To the editor:
According to the World Health Organization classification, the natural killer (NK)–cell neoplasms consist of 2 separate entities: aggressive NK–cell leukemia (ANKL) and extranodal NK-cell lymphoma, nasal type (ENKL).1 Both of them are aggressive diseases with poor survival.2 We have previously reported constitutively active nuclear factor-κB signaling in ENKL.3 Here, we report that this earlier finding has given us a promising lead for NK-cell neoplasm treatment. Bortezomib, the proteasome inhibitor, targets nuclear factor-κB activation and can be used to treat multiple myeloma and mantle-cell lymphoma.4 We show in this study that bortezomib also has anticancer activity against ANKL and ENKL. Our results from both in vitro cytotoxicity assay and in vivo animal model give substantial support for planning a high priority clinical trial.
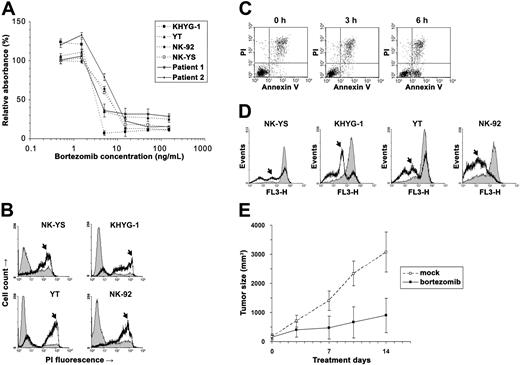
We used the cell viability assay to examine bortezomib's cytotoxicity on NK leukemia (KHYG-1, YT, and NK-92) and NK lymphoma (NK-YS) cell lines and short-term primary cultures from tumor biopsies of 2 patients with ENKL (Figure 1A).5 From the cytotoxicity results, we estimated that the median inhibitory concentrations (IC50) of bortezomib were 2.4 to 5 ng/mL in these neoplastic NK-cell lines and primary ENKL patient samples, approximately the same as in multiple myeloma.6 To further distinguish viable from nonviable cells, we stained the bortezomib-treated malignant NK cells with propidium iodide (PI) and found that they were permeable to PI and thus stainable by PI within 1 day of exposure (Figure 1B). Since the PI positivity is present only during late apoptosis, we speculated that these NK cells died very soon after exposure to the drug.
Bortezomib induced apoptosis in the neoplastic NK cells. (A) The NK lymphoma (NK-YS) and NK leukemia (KHYG-1, YT and NK-92) cell lines and short-term primary cultures from tumor biopsies of 2 patients with ENKL were treated with 0.5 to 150 ng/mL bortezomib for 24 hours. Viable cells were measured in triplicate with the MTS assay (Promega, Madison, WI), and results are presented as relative absorbance equal to the percentage of the average reading in untreated cells (±1 standard deviation). (B) After NK-cell lines were treated with 15 ng/mL bortezomib for 24 hours, the cells were collected and stained immediately with 5 μg/mL PI (Sigma, St Louis, MO) to distinguish viable cells from nonviable cells. The fluorescence was measured using the flow cytometer FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed by the WinMDI v2.8 software (Joseph Trotter, http://facs.scripps.edu/). Histograms of PI fluorescence were overlaid to show the altered distribution between before (thin line) and after (thick line) incubation with bortezomib. Because the dead cells are permeable to PI and thus stainable by PI, they correspond to the population with high fluorescence signals in the chart (arrows). (C) After treatment with 5 ng/mL bortezomib, KHYG-1 cells were stained with Annexin V/PI (BD Biosciences) and examined cytometrically. A population of apoptotic cells appeared in 6 hours with the Annexin V+/PI− phenotype. (D) After treatment with 15 ng/mL bortezomib, neoplastic NK cells were collected at different times and stained with Mitotracker Red (Molecular Probes, Eugene, OR) for Δmψ detection with flow cytometry. Histograms were overlaid to show the altered distribution (arrows) between the time before (thin line) and after 9-hour incubations with bortezomib (thick line). (E) Bortezomib inhibited YT cells in vivo. Four-week-old nude mice (n = 20) were injected subcutaneously in a single flank with 4 × 106 YT cells. Implanted tumors successfully engrafted in 10 mice. The tumor-bearing animals were injected intraperitoneally with bortezomib (n = 5) at 1 mg/kg twice a week or with vehicle only (n = 5). The subcutaneous tumor was measured with a caliper, and tumor size was calculated by the following formula: volume = 0.166 × π × length × width.2 The experiment lasted for 2 weeks until the animals were killed due to big tumor size (< 20 mm) in mock-treated mice. Means (±1 standard deviation) are shown. We compared the data by t test and found the result statistically significant (P = .001).
Bortezomib induced apoptosis in the neoplastic NK cells. (A) The NK lymphoma (NK-YS) and NK leukemia (KHYG-1, YT and NK-92) cell lines and short-term primary cultures from tumor biopsies of 2 patients with ENKL were treated with 0.5 to 150 ng/mL bortezomib for 24 hours. Viable cells were measured in triplicate with the MTS assay (Promega, Madison, WI), and results are presented as relative absorbance equal to the percentage of the average reading in untreated cells (±1 standard deviation). (B) After NK-cell lines were treated with 15 ng/mL bortezomib for 24 hours, the cells were collected and stained immediately with 5 μg/mL PI (Sigma, St Louis, MO) to distinguish viable cells from nonviable cells. The fluorescence was measured using the flow cytometer FACSCalibur (BD Biosciences, San Jose, CA). Data were analyzed by the WinMDI v2.8 software (Joseph Trotter, http://facs.scripps.edu/). Histograms of PI fluorescence were overlaid to show the altered distribution between before (thin line) and after (thick line) incubation with bortezomib. Because the dead cells are permeable to PI and thus stainable by PI, they correspond to the population with high fluorescence signals in the chart (arrows). (C) After treatment with 5 ng/mL bortezomib, KHYG-1 cells were stained with Annexin V/PI (BD Biosciences) and examined cytometrically. A population of apoptotic cells appeared in 6 hours with the Annexin V+/PI− phenotype. (D) After treatment with 15 ng/mL bortezomib, neoplastic NK cells were collected at different times and stained with Mitotracker Red (Molecular Probes, Eugene, OR) for Δmψ detection with flow cytometry. Histograms were overlaid to show the altered distribution (arrows) between the time before (thin line) and after 9-hour incubations with bortezomib (thick line). (E) Bortezomib inhibited YT cells in vivo. Four-week-old nude mice (n = 20) were injected subcutaneously in a single flank with 4 × 106 YT cells. Implanted tumors successfully engrafted in 10 mice. The tumor-bearing animals were injected intraperitoneally with bortezomib (n = 5) at 1 mg/kg twice a week or with vehicle only (n = 5). The subcutaneous tumor was measured with a caliper, and tumor size was calculated by the following formula: volume = 0.166 × π × length × width.2 The experiment lasted for 2 weeks until the animals were killed due to big tumor size (< 20 mm) in mock-treated mice. Means (±1 standard deviation) are shown. We compared the data by t test and found the result statistically significant (P = .001).
We next identified the starting point of apoptosis in these neoplastic NK cells. KHYG-1 cells were collected every 3 hours after exposure to bortezomib and stained with Annexin V and PI. A population of apoptotic cells with the Annexin V+/PI− phenotype was present in the KHYG-1 cells within 6 hours (Figure 1C). As the mitochondrial pathway is reported to be critical for bortezomib-induced apoptosis,7 we measured mitochondrial membrane potential (Δmψ) in all of the NK-cell lines. Each of them showed a mild decrease of Δmψ at 6 hours, becoming more evident at 9 hours (Figure 1D). It appeared that apoptosis began within 6 hours of exposure to bortezomib.
These in vitro assays show that bortezomib is effective in killing NK-cell neoplasms, but would the same be found in vivo in an animal model? We implanted YT cells into nude mice and injected the mice intraperitoneally with 1 mg/kg bortezomib, a safe and moderate dose used in the previous mouse studies of this drug.8,–10 We monitored the animals by recording their tumor growth and health conditions. Overall, the animals tolerated the drug toxicity well, and at this safe dose, bortezomib dramatically inhibited tumor growth (P = .001; Figure 1E).
In this study, we have shown bortezomib to be active against the NK-cell neoplasms ANKL and ENKL. With consistent apoptosis induction in 4 NK-cell lines and activity against 2 primary ENKL patient samples, bortezomib should be assessed for its activity against ANKL and ENKL in clinical trials of these diseases.
Authorship
Correspondence: Gopesh Srivastava, Department of Pathology, The University of Hong Kong, Queen Mary Hospital, Hong Kong; e-mail: gopesh@pathology.hku.hk; or Raymond H. Liang, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong; e-mail: rliang@hkucc.hku.hk.
This work was supported by a grant from the Research Grants Council of Hong Kong Special Administrative Region, P. R. China (HKU 7627/06M, G.S. and R.H.L.). We thank Millennium Pharmaceuticals (Cambridge, MA) for providing bortezomib for this study (supplier agreement no. 211837).
This study was approved by the Institutional Review Board of the University of Hong Kong (IRB Reference Number: UW 05–070 T/733), and the Committee on the Use of Live Animals in Teaching and Research, The Faculty of Medicine, The University of Hong Kong (CULATR 1098–05).
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal