Mutations in the receptor tyrosine kinase Flt3 represent a very common genetic lesion in acute myeloid leukemia (AML). Internal tandem duplication (ITD) mutations clustered in the juxtamembrane domain are the most frequent and best characterized mutations found in Flt3. Oncogenic activation of Flt3 by ITD mutations is known to activate aberrant signaling including activation of STAT5 and repression of myeloid transcription factors Pu.1 and c/EBP-alpha. However, the mechanisms of STAT5 activation by Flt3-ITD remain unclear. Using small molecule inhibitors and cell lines deficient for Src family kinases or Jak2 or Tyk2, here we show that Flt3-ITD–induced STAT5 activation is independent of Src or Jak kinases. Also, overexpression of SOCS1, an inhibitor of Jak kinases, inhibited IL-3– but not Flt3-ITD–mediated STAT5 activation. Furthermore, in vitro kinase assays revealed that STAT5 is a direct target of Flt3. Taken together, our data provide the mechanistic basis of STAT5 activation by Flt3-ITD.
Introduction
Previously, we and others have reported aberrant activation of STAT5 by Flt3-ITD mutations.1,,,,,–7 Activation of STAT5 is increasingly recognized as important for self-renewal of hematopoietic stem cells.5,8 Constitutive activation of STAT5 has also been observed in human leukemias.9 In animal models, activation of STAT5 has been shown to be essential for induction of leukemias by Tel-Jak2 and recently by Bcr-Abl and Flt3-ITD.10,11 Therefore, elucidation of mechanisms of STAT5 activation by Flt3-ITD may help to selectively target oncogenic signals of Flt3-ITD
Materials and methods
Cell lines
SYF (Src, Yes, and Fyn) cells are derived from Src, Yes, and Fyn knockout mice and express no Src family kinases.12 γ2A and U1A cell lines are deficient for Jak2 and Tyk2 kinases, respectively.13 All cell lines, except 32D, were cultured in DMEM medium supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin. The 32D cells were cultured and maintained as described earlier.1,3,4
Preparation of retroviruses, generation of cell lines, and sources of antibodies and reagents have been described in the supplementary materials and methods, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Purification of recombinant proteins and in vitro kinase assays
His-tagged murine STAT5 and GST fusion proteins with Flt3 or PDGFRB kinase were expressed in Sf9 cells and purified by standard procedures. In vitro kinase assays were performed using about 0.5 μg STAT5 and 0.5 μg GST-Flt3 or GST-PDGFRB in 20 mM Hepes, 5 mM MnCl2, 0.2 mM Na-vanadate, in the absence or presence of 1 mM ATP at 30°C for 20 minutes.
Results and discussion
Flt3-ITD–induced STAT5 activation is independent of Src or Jak kinases
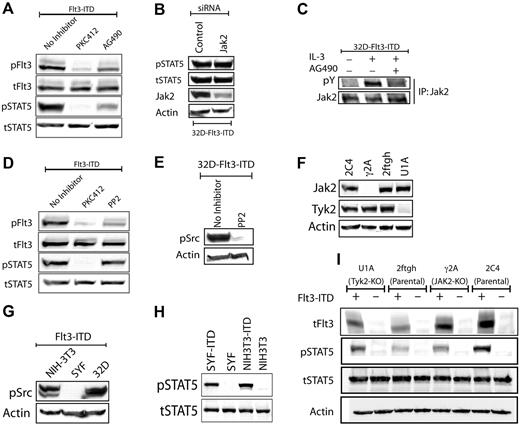
STAT5 is generally activated by one of the 3 mechanisms: (1) by Jak kinases, (2) by Src family kinases (SFK), or (3) directly by RTKs such as the EGFR, PDGFRB, or insulin receptors.14,15 Although phosphorylation of Jak2 or Tyk2 by Flt3-ITD has been shown in previous studies, the role of Jak kinases in Flt3-ITD signaling remains controversial and inconclusive.16,17 To systematically explore the mechanisms of STAT5 activation by Flt3-ITD, we first tested chemical inhibitors to inhibit SFKs or JAKs.18,19 Treatment of 32D-Flt3-ITD with AG490 resulted in a decrease of STAT5 phosphorylation, but also in proportional inhibition of Flt3 autophosphorylation, making the interpretation of this result difficult (Figure 1A). Therefore, we analyzed the effects of Jak2 knockdown using siRNA, which did not have any effect on Flt3-ITD–mediated STAT5 activation (Figure 1B). Failure of Flt3-ITD to induce phosphorylation of any of the 4 Jak kinases (Figure 1C and data not shown) also indicated minor, if any, involvement of JAKs in Flt3-ITD–induced STAT5 activation. The SFK inhibitors PP-2 and PP-1 had marginal effect on STAT5 phosphorylation by Flt3-ITD (Figure 1D and data not shown). Also, they inhibited Flt3 autophosphorylation, indicating off-target effects of the compounds (Figure 1D and data not shown). It has been published earlier that 32D cells express at least 7 different SFKs (Src, Yes, Fyn, Lyn, Hck, Lck, Fgr).20,21 However, PP2 effectively abolished all SFK activity, as indicated by Western blot analyses with an antibody that recognizes the activation state–specific consensus phosphorylation site in all these proteins (Figure 1E). Despite complete inhibition of SFK or Jak2 (Figure 1C), both inhibitors failed to interfere with Flt3-ITD–induced STAT5 activation. Taken together, the SFK and Jak2 inhibitors effectively inhibited their respective targets with only minor effects on Flt3-ITD–induced STAT5 activation that could largely be attributed to their off-target effects.
Flt3-ITD–induced STAT5 activation is independent of Jak or Src kinases. (A) Flt3-ITD phosphorylates STAT5 in AG490-resistant manner. The 32D Flt3-ITD cells were starved for 6 hours in the presence or absence of PKC412 (100 nM) or AG490 (50 μM). Cell lysates were immunoblotted against activation-specific phospho-Flt3 (Y591) or STAT5 (Y694) antibodies. Equal loading of proteins was confirmed by reprobing with antibodies recognizing total proteins. (B) siRNA-mediated down-regulation of Jak2 does not influence Flt3-ITD–mediated STAT5 phosphorylation. 32D Flt3-ITD cells were electroporated with a mixture of 4 siRNAs directed against Jak2, using the Amaxa nucleofection technology (Cologne, Germany). The cells were grown in the absence of IL-3 for the next 30 hours and cell lysates were analyzed for STAT5 activation as described in panel A. Effective down-regulation of Jak2 was verified by staining the membranes with anti-Jak2 antibody. (C) Jak2 phosphorylation is induced by IL-3 but not by Flt3-ITD and can be inhibited by AG490. The 32D cells expressing Flt3-ITD were starved in the absence or presence of AG490 (50 μM) for 6 hours. Subsequently, cells were left unstimulated or were stimulated with IL-3 for 10 minutes at 37°C. Cell lysates were prepared and Jak2 was immunoprecipitated using Jak2 antibodies. Phosphorylation of Jak2 was analyzed using a phosphotyrosine-specific antibody (pY100). The membrane was reprobed with an antibody that recognizes total Jak2. (D) Flt3-ITD–mediated STAT5 phosphorylation is not inhibited by PP2. The 32D Flt3-ITD cells were starved overnight in the presence or absence of PKC412 (100 nM) or PP2 (10 μM). Signaling analyses were performed as described in panel A. (E) PP2 inhibits phosphorylation of Src family kinases. Flt3-ITD–expressing 32D cells were starved overnight in the presence or absence of PP2 (10 μM). Activation of Src kinases was analyzed using a pan-phospho-Src family antibody that recognizes Src family kinases (including the hematopoietic cell type–specific Lyn and Hck kinases) when phosphorylated on the activation loop tyrosine residue analogous to tyrosine 416 (Y416) of c-Src. (F) Src family kinases (SFKs) are dispensable for Flt3-ITD–mediated STAT5 activation. SFK-deficient SYF or control NIH3T3 stably expressing Flt3-ITD were starved and analyzed for signaling activation as described in panel A. (G) No phosphorylation of SFKs is observed in SYF cells. SYF, 32D, or NIH-3T3 cells expressing Flt3-ITD were analyzed for the activation of SFKs as described in panel E. (H-I) Jak2 or Tyk2 is not involved in Flt3-ITD–mediated STAT5 activation. Jak2 (γ2A)– or Tyk2 (U1A)–deficient and control cells (2C4 and 2ftgh) stably expressing Flt3-ITD were serum starved and lysates were analyzed for activation of STAT5 as described in panel A. Deficiency for Jak2 or Tyk2 in γ2A or U1A cells, respectively, was confirmed by probing total cell lysates with Jak2 or Tyk2 antibodies.
Flt3-ITD–induced STAT5 activation is independent of Jak or Src kinases. (A) Flt3-ITD phosphorylates STAT5 in AG490-resistant manner. The 32D Flt3-ITD cells were starved for 6 hours in the presence or absence of PKC412 (100 nM) or AG490 (50 μM). Cell lysates were immunoblotted against activation-specific phospho-Flt3 (Y591) or STAT5 (Y694) antibodies. Equal loading of proteins was confirmed by reprobing with antibodies recognizing total proteins. (B) siRNA-mediated down-regulation of Jak2 does not influence Flt3-ITD–mediated STAT5 phosphorylation. 32D Flt3-ITD cells were electroporated with a mixture of 4 siRNAs directed against Jak2, using the Amaxa nucleofection technology (Cologne, Germany). The cells were grown in the absence of IL-3 for the next 30 hours and cell lysates were analyzed for STAT5 activation as described in panel A. Effective down-regulation of Jak2 was verified by staining the membranes with anti-Jak2 antibody. (C) Jak2 phosphorylation is induced by IL-3 but not by Flt3-ITD and can be inhibited by AG490. The 32D cells expressing Flt3-ITD were starved in the absence or presence of AG490 (50 μM) for 6 hours. Subsequently, cells were left unstimulated or were stimulated with IL-3 for 10 minutes at 37°C. Cell lysates were prepared and Jak2 was immunoprecipitated using Jak2 antibodies. Phosphorylation of Jak2 was analyzed using a phosphotyrosine-specific antibody (pY100). The membrane was reprobed with an antibody that recognizes total Jak2. (D) Flt3-ITD–mediated STAT5 phosphorylation is not inhibited by PP2. The 32D Flt3-ITD cells were starved overnight in the presence or absence of PKC412 (100 nM) or PP2 (10 μM). Signaling analyses were performed as described in panel A. (E) PP2 inhibits phosphorylation of Src family kinases. Flt3-ITD–expressing 32D cells were starved overnight in the presence or absence of PP2 (10 μM). Activation of Src kinases was analyzed using a pan-phospho-Src family antibody that recognizes Src family kinases (including the hematopoietic cell type–specific Lyn and Hck kinases) when phosphorylated on the activation loop tyrosine residue analogous to tyrosine 416 (Y416) of c-Src. (F) Src family kinases (SFKs) are dispensable for Flt3-ITD–mediated STAT5 activation. SFK-deficient SYF or control NIH3T3 stably expressing Flt3-ITD were starved and analyzed for signaling activation as described in panel A. (G) No phosphorylation of SFKs is observed in SYF cells. SYF, 32D, or NIH-3T3 cells expressing Flt3-ITD were analyzed for the activation of SFKs as described in panel E. (H-I) Jak2 or Tyk2 is not involved in Flt3-ITD–mediated STAT5 activation. Jak2 (γ2A)– or Tyk2 (U1A)–deficient and control cells (2C4 and 2ftgh) stably expressing Flt3-ITD were serum starved and lysates were analyzed for activation of STAT5 as described in panel A. Deficiency for Jak2 or Tyk2 in γ2A or U1A cells, respectively, was confirmed by probing total cell lysates with Jak2 or Tyk2 antibodies.
Given the cross-inhibitory nature of the inhibitors, we used cell lines deficient for SFK,12 Jak2 or Tyk2,13 the 2 Jak kinases commonly involved in STAT5 activation. Expression of Flt3-ITD in SFK-deficient SYF or control NIH3T3 fibroblasts led to activation of STAT5 at similar levels (Figure 1F and G), indicating SFK-independent activation of STAT5 by Flt3-ITD. Since fibroblasts do not express hematopoietic cell–specific SFKs, including Hck, Lyn, or Fgr, these cells lack all SFK family members. Western blot analysis with a pan-phospho-SFK antibody verified that SYF cells were indeed lacking activation of SFKs (Figure 1G). Next, to assess the role of Jak2 or Tyk2, we transfected Flt3-ITD into cell lines deficient for Jak2 (γ2A) or Tyk2 (U1A) (Figure 1H), and compared STAT5 activation levels with their respective parental cell lines 2C4 and 2ftgh. Interestingly, activation of STAT5 by Flt3-ITD is dependent on neither Jak2 nor Tyk2 kinases (Figure 1I).
Effect of SOCS1 on Flt3-ITD–mediated growth of 32D cells
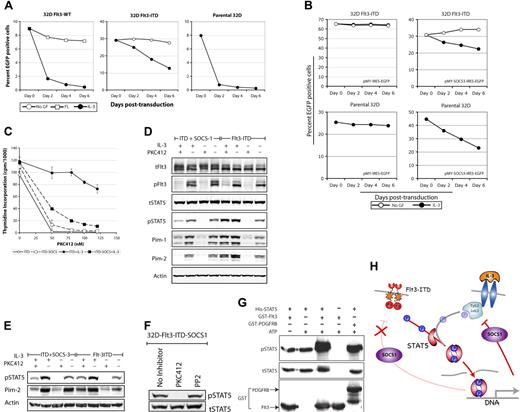
Since redundant functions of different Jak family members may account for our results in cells deficient for single Jaks, we analyzed the effects of SOCS1, a potent molecular inhibitor of Jak family members, on STAT5 activation by Flt3-ITD.22,23 SOCS1 is a transcriptional target and negative regulator of STAT5 activation.22,23 Several SOCS family members including SOCS1 and SOCS3 were found to be up-regulated in Flt3-ITD–expressing cell lines (C.C., H.S. unpublished observation, February, 2006; and Mizuki et al4 ). To define the role of Jaks in Flt3-ITD signaling, 32D, 32D-Flt3-ITD, or 32D-Flt3-WT cells were transduced with a retroviral construct encoding SOCS1 and EGFP.24 Surprisingly, overexpression of SOCS1 had no effect on growth of Flt3-ITD cells, as the proportion of SOCS1-transduced EGFP-positive cells remained constant over time (Figure 2A). Also, SOCS1 was found to have marginal effect on FL-stimulated growth of Flt3-WT cells (Figure 2A). In contrast, overexpression of SOCS1 severely inhibited IL-3–mediated growth and resulted in drastic reduction of SOCS1-positive cells, suggesting selective inhibitory effects of SOCS1 on IL-3– but not on Flt3–mediated growth (Figure 2A). Similar to earlier reports using BAF/3 cells,24 SOCS1-transduced 32D cells underwent rapid apoptosis in the presence of IL-3 (data not shown). Furthermore, addition of IL-3 to Flt3-ITD-SOCS1 cells also resulted in a slower decrease of the proportion of SOCS1-positive cells, indicating that expression of Flt3-ITD could indeed rescue 32D cells from SOCS1-induced inhibition of IL-3–mediated growth.
Flt3-ITD directly phosphorylates STAT5 in a SOCS1- and SOCS3-resistant manner. (A-B) Flt3-ITD cells are resistant to SOCS1- or SOCS3-induced growth inhibition. Parental 32D or 32D cells expressing Flt3-WT or Flt3-ITD were transduced with retroviruses encoding SOCS1 or SOCS3 and EGFP or vector control (pMY-IRES-EGFP). Thirty hours after transduction, cells were washed and cultured with or without the indicated cytokines for up to one week. The proportion of EGFP-positive cells was analyzed by flow cytometry. (C) 32D Flt3-ITD or Flt3-ITD-SOCS1 cells were starved for 12 hours. Subsequently, cells were exposed to IL-3 or left unstimulated in the presence or absence of the indicated PKC412 concentrations. Each data point represents the mean of 3H thymidine incorporation of 3 samples ± standard deviation. (D-E) SOCS1 or SOCS3 does not inhibit Flt3-ITD–mediated STAT5 activation. The 32D Flt3-ITD, Flt3-ITD-SOCS1, or Flt3-ITD-SOCS3 cells were starved overnight in the presence or absence of IL-3, supplemented with or without PKC412. On the next day, cell lysates were prepared and signaling analyses were performed as mentioned in the legend to Figure 1A. Activation of STAT5 was further confirmed in Flt3-ITD-SOCS1–expressing cells by analyzing the expression of the STAT5 target genes Pim-1 and Pim-2. (F) SFKs are dispensable for Flt3-ITD–mediated STAT5 phosphorylation in the absence of Jak activity. The 32D-Flt3-ITD-SOCS1 cells (with Jak kinase inhibition by SOCS1) were treated with PP2 to inhibit SFKs. Cell lysates were analyzed for STAT5 phosphorylation as described in the legend to Figure 1A. (G) Flt3 directly phosphorylates STAT5. In vitro kinase assays were performed using purified recombinant STAT5 alone, or together with GST-Flt3 or GST-PDGFRB kinase. The phosphorylation of STAT5 was measured with a phospho-STAT5 antibody and loading of STAT5 and Flt3 or PDGFRB was confirmed by reprobing the membrane with a STAT5 or GST antibody. (H) SOCS1 inhibits IL-3 but not Flt3-ITD–mediated STAT5 activation. Activation of STAT5 by Flt3-ITD or IL-3 leads to induction of STAT5 target genes, including SOCS proteins. Up-regulation of SOCS proteins results in inhibition of Jak kinases of STAT5 activation by IL-3. In contrast, Jak-independent STAT5 activation by Flt3-ITD is not inhibited by SOCS proteins such as SOCS1 and SOCS3.
Flt3-ITD directly phosphorylates STAT5 in a SOCS1- and SOCS3-resistant manner. (A-B) Flt3-ITD cells are resistant to SOCS1- or SOCS3-induced growth inhibition. Parental 32D or 32D cells expressing Flt3-WT or Flt3-ITD were transduced with retroviruses encoding SOCS1 or SOCS3 and EGFP or vector control (pMY-IRES-EGFP). Thirty hours after transduction, cells were washed and cultured with or without the indicated cytokines for up to one week. The proportion of EGFP-positive cells was analyzed by flow cytometry. (C) 32D Flt3-ITD or Flt3-ITD-SOCS1 cells were starved for 12 hours. Subsequently, cells were exposed to IL-3 or left unstimulated in the presence or absence of the indicated PKC412 concentrations. Each data point represents the mean of 3H thymidine incorporation of 3 samples ± standard deviation. (D-E) SOCS1 or SOCS3 does not inhibit Flt3-ITD–mediated STAT5 activation. The 32D Flt3-ITD, Flt3-ITD-SOCS1, or Flt3-ITD-SOCS3 cells were starved overnight in the presence or absence of IL-3, supplemented with or without PKC412. On the next day, cell lysates were prepared and signaling analyses were performed as mentioned in the legend to Figure 1A. Activation of STAT5 was further confirmed in Flt3-ITD-SOCS1–expressing cells by analyzing the expression of the STAT5 target genes Pim-1 and Pim-2. (F) SFKs are dispensable for Flt3-ITD–mediated STAT5 phosphorylation in the absence of Jak activity. The 32D-Flt3-ITD-SOCS1 cells (with Jak kinase inhibition by SOCS1) were treated with PP2 to inhibit SFKs. Cell lysates were analyzed for STAT5 phosphorylation as described in the legend to Figure 1A. (G) Flt3 directly phosphorylates STAT5. In vitro kinase assays were performed using purified recombinant STAT5 alone, or together with GST-Flt3 or GST-PDGFRB kinase. The phosphorylation of STAT5 was measured with a phospho-STAT5 antibody and loading of STAT5 and Flt3 or PDGFRB was confirmed by reprobing the membrane with a STAT5 or GST antibody. (H) SOCS1 inhibits IL-3 but not Flt3-ITD–mediated STAT5 activation. Activation of STAT5 by Flt3-ITD or IL-3 leads to induction of STAT5 target genes, including SOCS proteins. Up-regulation of SOCS proteins results in inhibition of Jak kinases of STAT5 activation by IL-3. In contrast, Jak-independent STAT5 activation by Flt3-ITD is not inhibited by SOCS proteins such as SOCS1 and SOCS3.
To further characterize the effect of SOCS1 on Flt3-ITD–mediated proliferation, we generated stable SOCS1-overexpressing Flt3-ITD bulk cultures. These cells were able to grow factor independently, similar to Flt3-ITD cells without SOCS1 (data not shown). Furthermore, in thymidine incorporation assays, both Flt3-ITD and Flt3-ITD-SOCS1 cell lines proliferated factor independently but required Flt3 kinase activity, as the Flt3-specific inhibitor PKC412 inhibited the proliferation of both cell lines (Figure 2C). Interestingly, addition of IL-3 could rescue the effects of PKC412 on Flt3-ITD but not on Flt3-ITD-SOCS1 cells, again confirming Jak-independent proliferation of 32D-Flt3-ITD. Similar to SOCS1, the overexpression of another Jak family inhibitor, SOCS3, inhibited IL-3– but not Flt3-ITD–mediated growth (Figure 2B).
Effect of SOCS1 and SOCS3 on Flt3-ITD–mediated STAT5 activation
To further characterize the effects of SOCS1 and SOCS3 on Flt3-ITD–induced STAT5 activation, a comprehensive signaling analysis was performed on Flt3-ITD versus Flt3-ITD-SOCS1 or Flt3-ITD-SOCS3 cell lines. Flt3-ITD, Flt3-ITD-SOCS1, and Flt3-ITD-SOCS3 activated STAT5 comparatively, which could be inhibited by PKC412 (Figure 2D-E). Addition of IL-3 resulted in a slight increase of STAT5 phosphorylation and induction of the STAT5 target genes Pim-1 and Pim-2 in Flt3-ITD but not in Flt3-ITD-SOCS1 cells. Addition of IL-3 also rescued the inhibitory effects of PKC412 on STAT5 activation in Flt3-ITD but not in Flt3-ITD-SOCS1 cells. These results suggest different mechanisms of STAT5 activation by Flt3-ITD and IL-3, and demonstrate Jak-independent activation of STAT5 by Flt3-ITD. Interestingly, simultaneous inhibition of SFK and Jak kinases also had no effect on Flt3-ITD–mediated STAT5 phosphorylation, suggesting that SFK and Jaks are dispensable for Flt3-mediated STAT5 activation (Figure 2F).
Our finding that Flt3-ITD strongly up-regulates endogenous negative regulators of STAT5 such as SOCS proteins and activates STAT5 in a SOCS-resistant manner is reminiscent to earlier findings on SOCS1-resistant transformation by v-Abl.25 Thus, similar to v-Abl, up-regulation of SOCS proteins by Flt3-ITD may skew cytokine signaling in acute myeloid leukemia (AML) blasts.
STAT5 is a direct target of Flt3 in vitro
After confirming SFK- and Jak-independent activation of STAT5 by Flt3-ITD, we asked whether STAT5 is a direct target of Flt3. Therefore, we analyzed whether recombinant Flt3 could phosphorylate recombinant STAT5 in vitro, using a phospho-specific STAT5 (Y694) antibody to detect STAT5 phosphorylation at a functionally relevant site. As shown in Figure 2G, Flt3 kinase directly phosphorylated STAT5 at similar levels as the PDGFRB, which we used as a positive control, since it is known to directly phosphorylate STAT5.14
Taken together, our data provide the mechanistic basis of STAT5 activation by oncogenic Flt3-ITD mutations and show that Flt3-ITD directly activates STAT5 in a SFK- and Jak-independent manner.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Se 600/3–1, SFB293), Deutsche Krebshilfe (10–2258, 10–1539, 10–6697, 10–2-100-Do2), the Fritz Thyssen Stiftung (Az.10.05.2178), the Deutsche Jose-Carreras-Leukämie-Stiftung (DJCLS R05/13, DJCLS R03/19F), and the Medical Faculty of the University of Münster (IZKF Ser2/038/06, IMF Sa 110404).
We are grateful to George Stark and P. Soriano for providing Jak2-, Tyk2-, or SYF-deficient cells and to Toshio Kitamura, Justus Duyster, Edith Pfitzner, and Lars-Arne Haldosen for providing plasmid constructs used in this study.
Authorship
Contribution: H.S. and C.C. conceptualized the original idea and designed experiments; A.U. and F.-D.B. purified the recombinant proteins and performed in vitro kinase assays; C.C. carried out all the other experiments; C.C., H.S., C.B., J.S., L.T., B.S., W.E.B., and C.-M.T. were involved in discussions, data analysis, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hubert Serve, Department of Medicine, Hematology and Oncology, University Hospital Münster, Albert-Schweitzer-Strasse 33, 48129 Münster, Germany; e-mail: serve@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal