Abstract
18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) is a noninvasive, 3-dimensional imaging modality that has become widely used in the management of patients with malignant lymphomas. This technology has been demonstrated to be more sensitive and specific than either 67gallium scintigraphy or computerized tomography, providing a more accurate distinction between scar or fibrosis and active tumor. PET scans have been evaluated in pretreatment staging, restaging, monitoring during therapy, posttherapy surveillance, assessment of transformation, and, more recently, as a surrogate marker in new drug development. Data to support these various roles require prospective validation. Moreover, caution must be exercised in the interpretation of PET scans because of technical limitations, variability of FDG avidity among the different lymphoma histologic subtypes, and in the large number of etiologies of false-negative and false-positive results. Recent attempts to standardize PET in clinical trials and incorporation of this technology into uniformly adopted response criteria will hopefully lead to improved outcome for patients with lymphoma.
Introduction
Approximately 61 190 new cases of non-Hodgkin lymphoma (NHL) and 8190 cases of Hodgkin lymphoma (HL) will be diagnosed in the United States in 2007, and more than 18 660 NHL patients and 1070 HL patients will die from their disease.1 Clinical trials directed at improving patient outcome rely on accurate staging and assessment of response. Functional imaging with 18-fluoro-deoxyglucose (FDG) positron emission tomography (PET) and, more recently, with PET/computed tomography (CT), increases the sensitivity and specificity of disease assessment and may also predict outcome and direct future therapies
Conventional imaging techniques for lymphoma evaluation
CT scanning
For decades, CT scans were considered sufficiently reliable for staging and restaging of lymphoma. CT provides relatively high sensitivity and specificity in pretreatment staging,2,3 but has low specificity in response assessment following therapy.4-7 For example, patients with bulky disease prior to therapy often exhibit a residual mass after treatment. CT scans determine the size and location of masses, but are unable to distinguish viable tumor from necrotic or scar tissue.4-6 Fuks et al4 reported that following combination chemotherapy for 100 patients with NHL there were 33 complete and 38 partial remissions. In 20 of the latter, all clinical evidence suggested a complete remission; however, lymphangiogram, gallium scan, abdominal CT scan, or ultrasound suggested residual disease. Only 20% of these cases had persistent disease at restaging laparotomy. Surbone et al6 reported that of 241 patients with aggressive lymphoma, 30% had an abdominal mass at diagnosis with a residual mass in 40% at the time of clinical complete remission. Of 22 patients with pathologic evaluations, the specimen was negative in 95%, none of whom relapsed at a median follow up of 31 months.8 Radford et al9 observed residual mediastinal abnormalities on chest x-ray in 64% of 110 patients with HL at the completion of treatment, more commonly in patients with prior bulky disease. Partial or complete regression of the abnormalities occurred in 59% of patients at one year following completion of therapy. The presence of residual adenopathy did not predict relapse.
67Gallium scanning
67Gallium scintigraphy is a metabolic study that relies on the accumulation of the isotope into viable lymphoma cells via binding to transferrin receptors. 67Ga is useful in assessing response in lymphoma, improving on the specificity of CT.10-15 Even-Sapir et al12 reviewed 107 scans from 101 patients and found that 67Ga scan distinguished lymphoma from benign tissue with a sensitivity of 90%, a specificity of 93%, and positive and negative predictability of 84% and 96%, respectively. Other investigators found substantially lower positive and negative predictive values for 67gallium scintigraphy, with positive and negative predictive values of 67Ga scanning following treatment of approximately 70% to 80% and 65% to 85%, respectively.13,16 67Ga scanning was not widely adopted because its spatial resolution, specificity, and sensitivity were low for indolent lymphomas and abdominal disease due to physiologic bowel uptake. The time involved in performing the scans (7-14 days after 67Ga injection) and lack of a uniform approach to imaging with 67Ga, which should include the use of single-photon-emission computer tomography (SPECT) and appropriately high doses of radioactivity, further limited its use.
FDG-PET
PET is a noninvasive, 3-dimensional, metabolic imaging technique that uses a radiopharmaceutical to target a specific physiologic process (eg, glucose metabolism, amino acid metabolism, DNA synthesis). The most widely used pharmaceutical is the radiolabeled glucose analog fluorine-18-deoxyglucose (FDG). FDG is transported into cells and phosphorylated in a similar manner to glucose. However, because FDG-6-phosphate is not a substrate for glucose-6-phosphate isomerase and because FDG-6-phosphate is typically not dephosphorylated in tumors, it becomes trapped in the cell and reaches a near equilibrium state at approximately 60 minutes after injection. The positron-emitting 18F isotope to which FDG is linked decays, and the emitted positron annihilates after “bumping” into an electron, generating 2 511-KeV photons emitted in nearly opposite directions that are detected by the PET scanner.
Visual assessment is usually used to interpret PET scans, defining positive PET findings as focal or diffuse FDG uptake above the surrounding background in a location incompatible with normal anatomy/physiology, except for a few exceptions, primarily in the posttherapy setting. However, the standardized uptake value (SUV), representing the ratio of the tumoral tracer concentration to the average tracer concentration in the entire body, is often used as a semiquantitative measure of the degree of FDG uptake and aids in the interpretation of PET scans.17-19
PET/CT combines a full-ring detector PET scanner with a multidetector helical CT such that the PET scan is acquired immediately after the CT scan. The images are fused to provide precise localization of abnormal lesions. PET/CT provides more sensitive and specific imaging than either modality alone,20-25 and is considerably faster than the combination of emission and transmission PET scans required to obtain attenuation-corrected PET images. PET/CT is essentially replacing stand-alone PET scanners.
PET(/CT) is the most important recent advance in noninvasive lymphoma assessment. PET shows high sensitivity and specificity in patients with HL and most subtypes of indolent and aggressive NHL. PET is superior to 67Ga scintigraphy in pretreatment staging and restaging of the various lymphoma subtypes, especially follicular lymphomas where the sensitivity of 67Ga scanning is low.15,26-28 Most importantly, FDG-PET is easier to perform than 67Ga scintigraphy, requiring only approximately 2 hours from the time of radiotracer injection. In addition, the increasing availability of PET(/CT) scanners has resulted in the widespread use of FDG-PET(/CT) in lymphoma evaluation.
Issues regarding the application of PET(/CT) in lymphoma
Current applications of PET(/CT) in lymphoma may be divided into pretreatment staging, restaging, therapy monitoring, posttherapy surveillance, and assessment of transformation. In the United States, staging and restaging PET scans as well as those performed to assess transformation from an indolent to an aggressive NHL are reimbursed by the Center for Medicare and Medicaid Services (CMS), essentially without restriction, whereas PET for monitoring therapy and posttherapy surveillance is not yet approved. CMS will, however, provide coverage for scans obtained within specifically defined clinical trials, for example those conducted by National Cancer Institute cooperative groups, or a prospective registry, such as the National Oncologic PET Registry (NOPR) administered by the American College of Radiology Investigative Network (ACRIN).
Should PET replace Ann Arbor staging for pretreatment evaluation?
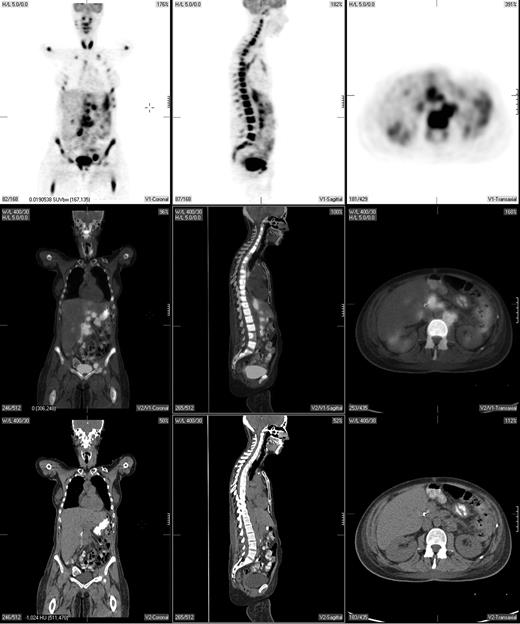
Pretreatment staging determines the extent of disease and helps direct therapy. The Ann Arbor system was initially developed to distinguish patients who might be candidates for radiation therapy from those who would benefit from systemic treatment.29 Traditionally, the Ann Arbor staging system was based on physical examination and bone marrow evaluation but, more recently, CT scans have been incorporated. PET may provide complementary information to conventional staging methods, such as dedicated intravenous contrast-enhanced CT (CECT) and bone marrow biopsy. PET is highly sensitive in detecting nodal and extranodal involvement by most histologic subtypes of lymphoma prior to and following treatment (Figure 1).2,3,20,30-43 Most common types of lymphoma (eg, diffuse large B-cell NHL, follicular NHL, mantle cell NHL, HL) are routinely FDG avid with a sensitivity that exceeds 80% and a specificity of about 90%, which is superior to CT.2,3,33
A pretreatment PET/CT scan in a 48-year-old female patient with Burkitt lymphoma showing widespread nodal and extranodal disease including periaortic, iliac, and mediastinal lymphadenopathy in addition to extensive involvement of the bone/bone marrow, both thyroid lobes and focal liver involvement.
A pretreatment PET/CT scan in a 48-year-old female patient with Burkitt lymphoma showing widespread nodal and extranodal disease including periaortic, iliac, and mediastinal lymphadenopathy in addition to extensive involvement of the bone/bone marrow, both thyroid lobes and focal liver involvement.
PET and CT are concordant in staging 80% to 90% of patients with diffuse large B-cell lymphoma, follicular lymphoma, and probably also mantle cell lymphoma.33,35 In the 10% to 20% of patients in whom a discordance is observed, PET typically results in upstaging due to the additional presumed sites of disease detected by PET alone such as lymph nodes of 1 cm or smaller in short axis by CT and splenic and hepatic infiltration. In contrast, concordance of PET and CT in determining clinical stage occurs in only about 60% to 80% of patients with HL. Discordant findings occur with a comparable frequency (eg, 10%-20%) in both directions.34,37-42 Although most studies show that PET-negative/CT-positive findings are less common than the converse, it is clear that PET alone cannot replace CT for pretreatment staging of HL.34,37,39,41
PET can detect focal or multifocal bone/bone marrow involvement in lymphoma patients with a negative iliac crest bone marrow biopsy, subsequently confirmed by histopathology or magnetic resonance imaging (MRI)44-46 However, PET alone is unreliable in detecting bone marrow involvement, particularly of limited extent (ie, ≤ 10%-20% of marrow space); estimates of PET sensitivity for detecting marrow infiltration in NHL and HL based on a recently reported meta-analysis were 43% (95% CI, 28-60) and 76% (95% CI, 47-92), respectively.46 While PET may also detect extensive diffuse bone/bone marrow involvement, these patients typically have a positive bone marrow biopsy. Moreover, diffusely increased bone marrow uptake on PET may be due to reactive myeloid hyperplasia, and, therefore, such uptake should be interpreted with caution.45 PET-positive bone/bone marrow findings should be confirmed by biopsy or MRI if a change in treatment is planned based on these findings. Thus, PET cannot substitute for bone marrow biopsy in lymphoma staging.
In a meta-analysis of FDG-PET in the staging of patients including mostly diffuse large B-cell NHL and HL, with some follicular lymphomas,43 the pooled sensitivity for 14 studies with patient-based data was 90.9% (95% CI, 88.0-93.4), with a false-positive rate of 10.3% (95% CI, 7.4-13.8). The maximum joint sensitivity and specificity was 87.8% (95% CI, 85.0-90.7), with an apparently higher sensitivity and false-positive rate in patients with HL compared with NHL of various subtypes. The pooled sensitivity for 7 studies with lesion-based data was 95.6% (95% CI, 93.9-97.0), with a false-positive rate of only 1.0% (95% CI, 0.6-1.3). The maximum sensitivity and specificity was 95.6% (95% CI, 93.1-98.1). Thus, PET detects more occult lymphomatous sites than CECT and bone marrow biopsy.20,32,33,35,41,42,44
Nevertheless, PET is currently not standard in lymphoma staging primarily because of the generally small percentage of patients (∼ 15%-20%) in whom PET detects additional disease sites with modification of clinical stage, and even fewer patients (∼ 10%-15%) in whom this modification alters patient management or outcome. For example, Jerusalem et al30 reported that whereas PET identified more lymph nodes than physical examination or CT in 15 of 60 patients with NHL and HL, in only 2 was there a change in stage (IA to IIA in one HL patient, and II to III in one low-grade NHL patient) with no change in treatment. Radford et al,9 Buchmann et al,33 and Rodriguez-Vigil et al47 reported that PET altered clinical stage and patient management in only 8% of patients with untreated NHL and HL. The prognosis and choice of therapy were modified in 1 patient who was upstaged from II to III, and in 3 patients from I to II. All 4 patients received more aggressive first-line therapy, although the details were not provided.
PET/CT offers distinct advantages in both the staging and restaging settings compared with enhanced full-dose diagnostic CT or PET alone. PET/CT performed even without intravenous contrast (unenhanced PET/CT) with the CT portion typically acquired as low-dose CT (40-80 mAs) is more sensitive and specific than contrast-enhanced full-dose CT for evaluation of nodal and extranodal lymphomatous involvement.20,21,48 Schaefer et al20 reported that in patients with HL or high-grade NHL the sensitivity of PET/CT and contrast-enhanced CT for lymph node involvement was 94% and 88%, respectively, while the specificity was 100% and 86%, respectively. For organ involvement, the sensitivity of PET/CT and contrast-enhanced CT was 88% and 50%, respectively, while the specificity was 100% and 90%, respectively. Tatsumi et al21 evaluated 1537 anatomic sites in 20 patients with HL and 33 patients with NHL on an unenhanced low-dose PET/CT scanner. There were 1489 sites concordant between PET and CT, and among the 48 discordant sites, PET correctly identified 40 sites as true positives or true negatives by biopsy or clinical follow up.
Preliminary data suggest that the CT portion of the PET/CT examination for initial staging using intravenous contrast permits a more accurate assessment of the liver and spleen compared with unenhanced CT.19 A recently published study showed no significant difference between the typically acquired unenhanced low-dose PET/CT (80 mAs) and a contrast-enhanced full dose PET/CT acquired with up to 300 mAs in the assessment of nodal and extranodal lymphoma at initial staging.44 However, the enhanced full-dose PET/CT resulted in fewer indeterminate findings and identified a larger number of extranodal sites compared with unenhanced, low-dose PET/CT. The authors attributed this slight advantage to the use of intravenous contrast rather than the use of high-dose x-ray. In aggregate, the published data suggest that enhanced low-dose PET/CT may represent a reasonable choice as a single imaging modality for staging routinely FDG-avid lymphomas. The increased radiation associated with PET would be, in part, offset by the reduced radiation dose associated with the low-dose compared with full-dose CT.
Whereas pretreatment PET(/CT) scanning is currently not standard in pretreatment staging of lymphoma, it is strongly encouraged in patients with HL and diffuse large B-cell NHL to facilitate the interpretation of equivocal posttherapy PET(/CT) scans in these patients (Table 1).19,49
Recommended timing of PET (PET/CT) scans in lymphoma clinical trials
| Histology . | Before treatment . | Middle of treatment . | Response assessment . | Surveillance after tx . |
|---|---|---|---|---|
| Routinely FDG avid | ||||
| DLBCL | Yes* | Clinical trial | Yes | No |
| HL | Yes* | Clinical trial | Yes | No |
| Follicular NHL | No† | Clinical trial | No† | No |
| MCL | No† | Clinical trial | No† | No |
| Variably FDG avid | ||||
| Other aggressive NHLs | No† | Clinical trial | No †‡ | No |
| Other indolent NHLs | No† | Clinical trial | No †‡ | No |
| Histology . | Before treatment . | Middle of treatment . | Response assessment . | Surveillance after tx . |
|---|---|---|---|---|
| Routinely FDG avid | ||||
| DLBCL | Yes* | Clinical trial | Yes | No |
| HL | Yes* | Clinical trial | Yes | No |
| Follicular NHL | No† | Clinical trial | No† | No |
| MCL | No† | Clinical trial | No† | No |
| Variably FDG avid | ||||
| Other aggressive NHLs | No† | Clinical trial | No †‡ | No |
| Other indolent NHLs | No† | Clinical trial | No †‡ | No |
tx indicates transplantation; DLBCL, diffuse large B-cell lymphoma; and MCL, mantle cell lymphoma.
Recommended but not required before treatment.
Recommended only if ORR/CR is a primary study end point.
Recommended only if PET is positive before treatment.
PET may be of particular value prior to therapy for patients who appear to have stage I or II disease and for whom radiation therapy is being considered. Additional sites of involvement would result in altering the treatment to systemic therapy. Thus, while PET may identify additional lesions during staging, prospective trials are needed to document an impact on patient outcome.
Should PET be used for restaging of lymphoma?
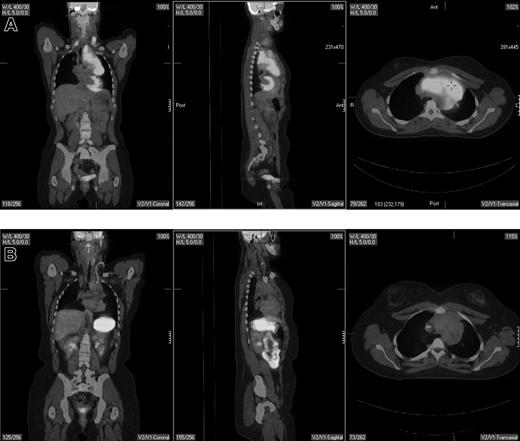
The clearest role for PET is in restaging patients following the completion of therapy.18,50-55 Restaging PET scanning is performed either for a final response assessment, typically within 6 to 8 weeks of therapy conclusion, or to determine the extent of known or suspected recurrence anytime after therapy. PET is more accurate than CT in this setting (Figure 2), largely related to its superiority in distinguishing between viable tumor and necrosis or fibrosis in residual mass(es). Jerusalem et al50 prospectively evaluated 54 patients with NHL (n = 35) and HL (n = 19), 24 with residual CT masses. All 6 patients with a positive PET scan relapsed compared with 5 (26%) of 19 CT+/PET− patients and 3 (10%) of 29 CT−/PET− patients. Eight of 48 patients relapsed despite a negative PET scan, suggesting the possibility of either residual disease below the resolution of the scanner or a false-negative result. Zinzani et al51 reported that all 13 aggressive NHL and HL patients with CT+PET+ residual abdominal masses relapsed (11 within 8 months) compared with only 1 of 24 CT+/PET− patients, who relapsed within 4 months at a previously involved site of disease. Spaepen et al53 reported on 93 patients with NHL; 56 of 67 patients with a normal PET scan after first-line chemotherapy remained in a complete remission (CR) at a median follow up of 653 days, compared with a relapse in all 26 patients with an abnormal PET scan occurring at a median of 73 days. In a retrospective analysis, Spaepen et al54 reported that 50 of 55 patients with a negative PET scan after completion of first-line treatment for HL remained in a complete remission at a median follow up of 955 days compared with all 5 patients with persistent abnormal FDG uptake who relapsed (median progression-free survival [PFS], 296 days).
Pretherapy and posttherapy fused PET/CT images in a 17-year-old female patient with nodular sclerosis Hodgkin disease. The pretherapy images (top) showed bilateral supraclavicular, anterior mediastinal, and left hilar lymphadenopathy by both PET and CT. Posttherapy PET/CT performed 4 weeks following completion of 6 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) showed resolution of disease in the supraclavicular and left hilar region but continued to show a residual 6.2 × 3.4-cm mass in the anterior mediastinum that was PET negative. Multiple biopsies of the mass showed only fibrous tissue with no evidence of lymphoma. The patient is currently without evidence of disease after 4 years of follow-up.
Pretherapy and posttherapy fused PET/CT images in a 17-year-old female patient with nodular sclerosis Hodgkin disease. The pretherapy images (top) showed bilateral supraclavicular, anterior mediastinal, and left hilar lymphadenopathy by both PET and CT. Posttherapy PET/CT performed 4 weeks following completion of 6 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) showed resolution of disease in the supraclavicular and left hilar region but continued to show a residual 6.2 × 3.4-cm mass in the anterior mediastinum that was PET negative. Multiple biopsies of the mass showed only fibrous tissue with no evidence of lymphoma. The patient is currently without evidence of disease after 4 years of follow-up.
The studies by Jerusalem et al50 and Spaepen et al53,54 used non–attenuation-corrected PET images, where mild FDG-PET uptake, particularly in deep-seated lymph nodes or nodal masses, may have gone undetected, since they fail to correct for absorption of photons through body tissues to obtain a true measure of accumulated activity. Weihrauch et al52 and Juweid et al,18 on the other hand, used currently standard attenuation-corrected PET to evaluate the predictive value of PET in HL and aggressive NHL, respectively. In a prospective series including 29 patients Weihrauch et al52 showed that 16 of 19 HL patients with PET-negative mediastinal tumor after first-line therapy remained in remission at a median follow up of 28 months, while 6 of 10 PET-positive patients progressed. Thus, the positive predictive value (PPV) of PET (the ability of a positive PET scan to predict persistent disease or future relapse) in this study was only 60%, while the negative predictive value (NPV) (the ability of a negative PET scan to exclude persistent disease or future relapse) was 84%. Juweid et al18 found in a retrospective evaluation of 54 patients with aggressive NHL a PPV of 74% and a NPV of 83% for attenuation-corrected PET scans.
In general, PET has a consistently high NPV averaging about 85% across studies including patients with HL and/or diffuse large B-cell NHL.18,50-55 The approximately 15% false-negative rate with PET is mostly related to its inability to detect microscopic disease resulting in future relapse. The PPV of PET is generally lower and considerably more variable averaging about 70% to 80%, with generally lower average values in patients with HL (∼ 65%) compared with NHL (∼ 85%).18,50-54 The reported generally lower PPV of PET in Hodgkin lymphoma compared with aggressive NHL is likely related to the substantial fraction of Hodgkin lymphoma patients who received radiation therapy, either alone or combined with chemotherapy, prior to undergoing PET.52 Postradiation inflammatory changes can lead to a false-positive PET scan. Still, the PPV of PET is substantially higher than CT, which has a reported PPV in patients with aggressive NHL of approximately 40% to 50% and in HL of only approximately 20%. The NPV of PET is similar to that of CT resulting in a considerably higher accuracy of PET for response assessment compared with CT (approximately 80% vs 50%).
What is the role of PET prior to stem-cell transplantation?
Patients whose tumors are FDG avid prior to stem-cell transplantation should be considered for alternative treatments because of the high risk for relapse and poor prognosis.56-60 Schot et al60 incorporated PET into 2 clinical risk scores, the secondary age-adjusted International Prognostic Index (sAA-IPI) for patients with recurring aggressive NHL and the recurring Hodgkin score (rHPS) for patients with recurring HL, before and after 2 cycles of reinduction chemotherapy prior to autologous stem-cell transplantation (ASCT). They were able to identify 4 risk groups that predicted success rates of ASCT ranging from 80% to 100% in patients with a low combined risk score to 0% to 7% in patients with a high combined risk score.
Should PET be used for monitoring response to therapy?
The International Prognostic Index (IPI) and the follicular lymphoma IPI (FLIPI) are currently used clinical prognostic indices for diffuse large B-cell lymphoma and follicular lymphoma, respectively.61,62 Molecular genetic profiling may also identify clinically meaningful prognostic groups within these risk categories.63 However, each of these prognostic models uses static pretreatment characteristics to predict the likelihood of response and survival in a given patient. PET, on the other hand, relies on the dynamic properties of the tumor mass both during and after treatment to predict outcome. PET for therapy monitoring is performed after 1 to 4 cycles of a 6- to 8-cycle chemotherapy or chemoimmunotherapy regimen to provide an early assessment of response that might result in altering patient management and outcome. Römer et al64 first correlated PET results with clinical outcome in 11 patients with newly diagnosed high-grade NHL. FDG uptake decreased by 60% by 7 days after initiation of chemotherapy, and 79% by 42 days. Lower SUV values and FDG accumulation rates after 2 cycles of chemotherapy were associated with stable complete remissions at 16 months (P = .018).
Numerous studies have confirmed that midtreatment PET scans predict clinical outcome.65-72 Spaepen et al66 reported that none of 33 patients with a positive PET after 3 or 4 cycles of chemotherapy for aggressive lymphoma experienced a durable complete response compared with 31 of 37 patients with a negative scan, who remained in a complete remission at a median follow-up of 1107 days. Haioun et al67 treated 90 patients with aggressive NHL and prospectively assessed PET before chemotherapy, after 2 cycles, and following completion of treatment. Early PET results predicted complete response rate, event-free survival, and overall survival, irrespective of IPI risk group or rituximab therapy. Hutchings et al70 evaluated 77 newly diagnosed patients with HL at staging and after 2 cycles of chemotherapy. They found that early PET results were as accurate as studies performed later in the treatment and were superior to CT scanning. Kostakoglu et al72 showed that a positive PET scan even after 1 cycle of chemotherapy was associated with a shorter median progression-free survival than the median not reached at 18 months for those with a negative PET.
To date, no clinical trials in patients with lymphoma have demonstrated clinical benefit from altering therapy on the basis of interim PET results. Thus, midtreatment PET scans should be reserved for clinical trials addressing this important question (Table 1).
Should surveillance PET scanning be part of standard of care?
PET for posttherapy surveillance is performed following treatment in the absence of clinical, biochemical, or radiographic evidence of recurrent disease, with the goal of early detection of recurrence. Most studies, however, suggest that more than 80% of the time, it is the patient or the physician who first suspects early recurrence, even with routine screening including CT scans.73-77 Similarly, the role of PET in posttreatment surveillance of lymphoma remains unproven. Jerusalem et al78 prospectively evaluated 36 HL patients who underwent PET every 4 to 6 months for 2 years at the completion of treatment. One patient had residual disease and 4 relapsed during follow-up; however, in 2 of these patients, there were disease-related symptoms. Thus, PET identified disease before the onset of symptoms in only 3 of 36 patients with confirmed relapsed disease but resulted in false-positive studies in another 6 patients. Since there is no demonstrated improvement in outcome with early detection using imaging, history and physical examination remain the standard approach to follow-up.73,74,77,79 Nevertheless, Jerusalem et al78 used PET and not PET/CT, the latter associated with substantially fewer false-positive findings and also greater sensitivity compared with PET alone. Thus, whether PET/CT may prove helpful in surveillance scanning for patients who are at particular risk of early relapse needs to be validated in prospective clinical trials.
Can PET be used to identify aggressive transformation?
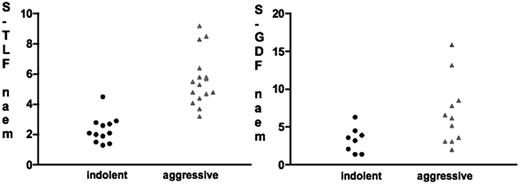
PET has been evaluated to confirm the clinical suspicion of transformation from an indolent to an aggressive histology. In this setting, PET may support the clinical suspicion and help select the optimal biopsy site for definitive histopathologic confirmation (ie, the one with the highest SUV). Nevertheless, FDG-PET is unlikely to replace biopsy in this setting because of the significant overlap in the degree of FDG uptake or SUV between indolent and aggressive lymphomas.80,81 Schöder et al81 reported that a SUVmax of more than 13 was associated with about a 90% probability of aggressive lymphoma, while a SUVmax of less than 6 was associated with a very high probability of indolent lymphoma. Unfortunately, the SUVmax ranges of more than 13 and less than 6 comprise only about half of the patients, the remaining half having equivocal SUVs. It is noteworthy that the use of F-18-fluorothymidine (FLT) as an in vivo marker of proliferative activity may be superior to FDG in differentiating between indolent and aggressive lymphomas (Figure 3) and, hence, may prove superior to FDG in assessment of transformation particularly when the FDG-SUV is in the equivocal range.82
Comparison between mean fluorothymidine (FLT) standardized uptake values (SUVs) and FDG SUVs in patients with indolent and aggressive non-Hodgkin lymphoma. The scattergrams demonstrate the superiority of FLT in differentiating between the 2 lymphoma types. Note the considerable overlap in FDG-SUVs between indolent and aggressive lymphoma. The only patient with an overlap in FLT-SUV was initially diagnosed with indolent lymphoma but was reclassified as high-grade lymphoma 3 weeks after PET imaging. (Reprinted from Buck et al82 with permission.)
Comparison between mean fluorothymidine (FLT) standardized uptake values (SUVs) and FDG SUVs in patients with indolent and aggressive non-Hodgkin lymphoma. The scattergrams demonstrate the superiority of FLT in differentiating between the 2 lymphoma types. Note the considerable overlap in FDG-SUVs between indolent and aggressive lymphoma. The only patient with an overlap in FLT-SUV was initially diagnosed with indolent lymphoma but was reclassified as high-grade lymphoma 3 weeks after PET imaging. (Reprinted from Buck et al82 with permission.)
What are the current limitations of PET scans?
False-positive and false-negative PET results have the potential to impact patient management. False positives arise because FDG is taken up in any process associated with increased glycolysis, for example, inflammation, infection, granulomatous disease such as sarcoidosis,83,84 and brown fat85 (Figure 4). Castellucci et al86 reported that 21.3% of positive PET scans reviewed (n = 134) had nontumoral uptake. Abnormal FDG uptake has also been associated with hyperplasia of the thymus in HL patients.87 Similarly, abnormal uptake has been associated with hyperplasia in the bone marrow and spleen in patients receiving granulocyte colony-stimulating factor after chemotherapy.88,89
To minimize the frequency of false-positive PET, the imaging subcommittee of the International Harmonization Project (IHP) recommended performing PET at least 3 weeks following chemotherapy and preferably 8 to 12 weeks after radiation therapy.19 These recommendations also included a standardized definition for a PET-positive residual mass. The lack of a systematic interpretation may have contributed to the wide variability in the reported PPV of PET in previous studies. According to the IHP definitions, residual masses or 2 cm or more in greatest transverse diameter (GTD) with FDG activity visually exceeding that of mediastinal blood pool structures are considered PET positive, whereas residual masses 1.1 to 1.9 cm are considered PET positive only if their activity exceeds surrounding background activity.
Using this definition, Olsen et al evaluated 50 consecutive patients with HL (n = 26) or aggressive NHL (n = 24) who underwent PET/CT within 3 to 12 weeks after therapy and had at least 1 year of follow up after treatment.90 A total of 51 residual masses were found in 28 patients (56%), 31 were 2 cm or more in greatest transverse diameter (GTD) and 20 were 1.1 to 1.9 cm in GTD but with a short axis diameter more than 1 cm. The proposed IHP interpretation resulted in high predictive value in posttreatment evaluation of residual masses in both histologies. The 2-year event-free survival in patients with PET-positive residual masses by the IHP definitions19 was 0% compared with 95% in patients with PET-negative residual masses and 85% in patients without residual masses.
False-negative PET scans may result from lesions below the resolution of the scanner, generally 5 to 10 mm. Because PET scanners differ in how they acquire, reconstruct, and analyze images, serial scans obtained in the same patient on different scanners can yield inconsistent results.91
The timing of imaging tumors is equally important, and scans obtained too soon after the injection of FDG can miss tumors. Kubota et al92 reported that delaying PET imaging 2 hours after the injection of FDG improved detection of various tumor types, including lymphoma. Finally, whereas normal physiologic uptake of FDG into the brain, heart, and digestive tract may obscure underlying tumor, hyperglycemia may decrease the amount of FDG uptake into tumor.
There is also variability of FDG avidity among histologies of lymphoma. Elstrom et al93 reported that PET detected at least one site of disease in 100% of patients with large cell lymphoma and mantle cell lymphoma and in 98% of patients with HL and follicular lymphoma. However, only 67% of marginal zone lymphomas and 40% of peripheral T-cell lymphomas were FDG avid. Jerusalem et al35 reported that PET detected 58% fewer abnormal lymph node areas in patients with small lymphocytic lymphoma than CT in contrast to detecting 40% more abnormal lymph node areas in patients with follicular NHL. Hoffmann et al94 reported the absence of FDG uptake in 10 patients with extranodal marginal zone lymphomas while nodal marginal zone lymphoma was FDG avid,95 and that that were positive tended to have plasmacytic features.96 In a retrospective series of 42 patients with extranodal marginal zone, Beal et al97 found that 81% had focal uptake at tumor sites; however, they considered SUVs as low as 1.4 as positive. Avidity of T-NHLs is also variable.98 Direct communication between the clinician and nuclear medicine physician is critical to minimizing the likelihood of a misleading PET interpretation.
Should PET be integrated into standard response criteria?
The International Working Group (IWG) criteria for response assessment of non-HLs were developed to enable researchers to compare the results of clinical trials and to promote the identification of new, effective therapies.99 CT was the most widely used imaging modality used at the time for response assessment, although SPECT gallium was recommended. The impact of PET on the IWG criteria was first evaluated by Juweid et al in a retrospective analysis of 54 patients with aggressive NHL who underwent PET and CT 1 to 16 weeks after completing anthracycline-based chemotherapy.18 PET increased the number of complete remissions from 17 to 35, and the complete remission unconfirmed (CRu) category was eliminated. The hazard ratio between partial remission (PR) and CR or between PR and CR/CRu was higher by IWG-PET than by IWG. In a multivariate model including both classification systems, only IWC + PET was a statistically significant independent predictor of PFS (P = .008).
While PET is an integral part of the revised response criteria, its use should be limited to the appropriate histologies and for the approved indications (Tables 1, 2).49
Response definitions for clinical trials
| Response . | Definition . | Nodal masses . | Spleen, liver . | Bone marrow . |
|---|---|---|---|---|
| Complete remission (CR) | Disappearance of all evidence of disease | (a) FDG avid or PET+ prior to therapy: mass of any size permitted if PET−. (b) Variably FDG avid or PET−: regression to normal size on CT | Not palpable, nodules disappeared | Infiltrate cleared on repeat biopsy; if indeterminate by morphology, immunohistochemistry should be negative |
| Partial remission (PR) | Regression of measurable disease and no new sites | ≥ 50% decrease in SPD of up to 6 largest dominant masses. No increase in size of other nodes. (a) FDG avid or PET+ prior to therapy: one or more PET+ at previously involved site. (b) Variably FDG avid or PET−: Regression on CT | ≥ 50% decrease in SPD of nodules (for single nodule in greatest transverse diameter); no increase in size of liver or spleen | Irrelevant if positive prior to therapy; cell type should be specified |
| Stable disease (SD) | Failure to attain CR/PR or PD | (a) FDG avid or PET+ prior to therapy: PET+ at prior sites of disease and no new sites on CT or PET. (b) Variably FDG avid or PET−: no change in size of previous lesions on CT | — | — |
| Relapsed or progressive disease | Any new lesion or increase from nadir by ≥ 50% of previously involved sites | Appearance of a new lesion > 1.5 in any axis, ≥ 50% increase in the longest diameter of a previously identified node, > 1 cm in short axis or in the SPD of more than one node. Lesions PET+ if FDG avid lymphoma or PET+ prior to therapy | ≥ 50% increase from nadir in the SPD of any previous lesions | New or recurrent involvement |
| Response . | Definition . | Nodal masses . | Spleen, liver . | Bone marrow . |
|---|---|---|---|---|
| Complete remission (CR) | Disappearance of all evidence of disease | (a) FDG avid or PET+ prior to therapy: mass of any size permitted if PET−. (b) Variably FDG avid or PET−: regression to normal size on CT | Not palpable, nodules disappeared | Infiltrate cleared on repeat biopsy; if indeterminate by morphology, immunohistochemistry should be negative |
| Partial remission (PR) | Regression of measurable disease and no new sites | ≥ 50% decrease in SPD of up to 6 largest dominant masses. No increase in size of other nodes. (a) FDG avid or PET+ prior to therapy: one or more PET+ at previously involved site. (b) Variably FDG avid or PET−: Regression on CT | ≥ 50% decrease in SPD of nodules (for single nodule in greatest transverse diameter); no increase in size of liver or spleen | Irrelevant if positive prior to therapy; cell type should be specified |
| Stable disease (SD) | Failure to attain CR/PR or PD | (a) FDG avid or PET+ prior to therapy: PET+ at prior sites of disease and no new sites on CT or PET. (b) Variably FDG avid or PET−: no change in size of previous lesions on CT | — | — |
| Relapsed or progressive disease | Any new lesion or increase from nadir by ≥ 50% of previously involved sites | Appearance of a new lesion > 1.5 in any axis, ≥ 50% increase in the longest diameter of a previously identified node, > 1 cm in short axis or in the SPD of more than one node. Lesions PET+ if FDG avid lymphoma or PET+ prior to therapy | ≥ 50% increase from nadir in the SPD of any previous lesions | New or recurrent involvement |
— indicates not applicable.
Future directions
The role of PET in the management of patients with lymphomas is currently being defined. Prospective studies are needed to determine whether PET should replace the Ann Arbor staging system for lymphoma. Although PET results correlate with outcome in aggressive NHL and HL, the role in other histologies is less well characterized. PET is currently being validated as a surrogate for clinical benefit in prospective randomized clinical trials, most notably CALGB 50303, a comparison of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) with R-EPOCH (rituximab, etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone) including genetic profiling in addition to PET scans.100 The newly published standardized guidelines for interpretation of PET in lymphoma should reduce variability among studies.19
Other issues remain to be addressed. For example, does altering therapy on the basis of a midtreatment PET favorably impact on outcome? If so, what is the optimal number of cycles of therapy before performing PET? Whether new radiotracers in development (eg, DNA synthesis, amino acid transport and protein metabolism, membrane lipid synthesis, and hypoxia) will be superior to FDG remains to be demonstrated.
PET as a biomarker has the potential to change the current model of drug development. The duration of phase 2 trials could be shortened if there was a lack of response on PET just as phase 3 trials could lead to accelerated approval if early clinical benefit was demonstrated on PET.100 In an era of evolving targeted therapies and gene expression profiling, tailoring therapy based on dynamic changes within the tumor itself is the logical next step. Facilitating the timely implementation of additional therapeutic interventions in nonresponders and increasing access to promising drugs to lymphoma patients nationwide could result in a significant improvement in patient outcome.
Authorship
Contribution: All authors contributed equally.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce D. Cheson, Georgetown University Hospital, 3800 Reservoir Rd, NW, Washington, DC 20007; e-mail:bdc4@georgetown.edu.