findings reported here highlight a novel immunotherapeutic approach using DCregs for the treatment of cGVHD. We have previously reported that the recipient-type DCregs protected mice from death as a result of aGVHD in murine fully MHC-mismatched alloBMT.15 In addition, human DCregs exhibited similar a phenotype and T-cell regulatory functions, including the generation of Ag-specific CD4+CD25+ TR cells from naive CD4+ T cells in vitro, to their murine counterparts.16 Therefore, the availability of human DCregs may provide an advantageous means of Ag-specific intervention for cGVHD as well as aGVHD in alloBMT. To test our hypothesis, the development of an immunotherapy with human DCregs is also being conducted in our laboratories. In addition, further insight into the molecular mechanism underlying the DCreg-mediated regulation of T cell-function may lead to a better understanding of the induction of peripheral tolerance.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Yamamoto for excellent assistance; Dr A. Yoshimura (Division of Molecular and Cellular Immunology, Medical Institute of Bioregulation, Kyushu University) for providing anti-TGF-β mAb; and all members of the Central Facility at the RIKEN Research Center for Allergy and Immunology for technical help in cell sorting.
Authorship
Contribution: K.S. designed the research project, analyzed data, and wrote the manuscript; S.F., Y.S., K.S., K.E., and T.F. performed the experiments; and M.K. and N.Y. contributed vital new reagents or analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katsuaki Sato, Laboratory for Dendritic Cell Immunobiology, Research Center for Allergy and Immunology, RIKEN Yokohama Institute, Suehiro-cho 1-7-22, Tsurumi, Yokohama, Kanagawa 230-0045, Japan; e-mail:katsuaki@rcai.riken.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal