To examine the efficacy of intensified maintenance chemotherapy, we conducted a prospective multicenter trial in adult patients with newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Of the 302 registered, 283 patients were assessable and 267 (94%) achieved complete remission. Predicted 6-year overall survival in all assessable patients and disease-free survival in patients who achieved complete remission were 83.9% and 68.5%, respectively. A total of 175 patients negative for PML-RARα at the end of consolidation were randomly assigned to receive either intensified maintenance chemotherapy (n = 89) or observation (n = 86). Predicted 6-year disease-free survival was 79.8% for the observation group and 63.1% for the chemotherapy group, showing no statistically significant difference between the 2 groups (P = .20). Predicted 6-year survival of patients assigned to the observation was 98.8%, which was significantly higher than 86.2% in those allocated to the intensified maintenance (P = .014). These results indicate that the intensified maintenance chemotherapy did not improve disease-free survival, but rather conferred a significantly poorer chance of survival in acute promyelocytic leukemia patients who have become negative for the PML-RARα fusion transcript after 3 courses of intensive consolidation therapy.
Introduction
The use of all-trans retinoic acid (ATRA) has markedly improved the therapeutic outcome in patients with acute promyelocytic leukemia (APL).1,–3 However, most patients treated with ATRA alone after achievement of complete remission (CR) eventually relapse, indicating that postremission chemotherapy is essential to obtain long-term survival.2,3 Noncross-resistance between ATRA and chemotherapeutic drugs has contributed to not only a high CR rate but also a decrease in the relapse rate, leading to a significant improvement in disease-free survival (DFS) and overall survival (OS) rates.4,,,,,,–11 Despite the impact of ATRA in the treatment of APL, approximately 10% to 30% of patients who were given intensive chemotherapy after achievement of CR still experienced relapse in several cooperative group studies.5,7,,,,–12
Before the introduction of ATRA in the treatment of APL, the efficacy of maintenance chemotherapy had been observed in patients with APL.13,14 In our previous study, the Japan Adult Leukemia Study Group (JALSG) APL92 study, patients with newly diagnosed APL received intensified maintenance therapy according to an earlier result of the AML87 study, which was performed before the use of ATRA.5 The AML87 study showed a significantly better DFS in patients who received 12 courses of intensified maintenance chemotherapy compared with those administered 4 courses of the same chemotherapy.15 However, it is not clear whether maintenance chemotherapy actually prevents relapse in APL patients treated with ATRA and chemotherapy, especially after they have become negative for the PML-RARα transcript at the end of intensive consolidation chemotherapy. If short-term therapy without maintenance shows DFS rates identical to those for long-term therapy with maintenance, it would be beneficial for patients' quality of life as well as for medical costs. To determine the value of intensified maintenance chemotherapy, this study was designed to compare DFS and survival in previously untreated APL patients who had become negative for PML-RARα transcript after 3 courses of intensive consolidation and were randomly allocated to either intensified maintenance chemotherapy or observation.
Patients and methods
Eligibility
Adult patients with previously untreated de novo APL were consecutively registered in the JALSG APL97 study. Eligible criteria were a diagnosis ofAPL with t(15;17) and/or the PML-RARα fusion gene; age from 15 to 70 years;Eastern Cooperative Oncology Group performance status between 0 and 3; and sufficient function of the heart (no severe abnormalities detected on ECGs and echocardiographs), lung (PaO2 > 60 mm Hg or SpO2 > 93%), liver (serum bilirubin level < 2.0 mg/dL), and kidney (serum creatinine level < 2.0 mg/dL). The study was approved by the Institutional Review Boards at each participating institution. Written informed consent was obtained from all patients before registration in accordance with the Declaration of Helsinki.
Assessment of disease
Morphologic diagnosis of APL was made according to the French-American-British classification and the bone marrow smears were centrally reviewed at the JALSG pathology committee. The diagnosis was confirmed by the presence of t(15;17) and/or the PML-RARα fusion gene. Bone marrow samples were obtained at diagnosis, after induction therapy, after each cycle of consolidation chemotherapy, and periodically during maintenance chemotherapy. The PML-RARα fusion gene was amplified using bone marrow samples obtained at diagnosis and after consolidation therapy by reverse-transcriptase polymerase chain reaction analysis.16,17 The detection limit of PML-RARα fusion transcript in this assay was 10−4.
Treatment regimens
Induction therapy.
Treatment was started as soon as a morphologic diagnosis of APL had been made. For remission induction therapy, patients received 45 mg/m2/d of ATRA orally divided into 3 doses given after meals daily until the day before the start of the first consolidation therapy. If patients had leukocyte counts below 3.0 × 109/L and APL cells below 109/L at the start of therapy, they were treated with ATRA alone (group A). ATRA at the same dosage combined with idarubicin (12 mg/m2/d by 30-minute intravenous infusion on days 1 and 2) plus cytarabine (Ara-C) (80 mg/m2/d by continuous intravenous infusion on days 1 through 5) was given to patients with initial leukocyte counts between 3.0 × 109/L and 10.0 × 109/L, and those with leukocyte counts below 3.0 × 109/L and APL cells above 109/L (group B). Patients with initial leukocyte counts of 10.0 × 109/L or more received idarubicin (12 mg/m2 on days 1 to 3) plus Ara-C (100 mg/m2 on days 1 to 5) in addition to ATRA (group C). During treatment with ATRA, if blast and promyelocyte counts in the peripheral blood were more than 109/L, an additional cycle of chemotherapy consisting of idarubicin (12 mg/m2 for 2 days) and Ara-C (80 mg/m2 for 5 days) was given. Patients in groups A and B who received an additional cycle of chemotherapy during induction were designated as groups AD and BD, respectively.
For prevention of bleeding, patients received transfusions of platelets and fresh frozen plasma to maintain platelet counts above 30 × 109/L or more and plasma fibrinogen level above 4.4 μmol/L (150 mg/dL) or more, respectively. If coagulation studies were abnormal, prophylactic use of heparin and/or other antifibrinolysis agents (dalteparin, gabexate mesilate, or nafamostat mesilate) was recommended. When retinoic acid (RA) syndrome occurred, ATRA was discontinued and 20 mg/kg of methylprednisolone was administered by 1-hour intravenous infusions for at least 3 days. RA syndrome was diagnosed in patients with unexplained fever, respiratory distress, weight gain, interstitial pulmonary infiltrate, and pleural or pericardial effusions, as previously described.18,–20 After resolution of the syndrome, ATRA was resumed at the same dosage.
Consolidation therapy.
After achieving CR, patients received 3 courses of consolidation chemotherapy. The first consolidation consisted of mitoxantrone (7 mg/m2) by 30-minute intravenous infusion on days 1 to 3, and Ara-C (200 mg/m2) by continuous infusion on days 1 to 5. The secondconsolidation contained Ara-C (140 mg/m2) for 5 days, etoposide (100 mg/m2)by 1-hour intravenous infusion for 5 days, and daunorubicin (50 mg/m2) by 30-minute infusion on days 1 through 3. The third consolidation consisted of Ara-C (140 mg/m2) for 5 days and idarubicin (12 mg/m2) for 3 days. Each consolidation course was given after recovery from the previous course, when polymorphonuclear cells were 1.5 × 109/L or more and platelets were 100 × 109/L or more. All patients received an intrathecal administration of methotrexate (MTX)(15 mg), Ara-C (40 mg), and prednisolone (10 mg) at the end of the second consolidation therapy.
Intensified maintenance chemotherapy.
After completion of consolidation therapy, patients negative for the PML-RARα transcript were randomly allocated either to receive 6 courses of intensified maintenance chemotherapy every 6 weeks or to observation. Randomization was stratified by age and initial leukocyte count, both of which were prognostic factors for DFS in the JALSG APL92 study.21 The first course of intensified maintenance therapy consisted of behenoyl Ara-C (BHAC) (170 mg/m2, 2-hour infusion, days 1 through 5), daunorubicin (30 mg/m2, 30-minute infusion, days 1 and 4) and mercaptopurine (6MP; 70 mg/m2, orally, days 1 through 7). The second consisted of BHAC and mitoxantrone (5 mg/m2, 30-minute infusion, days 1 and 2). The third consisted of BHAC, etoposide (80 mg/m2, 1-hour infusion, days 1, 3, and 5), and vindesine (2 mg/m2, bolus infusion, days 1 and 8). The fourth consisted of BHAC, aclarubicin (14 mg/m2, 30-minute infusion, days 1 through 4), and 6MP. The fifth and sixth courses were the same as the first and third, respectively. Patients who were positive for the PML-RARα fusion transcript at the end of consolidation chemotherapy received late ATRA therapy (45 mg/m2/day, orally after meals for 4 weeks) followed by maintenance therapy. These patients were also scheduled to receive allogeneic hematopoietic stem cell transplantation (HSCT) if there was a human leukocyte antigen-identical donor.
Definition and study end points
Hematologic response was evaluated by standard criteria generally used for chemotherapy.22,23 CR was defined as less than 5% of blasts and promyelocytes with normal erythropoiesis, thrombopoiesis, and granulopoiesis in the bone marrow, and neutrophil counts of more than 1.5 × 109/L and platelet counts of more than 100 × 109/L in the peripheral blood. Hematologic relapse was defined as the presence of more than 10% blasts plus abnormal promyelocytes in the marrow or the presence of any those cells in the peripheral blood or extramedullary sites. In addition, molecular relapse detected by the reverse-transcriptase polymerase chain reaction analysis of PML-RARα was also considered as a relapse event.
The primary end point of this study was survival and DFS of patients in CR who had become negative for the PML-RARα fusion transcript after the consolidation therapy and who were registered in the randomized study of the maintenance chemotherapy. OS for all patients was calculated from the first day of therapy to death or last visit. DFS for patients who had achieved CR was measured from the date of CR to relapse, death from any cause, or last visit. Survival and DFS in patients who were randomized to either observation or maintenance chemotherapy groups were measured from the date of random assignment to the same end points of these mentioned.
Statistical analyses
Baseline characteristics of the 2 randomized groups were compared using the chi-square test or Fisher exact test for categorical data, and the Wilcoxon rank-sum test for continuous data. Probabilities of survival and DFS were estimated using the Kaplan-Meier method and compared by the log-rank test. The follow-ups on these patients were updated on September 30, 2004. Patients who were lost to follow-up or were still alive at the time of data cutoff were censored at the last date they were known to be alive. Patients who underwent HSCT were also censored at the date of HSCT. Factors affecting survival and DFS were analyzed by the use of the Cox regression model to estimate a hazard ratio with 95% confidence intervals (CI). All analyses were performed according to the intent-to-treat principle. All statistical tests were 2-sided, and the significance level was set at .05. Statistical analyses were performed using SAS 8.2 (SAS Institute Japan, Tokyo, Japan).
Results
Patient characteristics
Between May 1997 and June 2002, 302 patients from 92 institutes participating in the JALSG were consecutively enrolled in the study. Of these, 19 were excluded because 4 were misdiagnosed, 2 were not consistent with the eligibility criteria, 7 were negative for t(15;17) or PML-RARα, and 6 had no test for t(15;17) or PML-RARα. Early death was not excluded, although 5 patients died of hemorrhage within 7 days. The characteristics of the 283 evaluable patients are listed in Table 1. Ages ranged from 15 to 70 years, with a median of 48 years. Eighteen patients (6%) had a variant form of French-American-Britishmorphology (M3v). The median leukocyte count was 1.7 × 109/L(range, 0.03 to 257 × 109/L) on admission. One hundred fifty-one patients started on ATRA alone during induction, and in 66 of these, chemotherapy was later added because of increased blasts and promyelocytes according to the protocol (groups A and AD; Table 1). One hundred twenty-five patients received both ATRA and chemotherapy from the beginning of therapy (groups B and C), and in 4 of group B an additional cycle of chemotherapy was later added because of increased blasts and promyelocytes (group BD).
Clinical features of patients at diagnosis
| Parameters . | Total . | Maintenance chemotherapy . | Observation . | P* . | |||
|---|---|---|---|---|---|---|---|
| No. (%) . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | Median (range) . | ||
| No. of patients | 283 | 89 | 86 | ||||
| Sex | |||||||
| Male | 158 (56) | 53 (60) | 47 (55) | .51 | |||
| Female | 125 (44) | 36 (40) | 39 (45) | ||||
| Age, years | 48 (15-70) | 49 (15-70) | 46 (16-67) | .70 | |||
| 15-29 | 49 (17) | 17 (19) | 15 (17) | ||||
| 30-49 | 106 (37) | 32 (36) | 34 (40) | .88 | |||
| 50-70 | 128 (45) | 40 (45) | 37 (43) | ||||
| FAB Morphology | |||||||
| M3 | 265 (93) | 80 (90) | 82 (95) | .25 | |||
| M3v | 18 (6) | 9 (10) | 4 (5) | ||||
| Leukocyte count, ×109/L | 1.7 (0.03-257) | 1.9 (0.03-152) | 2.1 (0.1-98) | .95 | |||
| Less than 3.0 | 174 (61) | 50 (56) | 47 (55) | ||||
| 3.0-10.0 | 58 (20) | 21 (24) | 20 (23) | .95 | |||
| 10.0 or higher | 51 (18) | 18 (20) | 19 (22) | ||||
| Platelet count, ×109/L | 30 (2-238) | 31 (4-230) | 23 (2-238) | .10 | |||
| Less than 10 | 39 (14) | 8 (9) | 16 (19) | ||||
| 10-40 | 140 (49) | 47 (53) | 41 (48) | .18 | |||
| 40 or higher | 104 (37) | 34 (38) | 29 (34) | ||||
| Induction therapy# | |||||||
| Group A | 85 (30) | 29 (33) | 29 (34) | 1.0 | |||
| Group AD | 66 (23) | 17 (19) | 16 (19) | ||||
| Group B+BD | 73 (26) | 23 (26) | 22 (26) | ||||
| Group C | 52 (18) | 20 (22) | 19 (22) | ||||
| Unknown | 7 (2) | NA | NA | ||||
| Parameters . | Total . | Maintenance chemotherapy . | Observation . | P* . | |||
|---|---|---|---|---|---|---|---|
| No. (%) . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | Median (range) . | ||
| No. of patients | 283 | 89 | 86 | ||||
| Sex | |||||||
| Male | 158 (56) | 53 (60) | 47 (55) | .51 | |||
| Female | 125 (44) | 36 (40) | 39 (45) | ||||
| Age, years | 48 (15-70) | 49 (15-70) | 46 (16-67) | .70 | |||
| 15-29 | 49 (17) | 17 (19) | 15 (17) | ||||
| 30-49 | 106 (37) | 32 (36) | 34 (40) | .88 | |||
| 50-70 | 128 (45) | 40 (45) | 37 (43) | ||||
| FAB Morphology | |||||||
| M3 | 265 (93) | 80 (90) | 82 (95) | .25 | |||
| M3v | 18 (6) | 9 (10) | 4 (5) | ||||
| Leukocyte count, ×109/L | 1.7 (0.03-257) | 1.9 (0.03-152) | 2.1 (0.1-98) | .95 | |||
| Less than 3.0 | 174 (61) | 50 (56) | 47 (55) | ||||
| 3.0-10.0 | 58 (20) | 21 (24) | 20 (23) | .95 | |||
| 10.0 or higher | 51 (18) | 18 (20) | 19 (22) | ||||
| Platelet count, ×109/L | 30 (2-238) | 31 (4-230) | 23 (2-238) | .10 | |||
| Less than 10 | 39 (14) | 8 (9) | 16 (19) | ||||
| 10-40 | 140 (49) | 47 (53) | 41 (48) | .18 | |||
| 40 or higher | 104 (37) | 34 (38) | 29 (34) | ||||
| Induction therapy# | |||||||
| Group A | 85 (30) | 29 (33) | 29 (34) | 1.0 | |||
| Group AD | 66 (23) | 17 (19) | 16 (19) | ||||
| Group B+BD | 73 (26) | 23 (26) | 22 (26) | ||||
| Group C | 52 (18) | 20 (22) | 19 (22) | ||||
| Unknown | 7 (2) | NA | NA | ||||
Baseline characteristics of the two randomized groups were compared with Chi-squqre test or Wilcoxon rank-sum test.
Patients in Group A were treated with ATRA alone; patients in Groups B and C were treated with ATRA plus idarubicin and cytarabine. Patients in Groups A and B who received an additional cycle of chemotherapy due to increased leukemic cells during induction were designated as Groups AD and BD, respectively. Four patients were in Group BD.
NA indicates not applicable.
Treatment outcome.
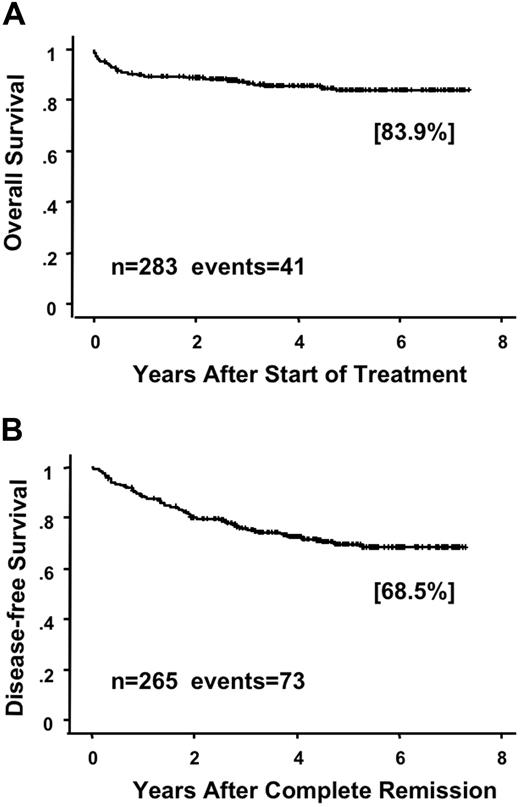
Of the 283 evaluable patients, 267 (94.3%) had CR at a median of 42 days (range, 14 to 98) after the start of therapy. During induction therapy, 60 (21%) patients showed signs of RA syndrome and 2 died of the syndrome. In addition, 65 (23%) patients developed organ bleeding, and 9 patients had fatal bleeding, including 5 early deaths within 7 days (Table 2). Thus, early death caused by bleeding was a major cause of induction failure. Although one patient had resistant leukemia, this patient received ATRA for only 16 days because of RA syndrome. Of the 267 patients who achieved CR, 258 (97%) completed the first course of consolidation, 250 (94%) completed the second, and 235 (88%) patients completed the third (Table 2 and Figure 1). After the consolidation, 5 patients underwent allogeneic HSCT at their first CR and 30 patients underwent HSCT after relapse. At a median follow-up of 64 months (range, 27 to 88 months), 60 (22%) of the 267 patients had relapsed and 18 had died. A further 16 (6%) patients died in CR, and 10 of those died of infection during myelosuppression after consolidation therapy (Table 2). The predicted 6-year OS rate in all 283 assessable patients was 83.9% (95% confidence interval [CI], 79.2% to 88.6%; Figure 2A). The predicted 6-year DFS rate in 265 CR cases was 68.5% (95% CI, 62.1% to 74.9%; Figure 2B).
Events occurring during the induction and consolidation therapy
| . | Induction . | Consolidation 1 . | Consolidation 2 . | Consolidation 3 . |
|---|---|---|---|---|
| No. of registered patients | 283 | 267 | 258 | 250 |
| Death during treatment | 13 | 0 | 4 | 6 |
| Infection | 1 | 0 | 4 | 6 |
| Bleeding | 9 | 0 | 0 | 0 |
| RA syndrome | 2 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 |
| Going off study by toxicity | 0 | 3 | 0 | 0 |
| Lost to follow-up | 2 | 6 | 3 | 7 |
| Relapse | 0 | 0 | 1 | 2 |
| Refractory | 1 | 0 | 0 | 0 |
| Stem cell transplantation | 0 | 0 | 0 | 0 |
| No. of completed patients | 267 | 258 | 250 | 235 |
| . | Induction . | Consolidation 1 . | Consolidation 2 . | Consolidation 3 . |
|---|---|---|---|---|
| No. of registered patients | 283 | 267 | 258 | 250 |
| Death during treatment | 13 | 0 | 4 | 6 |
| Infection | 1 | 0 | 4 | 6 |
| Bleeding | 9 | 0 | 0 | 0 |
| RA syndrome | 2 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 |
| Going off study by toxicity | 0 | 3 | 0 | 0 |
| Lost to follow-up | 2 | 6 | 3 | 7 |
| Relapse | 0 | 0 | 1 | 2 |
| Refractory | 1 | 0 | 0 | 0 |
| Stem cell transplantation | 0 | 0 | 0 | 0 |
| No. of completed patients | 267 | 258 | 250 | 235 |
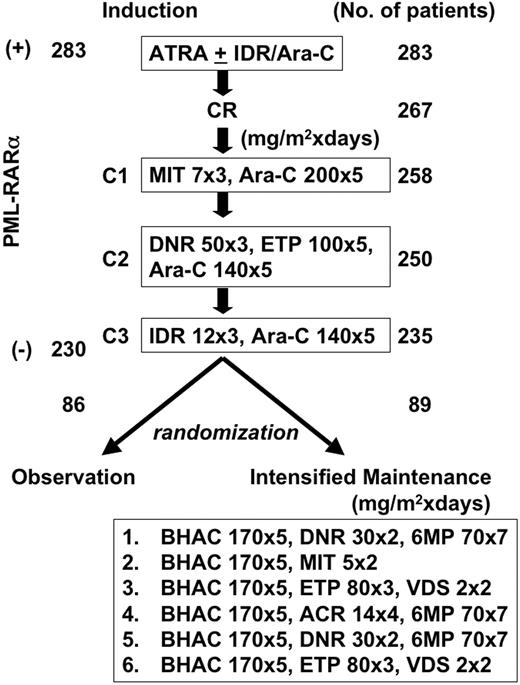
Study design. The number of patients who completed each step is indicated. C1, C2, and C3 were consolidation courses 1, 2, and 3. A total of 283 patients had t(15;17) and/or the PML-RARα transcript at the time of diagnosis, and 230 patients were negative for PML-RARα at the end of 3 courses of consolidation therapy. After completion of consolidation therapy, 175 patients who showed absence of PML-RARα transcript were randomized either to receive 6 courses of intensified maintenance chemotherapy (n = 89) or to observation (n = 86).
Study design. The number of patients who completed each step is indicated. C1, C2, and C3 were consolidation courses 1, 2, and 3. A total of 283 patients had t(15;17) and/or the PML-RARα transcript at the time of diagnosis, and 230 patients were negative for PML-RARα at the end of 3 courses of consolidation therapy. After completion of consolidation therapy, 175 patients who showed absence of PML-RARα transcript were randomized either to receive 6 courses of intensified maintenance chemotherapy (n = 89) or to observation (n = 86).
Overall survival and disease-free survival in patients enrolled in the JALSG APL97 study. Overall survival (A) in all assessable patients and disease-free survival (B) in patients who achieved CR are estimated by the Kaplan-Meier method.
Overall survival and disease-free survival in patients enrolled in the JALSG APL97 study. Overall survival (A) in all assessable patients and disease-free survival (B) in patients who achieved CR are estimated by the Kaplan-Meier method.
Randomized study with or without intensified maintenance therapy.
Among the 235 patients who completed 3 courses of consolidation and were evaluated for minimal residual disease, 5 (2.1%) were positive for the PML-RARα fusion transcript. Three of these subsequently relapsed and another patient received allogeneic HSCT. However, 230 patients (97.9%) showed no PML-RARα transcript in the bone marrow cells at the end of consolidation. A total of 55 patients negative for PML-RARα were not included in the randomized study for a variety of reasons. Of these, 33 patients refused the randomization because 20 did not want to receive further therapy and 13 wanted to receive additional chemotherapy; another 13 had residual toxicity from the consolidation and were considered as lack of tolerance to subsequent therapy (10 myelosuppression, 2 general fungal infection, and 1 heart disease); lost to follow-up in 3 patients; and unknown causes or no report in 6 patients. There was no significant difference in the 6-year DFS between 175 patients included in the randomized study (70.8%; 95% CI, 62.7% to 78.8%) and 55 patients not included (76.7%; 95% CI, 65.1 to 88.3%; P = .87). The 6-year OS was 92.1% (95% CI, 87.2% to 97.1%) in the patients enrolled in the randomized study and 93.1% (95% CI, 85.3% to 100%) in the patients not enrolled (P = .97).
A total of 175 patients who were negative for PML-RARα at the end of consolidation were randomly assigned to either observation (n = 86) or intensified maintenance chemotherapy (n = 89; Figure 1). Median interval from the recovery of myelosuppression after the third course of consolidation to the randomization was 20 days in both the maintenance and observation groups (P = .35). More than 90% of patients were allocated to either intensified maintenance chemotherapy or observation groups within 2 months after the consolidation. There was no significant difference between the 2 groups in patient profiles, including sex, age, French-American-British morphology, initial leukocyte count, platelet count, and induction therapy (Table 1).
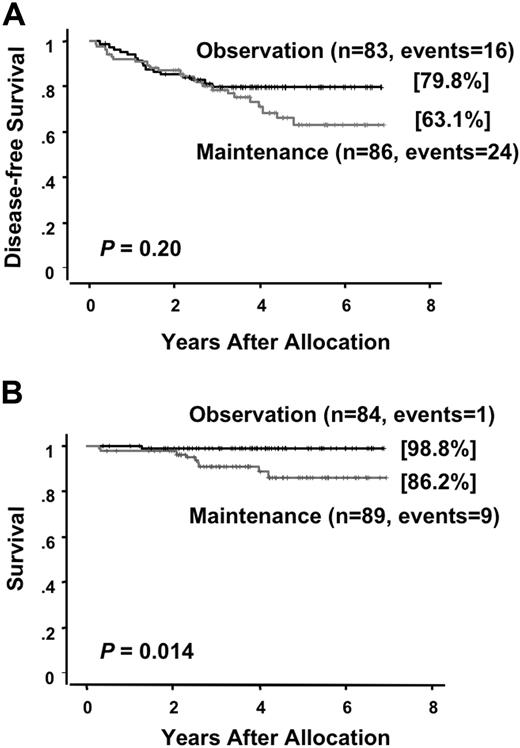
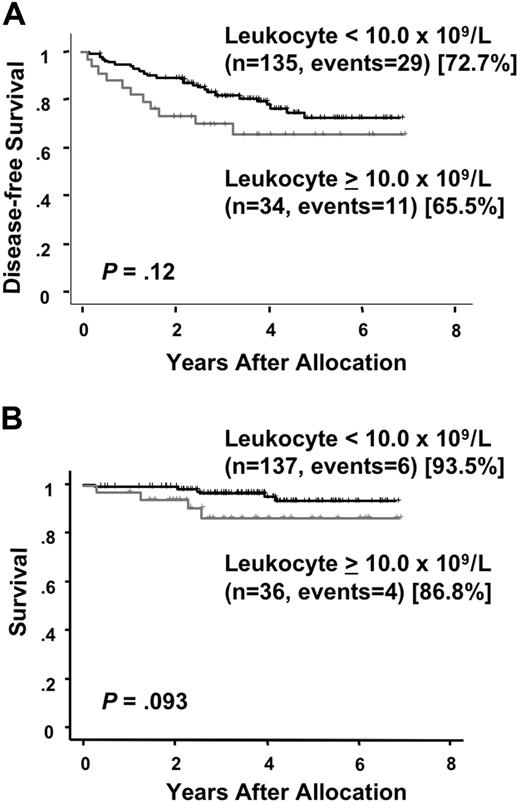
At a median follow-up time of 49 months (range, 24 to 81 months) after randomization, there were 25 (28%) relapses and 13 (15%) deaths among the 89 patients who were allocated to the intensified maintenance chemotherapy. Of the 86 patients who were assigned to the observation, 17 (20%) relapsed and 3 (3%) died. There was no therapy-related mortality during the intensified maintenance chemotherapy. All but 2 patients in the maintenance group died after relapse. In the chemotherapy group, one patient developed therapy-related myelodysplastic syndrome and another developed acute myeloid leukemia during their first CR of APL. By contrast, none of patients in the observation group developed therapy-related leukemia and all 3 patients died after relapse. A second CR was achieved in 13 of 24 (54%) in the chemotherapy group and 13 of 17 (76%) in the observation group (P = .19). The predicted 6-year DFS rates were 63.1% (95% CI, 50.2 to 76.0%) for patients assigned to the maintenance chemotherapy and 79.8% (95% CI, 71.0 to 88.7%) for patients assigned to the observation (Figure 3A). No statistically significant difference in DFS was observed in patients treated with or without the maintenance chemotherapy (P = .20). In the chemotherapy group, 8 patients showed late relapses occurring after at least 3 years of continuous CR, whereas no patients in the observation group showed a late relapse (Figure 3A; P = .006). Univariate analysis showed that an initial leukocyte count of more than 10.0 × 109/L and induction group C trended to be unfavorable prognostic factors for DFS (Table 3). The predicted 6-year survival in the observation group was 98.8% (95% CI, 96.3 to 100%), which was significantly higher than 86.2% (95% CI, 77.3 to 95.0%) in the intensified maintenance group (P = .014; Figure 3B). Univariate analysis revealed that induction group C and maintenance chemotherapy were significant unfavorable prognostic factors for survival (Table 4). Patients with initial leukocyte counts above 10.0 × 109/L showed a trend toward unfavorable DFS and survival, although this cohort was small (Figure 4A,B).
Disease-free survival and survival of randomized patients in the maintenance phase. Disease-free survival (A) and survival (B) are estimated from the date of randomization.
Disease-free survival and survival of randomized patients in the maintenance phase. Disease-free survival (A) and survival (B) are estimated from the date of randomization.
Effects of factors on disease-free survival
| Parameters . | No. of patients . | No. of relapses . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95%CI) . | P* . | |||
| Sex | ||||
| Female | 100 | 16 | 1 | |
| Male | 75 | 26 | 1.4 (0.7-2.7) | .29 |
| Age, years | ||||
| 15-50 | 98 | 24 | 1 | |
| 50-70 | 77 | 18 | 0.9 (0.5-1.7) | .75 |
| Leukocyte count, × 109/L | ||||
| Less than 10.0 | 138 | 29 | 1 | |
| 10.0 or higher | 37 | 13 | 1.7 (0.9-3.5) | .12 |
| Platelet count, × 109/L | ||||
| Less than 40 | 112 | 25 | 1 | |
| 40 or higher | 63 | 17 | 1.4 (0.7-2.6) | .32 |
| Induction therapy | ||||
| Group A | 58 | 10 | 1 | |
| Group AD | 33 | 6 | 1.0 (0.3-2.9) | .97 |
| Group B, BD | 45 | 12 | 1.8 (0.8-4.1) | .17 |
| Group C | 39 | 14 | 2.3 (1.0-5.3) | .05 |
| Maintenance chemotherapy | ||||
| No maintenance | 86 | 17 | 1 | |
| Maintenance | 89 | 25 | 1.5 (0.8-2.8) | .20 |
| Parameters . | No. of patients . | No. of relapses . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95%CI) . | P* . | |||
| Sex | ||||
| Female | 100 | 16 | 1 | |
| Male | 75 | 26 | 1.4 (0.7-2.7) | .29 |
| Age, years | ||||
| 15-50 | 98 | 24 | 1 | |
| 50-70 | 77 | 18 | 0.9 (0.5-1.7) | .75 |
| Leukocyte count, × 109/L | ||||
| Less than 10.0 | 138 | 29 | 1 | |
| 10.0 or higher | 37 | 13 | 1.7 (0.9-3.5) | .12 |
| Platelet count, × 109/L | ||||
| Less than 40 | 112 | 25 | 1 | |
| 40 or higher | 63 | 17 | 1.4 (0.7-2.6) | .32 |
| Induction therapy | ||||
| Group A | 58 | 10 | 1 | |
| Group AD | 33 | 6 | 1.0 (0.3-2.9) | .97 |
| Group B, BD | 45 | 12 | 1.8 (0.8-4.1) | .17 |
| Group C | 39 | 14 | 2.3 (1.0-5.3) | .05 |
| Maintenance chemotherapy | ||||
| No maintenance | 86 | 17 | 1 | |
| Maintenance | 89 | 25 | 1.5 (0.8-2.8) | .20 |
Factors affected on disease-free survival were analyzed by the Cox hazard regression model.
Effects of factors on survival
| Parameters . | No. of patients . | No. of deaths . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95%CI) . | P* . | |||
| Sex | ||||
| Female | 100 | 12 | 1 | |
| Male | 75 | 4 | 2.1 (0.6-8.3) | .27 |
| Age, years | ||||
| 15-50 | 98 | 8 | 1 | |
| 50-70 | 77 | 8 | 2.6 (0.7-10) | .16 |
| Leukocyte count, × 109/L | ||||
| Less than 10.0 | 138 | 11 | 1 | |
| 10.0 or higher | 37 | 5 | 2.8 (0.8-10) | .11 |
| Platelet count, × 109/L | ||||
| Less than 40 | 112 | 12 | 1 | |
| 40 or higher | 63 | 4 | 0.2 (0.03-1.8) | .16 |
| Induction therapy | ||||
| Group A | 58 | 1 | 1 | |
| Group AD | 33 | 4 | 3.8 (0.3-41) | .28 |
| Group B, BD | 45 | 5 | 2.8 (0.3-31) | .4 |
| Group C | 39 | 6 | 8.9 (1.0-76) | .05 |
| Maintenance chemotherapy | ||||
| No maintenance | 86 | 3 | 1 | |
| Maintenance | 89 | 13 | 8.6 (1.1-68) | .04 |
| Parameters . | No. of patients . | No. of deaths . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95%CI) . | P* . | |||
| Sex | ||||
| Female | 100 | 12 | 1 | |
| Male | 75 | 4 | 2.1 (0.6-8.3) | .27 |
| Age, years | ||||
| 15-50 | 98 | 8 | 1 | |
| 50-70 | 77 | 8 | 2.6 (0.7-10) | .16 |
| Leukocyte count, × 109/L | ||||
| Less than 10.0 | 138 | 11 | 1 | |
| 10.0 or higher | 37 | 5 | 2.8 (0.8-10) | .11 |
| Platelet count, × 109/L | ||||
| Less than 40 | 112 | 12 | 1 | |
| 40 or higher | 63 | 4 | 0.2 (0.03-1.8) | .16 |
| Induction therapy | ||||
| Group A | 58 | 1 | 1 | |
| Group AD | 33 | 4 | 3.8 (0.3-41) | .28 |
| Group B, BD | 45 | 5 | 2.8 (0.3-31) | .4 |
| Group C | 39 | 6 | 8.9 (1.0-76) | .05 |
| Maintenance chemotherapy | ||||
| No maintenance | 86 | 3 | 1 | |
| Maintenance | 89 | 13 | 8.6 (1.1-68) | .04 |
Factors affected on survival were analyzed by the Cox hazard regression model.
Disease-free survival and survival by initial leukocyte count. Disease-free survival (A) and survival (B) in patients with initial leukocyte counts above or below 10.0 × 109/L are estimated from the date of randomization.
Disease-free survival and survival by initial leukocyte count. Disease-free survival (A) and survival (B) in patients with initial leukocyte counts above or below 10.0 × 109/L are estimated from the date of randomization.
Discussion
The present randomized study demonstrated that intermittent intensified maintenance chemotherapy did not improve DFS, but rather worsened survival in patients with newly diagnosed APL who had become negative for the PML-RARα fusion transcript at the end of consolidation therapy.
In this study, ATRA and chemotherapy resulted in a high CR rate, improved OS, and DFS in patients with previously untreated APL. In our previous APL92 study, in which ATRA was used for the first time in the JALSG studies to newly diagnosed APL, the combination of ATRA plus chemotherapy induced CR in 333 of 369 (90%) assessable patients.24 The 6-year OS rate of all evaluable patients and the 6-year DFS rate of CR cases in the APL92 study were 65% and 59%, respectively. In both APL92 and APL97 studies, patients received ATRA only in the induction phase. Therefore, the improvement of OS and DFS in the present study can mostly be attributed to the intensification of chemotherapy during induction and consolidation. In the present study, idarubicin and Ara-C were used instead of daunorubicin and BHAC in the induction, and one of the anthracyclines in combination with Ara-C was given in each consolidation.25 Thus, the OS and DFS appear to depend on the intensities of chemotherapy in the treatments of APL. The high sensitivity of APL to anthracyclines is well-documented by several cooperative groups.26,27 In addition, there was a hypothesis that an anthracycline alone may be as effective as combinations of anthracycline and Ara-C.8,27,28 However, the interim analysis of the European APL2000 study showed the efficacy of Ara-C in induction and consolidation even in patients with leukocyte counts of less than 10.0 × 109/L.29 Therefore, despite significant improvement of therapeutic outcome in APL, concern still exists regarding which is the best chemotherapeutic strategy for APL.
PML-RARα generated by t(15;17) provides the most clinically relevant information in patients with APL.2,3,16,17 A number of patients who achieve molecular remission assessed by reverse-transcriptase polymerase chain reaction for PML-RARα after consolidation are predicted to obtain a long-term survival.7,30 However, detection of PML-RARα identifies patients at risk for relapse after consolidation. In addition, treatment of patients at the time of molecular relapse provides a survival advantage compared with treatment at overt hematologic relapse.31 In this study, 5 of 235 (2.1%) patients showed the PML-RARα fusion transcript after the consolidation therapy, and 3 of these relapsed subsequently. In contrast, 97.9% of patients were negative for PML-RARα transcript. In the GIMEMA-AIEOP study, 646 of 664 (97.3%) patients were negative for the PML-RARα fusion transcript at the end of consolidation.32 Because approximately half of APL patients are molecularly positive after induction,7,9 elimination of PML-RARα positive cells might be associated with intensive consolidation chemotherapy.
Our present results showed no benefit of moderately intensive and intermittent chemotherapy in the maintenance phase. This result is consistent with an earlier GIMEMA study before the availability of ATRA, in which patients randomized to maintenance therapy with low-dose 6MP and MTX did not have better outcomes than those randomized to the observation.27 However, the North American Intergroup trial showed a benefit for ATRA in both induction and maintenance therapy.6,12 In addition, the European APL93 study revealed that maintenance therapy with a combination of low-dose chemotherapy (6MP and MTX) and intermittent ATRA reduced the incidence of relapse.10 However, the recent GIMEMA-AIEOP study documented no difference in DFS in patients treated with maintenance consisting of either ATRA, 6MP/MTX, ATRA plus 6MP/MTX, or observation.32 Therefore, the role of maintenance chemotherapy in the treatment of APL remains to be determined. Because intensified maintenance chemotherapy in this study is apparently different from the continuous maintenance with low-dose 6MP and MTX, comparison with other studies of maintenance is difficult. It is very likely that the usefulness of maintenance therapy depends on the intensity of chemotherapy delivered during induction and consolidation phases. In the US Intergroup and European APL93 studies, patients were treated with only 2 cycles of consolidation,6,10 whereas patients received 3 cycles of consolidation both in the GIMEMA-AIEOP and our studies.32 Recently, we did not find a benefit for intensified maintenance therapy in patients with acute myeloid leukemia other than APL treated with intensive consolidation therapy.33 Our present study confirms that there is no beneficial effect of intensified maintenance chemotherapy in previously untreated APL patients who have become negative for the PML-RARα fusion transcript at the end of consolidation. In addition, there was a trend toward better DFS in patients with no maintenance chemotherapy. Patients in the maintenance chemotherapy group showed a significant number of late relapses occurring after at least 3 years of continuous CR compared with the observation group. This was a quite unexpected finding for us. Although the limited number of patients prohibits a robust conclusion, we speculated that intensified maintenance chemotherapy may impair potential immune surveillance to eradicate minimal residual leukemic cells in patients with molecularly undetectable residual leukemia. Further studies are required to investigate whether ATRA has a role in maintenance. The current JALSG APL204 study compares the efficacy of ATRA versus tamibarotene (Am80) in the maintenance phase.
It is interesting to note that patients assigned to the observation group showed a significantly better survival than those randomized to the maintenance group. Because the difference in DFS was not statistically significant and there was no chemotherapy-related death in the latter group, the difference in survival is thought to result from the difference in the second CR rates and CR durations. Although APL cells usually lack p-glycoprotein expression, multidrug resistance is generally acquired by the use of antileukemic agents.34 As the chemotherapy in the maintenance phase of this study mainly consisted of one of the anthracyclines and BHAC, accumulation of chemotherapeutic agents in patients in the maintenance group may induce drug resistance to additional chemotherapy. In addition, accumulated chemotherapy may induce an overall increased toxicity and lack of tolerance to subsequent therapy after relapse. Furthermore, it is of note that 2 patients in the maintenance group died of therapy-related leukemia in the first CR of APL. Occurrence of therapy-related leukemia in patients treated for APL is an emerging problem.35 Chemotherapeutic agents in the maintenance phase seem to increase the risk of therapy-related leukemia.
Although APL has become the most curable subtype of acute leukemia in adults, approximately 20% of patients still die of the disease because of early death or relapse.2,3 One of the unfavorable prognostic factors for DFS and survival, in the present study as well as in our previous and other studies, was high initial leukocyte count.9,21,36 In this study, the stratification by intensities of chemotherapy in the induction phase failed to improve DFS in patients with high initial leukocyte count (group C). Thus, patients with high leukocyte count will require an alternative approach to obtain long-term survival. Use of arsenic trioxide, Am80, and/or gemtuzumab ozogamicin during the front-line therapy may improve DFS and OS in these patients at high risk.37,,,–41
In conclusion, we did not find any beneficial effect of intensified maintenance chemotherapy in patients negative for PML-RARα at the end of consolidation chemotherapy. On the contrary, intensified maintenance chemotherapy unexpectedly conferred a significantly poor survival as well as an increased risk of therapy-related leukemia in these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the participating physicians in the Japan Adult Leukemia Study Group (JALSG) APL97 study for their cooperation.
This work was supported in part by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sport, Science, and Technology, and grants-in-aid for Cancer Research from the Japanese Ministry of Health, Labor, and Welfare.
Authorship
Contribution: N.A., S.O., T.N., and R.O. participated in the study design, analysis of the experiments, and writing of the manuscript. Y.K., H.K., M.O., Y.K., M.T., K.H., M.M., K.S., T.K., M.N., M.T., F.Y., A.T., and Y.K. were significant clinical contributors to the trial and have reviewed the manuscript. M.I. collected the data and performed statistical analysis.
A complete list of the Japan Adult Leukemia Study Group is provided in Document S1 as a data supplement to the online version of this article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Norio Asou, Department of Hematology, Kumamoto University School of Medicine, 1-1-1 Honjo, Kumamoto 860-8556, Japan; e-mail, ktcnasou@gpo.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal