The V617F JAK2 mutation reported in Ph-negative myeloproliferative diseases (MPDs) induces the constitutive activation of JAK2, which produces an increased phosphorylation of signal transducer activator of transcription (STAT). In this study, we have analyzed a series of 114 patients (54 with polycythemia vera [PV], 44 with essential thrombocythemia [ET], 12 with idiopathic myelofibrosis [IM], and 4 with myelofibrosis secondary to MPD) for the expression pattern of phosphorylated STAT-3 and STAT-5 (pSTAT-3 and pSTAT-5, respectively) by immunostaining bone marrow biopsies. We found 3 specific patterns of pSTAT-3 and pSTAT-5 expression, significantly different from the normal staining pattern: uniformly increased pSTAT-3 and pSTAT-5 expression in PV, increased pSTAT-3 and reduced pSTAT-5 expression in ET, and uniformly reduced pSTAT-3 and pSTAT-5 expression in IM. A moderate increase of pSTAT-3 and pSTAT-5 expression was observed in secondary forms of erythrocytosis and thrombocytosis. In all evaluated MPDs, the pSTAT-5 and pSTAT-3 expression pattern was not influenced by the presence of V617F JAK2 mutation. These findings underline the importance of bone marrow histology in the differential diagnosis of Ph-negative MPD and support the hypothesis that V617F mutation simply contributes with other molecular defects in allowing the PV, ET, or IM phenotype to emerge.
Introduction
The somatic point mutation in the pseudokinase domain of JAK2 tyrosine kinase has been recently found in most patients with polycythemia vera (PV) and in about half of cases of essential thrombocythemia (ET) and idiopathic myelofibrosis (IM).1,,,–5 The JAK2 is a cytoplasmic protein kinase associated with the receptors of many cytokines and growth factors, including thrombopoietin (Tpo) and erythropoietin (Epo) receptors. Engagement of the cytokine receptor with ligand results in the phosphorylation of both receptor and associated JAK, which, in turn, recruits and phosphorylates substrate molecules, including the signal transducers and activators of transcription (STATs). Gene-disruption studies in mice have provided growing knowledge concerning the different biologic effects, specific for the various STAT family members. Thus, the lack of erythropoietin-induced response in STAT-5 knock-out mice results in severe anemia, showing a clear role for STAT-5 in erythroid cell proliferation and differentiation.6 On the contrary, STAT-3 appears to have more complex functions. In fact, the ablation of the STAT-3 gene results in early embryonic lethality,7 while conditional STAT-3 deletion in specific tissues produces complex and seemingly contradictory effects in cellular proliferation, survival, apoptosis, and differentiation.8
The V617F mutation disrupts the inhibitory function of the pseudokinase domain, causes the constitutive activation of the kinase domain of JAK2,1 and induces the increased phosphorylation of STAT-5.1,4 Indeed, the presence of the V617F mutation can now explain the constitutive activation of STAT-3 and STAT-5 previously reported in PV patients.9,10
Considering that the detection of the V617F JAK2 mutation is likely to have a major impact on the way patients with myeloproliferative diseases (MPDs) are diagnosed, we have investigated if its presence could be revealed by the immunohistologic detection of phosphorylated STATs. Accordingly, we have examined phosphorylated STAT3 and STAT5 (pSTAT-3 and pSTAT-5) in bone marrow (BM) biopsies of patients with Ph-negative MPDs. Results obtained were then correlated to the presence of several MPD biologic markers, including V617F JAK2 mutation, endogenous erythroid colony (EEC) growth,11,12 PRV-1 RNA overexpression,13,14 and level of c-mpl expression.15,–17
Patients, materials, and methods
Patient samples were obtained following informed consent in accordance with the Declaration of Helsinki and with approval from the institutional review board of the Catholic University of Rome, Italy.
Patients
The study was carried out on BM biopsies obtained from 114 patients recorded at the Catholic University (Hematology Departments of Rome and of Campobasso) between January 2002 and June 2006. In 75 cases, the study was carried out retrospectively, while in 39 biopsies pSTAT-3 and pSTAT-5 expression was assessed prospectively, during the diagnostic procedure. The patient population included in the study consisted of 54 PV, 44 ET, 12 IM, and 4 myelofibrosis secondary to PV and ET patients. All patients with MPD were diagnosed according to the PVSG18 or WHO criteria.19 Thirty-nine patients with PV, 29 patients with ET, and 11 patients with IM were studied at the time of diagnosis. Clinical and hematologic features of patients are shown in Table 1. As a control group, we studied 22 patients undergoing bone marrow biopsy for non-Hodgkin lymphoma staging, 6 patients with secondary thrombocytosis (ST; 4 patients with infectious diseases and 2 patients who underwent splenectomy), and 6 patients with secondary erythrocytosis (SE) due to pulmonary diseases.
Clinical and laboratory findings of patients with polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis
| . | Polycythemia vera, n = 54 . | Essential thrombocythemia, n = 44 . | Idiopathic myelofibrosis, n = 12 . |
|---|---|---|---|
| Males/females | 37/17 | 14/30 | 8/4 |
| Age, y, mean | 54 | 55 | 66 |
| Median, range | 54 (24-83) | 56 (20-84) | 65 (57-79) |
| WBC, × 109/L, mean | 11.00 | 9.59 | 7.4 |
| Median, range | 9.8 (5.40-31.92) | 10.06 (4.35-15.12) | 6.61 (3.94-13.8) |
| Hb, g/dL, mean | 16.7 | 13.8 | 9 |
| Median, range | 17 (12.8-21.4) | 13.9 (11-16.4) | 7.8 (6.8-13.4) |
| Hct, %, mean | 52.3 | 43.1 | 27.4 |
| Median, range | 51.2 (38.9-66.1) | 42.7 (33.3-50.3) | 25.6 (19.4-40.2) |
| PLT, × 109/L, mean | 425 | 838 | 445 |
| Median, range | 377 (166-901) | 885 (403-1.484) | 456 (50-1.284) |
| Thrombosis, % | 17 (31) | 11 (25) | 3 (25) |
| . | Polycythemia vera, n = 54 . | Essential thrombocythemia, n = 44 . | Idiopathic myelofibrosis, n = 12 . |
|---|---|---|---|
| Males/females | 37/17 | 14/30 | 8/4 |
| Age, y, mean | 54 | 55 | 66 |
| Median, range | 54 (24-83) | 56 (20-84) | 65 (57-79) |
| WBC, × 109/L, mean | 11.00 | 9.59 | 7.4 |
| Median, range | 9.8 (5.40-31.92) | 10.06 (4.35-15.12) | 6.61 (3.94-13.8) |
| Hb, g/dL, mean | 16.7 | 13.8 | 9 |
| Median, range | 17 (12.8-21.4) | 13.9 (11-16.4) | 7.8 (6.8-13.4) |
| Hct, %, mean | 52.3 | 43.1 | 27.4 |
| Median, range | 51.2 (38.9-66.1) | 42.7 (33.3-50.3) | 25.6 (19.4-40.2) |
| PLT, × 109/L, mean | 425 | 838 | 445 |
| Median, range | 377 (166-901) | 885 (403-1.484) | 456 (50-1.284) |
| Thrombosis, % | 17 (31) | 11 (25) | 3 (25) |
Materials and methods
Cell separation, EEC growth, PRV-1 assay, and JAK-2 mutation analysis
All blood samples were collected after informed consent. Mononuclear cells, granulocytes, and T lymphocytes were isolated as previously described.14 Briefly, 15 mL peripheral blood collected in sodium citrate was mixed with 6% hydroxyethylsturch solution (HES; Plasmasteril, Fresenius AG, Germany) and sedimented for 90 minutes at room temperature. All nucleated cells were then recovered, washed twice, suspended in Ca++ and Mg++–free phosphate-buffered saline (PBS) and centrifuged over Ficoll Hypaque density gradient (Pharmacia Biotech, Uppsala, Sweden). Cells at the bottom of the vial, consisting of more than 95% granulocytes (as evaluated by morphologic examination after May-Grünwald staining), were then recovered and stored with and without TRIzol (Invitrogen, Milan, Italy) at −80°C until PRV-1 assay and JAK-2 mutation analysis. For EEC assay, the mononuclear cell fraction was suspended in RPMI (Hyclone, Cramlingtone, United Kingdom) and plated in methylcellulose (Methocult; Stem Cell Technologies, Vancouver, BC) at 3 × 105 cells/mL, with and without erythropoietin.
The PRV-1 assay was carried out on isolated granulocytes by qualitative reverse-transcription–polymerase chain reaction (RT-PCR), within few days of cells separation, as previously described.14 Briefly, RNA was extracted using TRIzol Reagent (Invitrogen) following the manufacturer's instructions, treated with RQ1 Rnase-free Dnase (Promega, Madison, WI), and subsequently reverted by incubating 0.5 to 1 μg RNA at 42°C for 50 minutes in a final volume of 20 μL RT buffer 1 × (50 mM Tris HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2) containing 100 units Moloney murine leukemia virus reverse transcriptase (Promega), 2.5 μM random hexamers, 1 mM dNTP mix, and 10 mM dithiothreitol. cDNA (3 μL) was amplified with specific primers for PRV-1 in 25 μL final volume, containing 1 unit Taq DNA polymerase (Promega), 0.5 mM of each primer, 200 mM of both dNTP and 1 × reaction buffer (10 mM Tris HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2). The PCR conditions were as follows: 1 cycle of 3 minutes at 95°C, followed by 30 cycles at 95°C for 40 seconds, 54°C for 40 seconds, 72°C for 40 seconds, and a final cycle of 3 minutes at 72°C. Amplified product was separated on 2% agarose gel and visualized with UV light after staining with ethidium bromide.14
The presence of the V617F JAK-2 mutation was investigated according to Baxter et al,2 as previously described.20 Briefly, after DNA extraction with DNAzol (Invitrogen), following the product's protocol, 80 ng DNA of patients was used to amplify mutated and unmutated exon 12 of JAK-2 in allele-specific PCR.20 PCR products were separated onto 3% agarose gel, stained with ethidium bromide, and visualized under UV light. A 203-bp fragment indicates the presence of 1849G>T mutation. The sensitivity of this method is less than 3% of positive cells.
The EEC growth, PRV-1 RNA overexpression, and V617F JAK-2 mutation were independently evaluated by 3 different investigators (L.T., T.C., and F.G.), blinded to diagnosis and to clinical and laboratory findings of the enrolled patients.
Immunohistochemical analysis
BM biopsies were obtained by using standard procedures, formalin-fixed and embedded in paraffin. Immunohistologic analysis for pSTAT-3 and pSTAT-5 was carried out by using the antihuman rabbit monoclonal antibody pSTAT-5 (Y694) (Epitomics, Burlingame, CA) and antihuman rabbit polyclonal antibody pSTAT-3 (Tyr705) (Cell Signaling Technology, Danvers, MA) after a step of antigen retrieval in microwave oven for 10 minutes in citrate buffer (0.01 M; pH 6) at 750 W. Slides were then incubated at a 1:50 dilution of primary antibody for 1 (pSTAT-5) or 2 (pSTAT-3) hours. Hydrogen peroxide, serum biotinylated immunoglobulins, and avidin-biotin complexes were used according to the manufacturer's instructions (Dako LSAB; Dakopatts, Glostrup, Denmark). After induction of the color reaction with freshly made diaminobenzidine solution (Dakopatts), slides were counterstained with hematoxylin. Immunohistochemical analysis for c-mpl was performed by using the monoclonal antibody (moAb) antihuman c-mpl (R&D System, Minneapolis, MN) as previously described.17
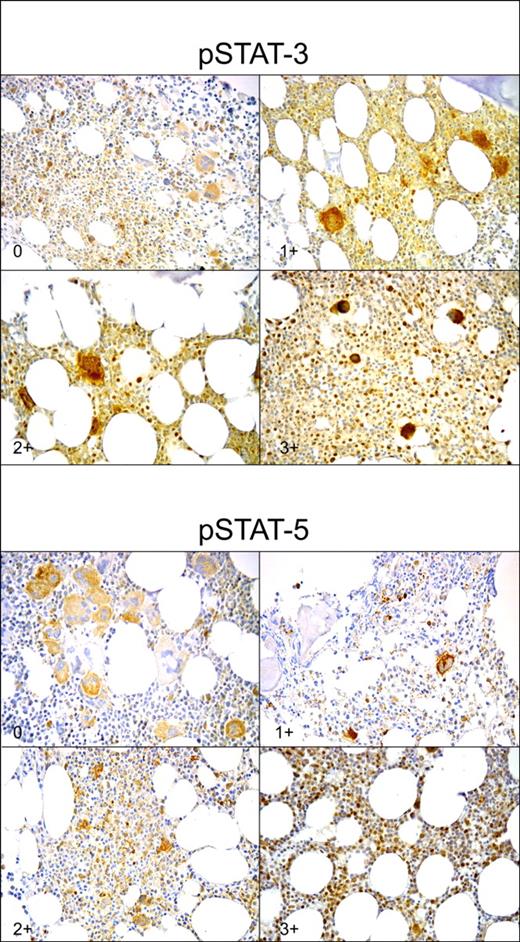
The pSTAT-3, pSTAT-5, and c-mpl staining intensities were evaluated by 2 pathologists (L.M.L. and M.M.) blinded to the diagnosis of individual patients, in assessment of control specimens. For each slide, a minimum of 10 fields was examined at 400 × magnification and at least 1000 cells were counted. In the case of specimens with sparse cellularity, the whole section was examined. The pSTAT-3 and pSTAT-5 staining intensities were classified in 4 different scores (from 0 to 3+), on the basis of the number of positive nuclei for high-magnification field. For each sample, interobserver variation was less than 3% and was omitted. Moreover, no significant differences in staining intensity were observed between older and more recent samples. The pSTAT-3 staining was scored 0 if fewer than 10 positive nuclei were present for high magnification field; 1+ if 10 to 20 positive nuclei were present; 2+ if 21 to 40 positive nuclei were present; and 3+ if more than 40 positive nuclei were found (Figure 1). The pSTAT-5 staining was scored 0 if 0 to 1 positive nucleus was present for high-magnification field; 1+ if 2 to 4 positive nuclei were present; 2+ if 5 to 9 positive nuclei were present; and 3+ if more than 10 positive nuclei were found (Figure 1). The c-mpl staining intensity was defined as normal (more than 80% megakaryocytes stained positive) or reduced (less than 80% megakaryocytes stained positive).17
Staining intensity of phosphorylated STAT-3 and STAT-5 (pSTAT-3 and pSTAT-5, respectively). pSTAT-3 staining was scored 0 if fewer than 10 positive nuclei were present in high magnification field (400×); 1+ if 10 to 20 positive nuclei were present; 2+ if 21 to 40 positive nuclei were present; and 3+ if more than 40 positive nuclei were present. pSTAT-5 staining was scored 0 if 0 to 1 positive nucleus were present in high magnification field; 1+ if 2 to 4 positive nuclei were present; 2+ if 5 to 9 positive nuclei were present; and 3+ if more than 10 positive nuclei were found. For each slide, a minimum of 10 high-magnification fields were examined (avidin-biotin complex immunoperoxidase method: original magnification, 400×).
Staining intensity of phosphorylated STAT-3 and STAT-5 (pSTAT-3 and pSTAT-5, respectively). pSTAT-3 staining was scored 0 if fewer than 10 positive nuclei were present in high magnification field (400×); 1+ if 10 to 20 positive nuclei were present; 2+ if 21 to 40 positive nuclei were present; and 3+ if more than 40 positive nuclei were present. pSTAT-5 staining was scored 0 if 0 to 1 positive nucleus were present in high magnification field; 1+ if 2 to 4 positive nuclei were present; 2+ if 5 to 9 positive nuclei were present; and 3+ if more than 10 positive nuclei were found. For each slide, a minimum of 10 high-magnification fields were examined (avidin-biotin complex immunoperoxidase method: original magnification, 400×).
Western blot analysis of pSTAT-3 and pSTAT-5
Cell samples were incubated with 1 mL lysis buffer consisting of 1% Triton X-100 in 50 mM Tris-HCl, 2 mM EDTA (pH 8), 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate. After incubation on ice for 30 minutes, cell lysates were centrifuged at 4°C and the supernatant proteins were recovered and measured by a Bio-Rad DC Protein Assay (Bio-Rad, Hemel Hempstead, United Kingdom). Protein solution was then dissolved in SDS sample buffer (Tris-HCl [pH 6.8], 10% SDS, 36% glycerine, 5% 2-mercaptoethanol, 0.03% bromophenol blue), boiled for 5 minutes, and then separated on SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Gels were blotted with transfer buffer (30 mM Tris, 240 mM glycine, 20% methanol) directly on pure nitrocellulose membrane (Bio-Rad) at 330 mA for 1 hour. Nonspecific binding sites on blots were blocked by incubation with 5% bovine serum albumin (BSA) in 10 mM Tris HCl, 0.15 M NaCl containing 0.05% Tween 20 (TBST) for 2 hours at room temperature on a rotating shaker. Blots were then probed with an anti–pSTAT-3 (1:500; BD Biosciences, Milano, Italy) and anti–pSTAT-5 (1:500; BD Biosciences) mouse moAb in TBST containing 1% BSA at 4°C for 18 hours, with gentle shaking, and were then incubated with goat antimouse HRP-conjugated antiserum (1:1000; BD Biosciences) in TBST for 1 hour at room temperature with gentle shaking. Blots were covered with enhancing chemiluminescence solution (GE Healthcare, United Kingdom) for 1 minute and exposed to Kodak XAR-5 x-ray film (Kodak, Rochester, NY) for 5 minutes. Nonspecific sites were blocked by incubation in TBST with 5% dry milk at room temperature for 2 hours with gentle shaking. Then, blots were incubated with a mouse moAb antiactin (Ab-5, 1:5000; BD Biosciences) in TBST with 3% dry milk at 4°C for 18 hours with gentle shaking, and incubated with goat antimouse HRP-conjugated antiserum (1:1000; BD Biosciences) in TBST at room temperature for 1 hour with gentle shaking. The pSTAT-3 and pSTAT-5 precipitates were subjected to densitometric analysis by using the Gel-Doc 2000 Quantity One program (Bio-Rad), after normalization with the actin intensity.
Statistical analysis
Statistical comparison of continuous variables was performed by the Kruskal-Wallis or the Mann-Whitney U test, as appropriate. Comparison of categorical variables was performed by the chi-square or Fisher exact test, as appropriate. P values more than .05 were considered statistically significant.
Results
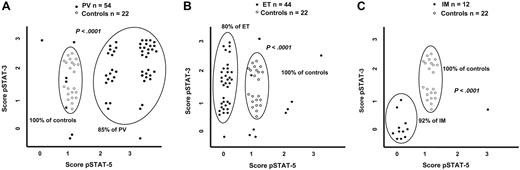
In all evaluated specimens, immunohistochemical staining showed that pSTAT-3 and pSTAT-5 were localized in the nuclei of precursor erythroid cells (mainly mature erythroblasts), of megakaryocytes and of granulocytic precursors. The different staining scores of pSTAT-3 and pSTAT-5 are shown in Figure 1. The staining was independent of specimen's cellularity, disease duration, and type of therapy. The various patterns of pSTAT-3 and pSTAT-5 staining observed in healthy controls, in PV, in ET and IM are shown in Figure 2. Results of EEC assay, PRV-1 RNA overexpression analysis, megakaryocyte c-mpl staining, and V617F JAK2 mutation analysis in PV, ET, and IM patients are shown in Table 2.
Comparison of pSTAT-3 and pSTAT-5 score distribution in MPDs and healthy controls. The different score of pSTAT-3 and pSTAT-5 in PV (A), ET (B), and IM (C) patients in comparison to pSTAT-3 and pSTAT-5 staining pattern observed in healthy controls.
Comparison of pSTAT-3 and pSTAT-5 score distribution in MPDs and healthy controls. The different score of pSTAT-3 and pSTAT-5 in PV (A), ET (B), and IM (C) patients in comparison to pSTAT-3 and pSTAT-5 staining pattern observed in healthy controls.
Expression of myeloproliferative markers in patients with polycythemia vera, essential thrombocythemia, and idiopathic myelofibrosis
| . | EECs . | PRV-1 . | c-Mpl . | V617F JAK2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Positive . | % . | Positive . | % . | Low . | % . | Positive . | % . | |
| PV, n = 41 | 31 | 76 | 33 | 80 | 37 | 90 | 33 | 81 |
| ET, n = 34 | 26 | 76 | 27 | 79 | 27 | 79 | 15 | 44 |
| IM, n = 5 | 3 | 60 | 0 | 0 | 5 | 100 | 3 | 60 |
| . | EECs . | PRV-1 . | c-Mpl . | V617F JAK2 . | ||||
|---|---|---|---|---|---|---|---|---|
| Positive . | % . | Positive . | % . | Low . | % . | Positive . | % . | |
| PV, n = 41 | 31 | 76 | 33 | 80 | 37 | 90 | 33 | 81 |
| ET, n = 34 | 26 | 76 | 27 | 79 | 27 | 79 | 15 | 44 |
| IM, n = 5 | 3 | 60 | 0 | 0 | 5 | 100 | 3 | 60 |
The number of evaluated patients is listed for each condition.
Controls
The number of pSTAT-3–expressing cells in normal bone marrow ranged from 10 to 40 elements for high-magnification field. Indeed, normal bone marrow samples exhibited score 1+ or 2+ (Figures 1–2). In contrast, pSTAT-5–expressing cells ranged from 2 to 4 for high magnification field (scored 1+) in all control samples (Figures 1–2). Bone marrow biopsies of patients with secondary polyglobulia and reactive thrombocytosis showed a slight increase in the number of pSTAT-3– and pSTAT-5–expressing cells (score 2+ in both cases), probably reflecting an increased “physiological” stimulation of receptors due to the high circulating levels of Epo or Tpo. Indeed, pSTAT-3 and pSTAT-5 expression pattern in secondary polyglobulia cannot differentiate these forms from PV (see Polycythemia vera), while secondary thrombocytosis presented similar pSTAT-3 expression but higher pSTAT-5 expression compared to ET (P = .003, data not shown). Lastly, bone marrow biopsies obtained from patients with secondary thrombocytosis due to splenectomy appeared undistinguishable from normal bone marrow biopsies (data not shown).
Polycythemia vera
Six (11%) of 54 patients with PV showed a weak pSTAT-3 expression (score 1+), 19 patients (35%) scored 2+, 26 patients (48%) strongly expressed pSTAT-3 (score 3+), and 3 patients (6%) showed a very low pSTAT-3 expression (score 0) (Figures 1–2A). In comparison to healthy controls, in PV patients the score 1+ of pSTAT-3 appeared significantly less frequently (P = .001), while score 3+ was significantly more represented (P > .001).
The vast majority of PV patients showed a high expression of pSTAT-5 (18 with score 2+ and 29 with score 3+, in total 87% of PV patients), while only 7 patients exhibited low or normal pSTAT-5 expression (1 patient scored 0 and 6 patients scored 1+) (Figure 2A). Indeed, in PV patients, compared to normal biopsies, score 1+ of pSTAT-5 was found more rarely (P > .001), while scores 2+ and 3+ were significantly more frequent (P = .001 and P > .001, respectively).
The staining pattern consisting of high pSTAT-5 expression (score 2+ and 3+) with normal or high pSTAT-3 expression (score 1+ to 3+) was typical of PV (46 [85%] of 54 cases), and was never found in normal bone marrow (P > .001, Figure 2A) nor in patients with ET (P > .001, data not shown). This staining pattern was not influenced by the presence of myeloid or erythroid predominance in bone marrow cellularity. Moreover, no clinical or hematologic difference was found between patients expressing the typical pSTAT-3 and pSTAT-5 staining pattern and those with low pSTAT-3 and/or pSTAT-5 levels.
Essential thrombocythemia
Thirty-three (79%) of 44 patients with ET showed a pSTAT-3 score 1+ and 2+; 7 patients (16%) showed a strong pSTAT-3 expression (score 3+); and only 4 patients (9%) showed a very low pSTAT-3 expression (score 0) (Figure 2B). The expression of pSTAT-3 in ET was similar to healthy controls, while it was significantly lower than in PV (P = .001).
On the contrary, 35 patients (80%) were negative for pSTAT-5 (score 0), 5 patients had score 1+ (11%), 3 patients score 2+ (7%), and 1 patient had score 3+ (2%). Indeed, ET was characterized by a reduced pSTAT-5 expression in the presence of normal or high pSTAT-3 expression (P > .001 in comparison to normal controls, Figure 2B). As reported for PV, the pSTAT-3 and/or pSTAT-5 staining was not influenced by the presence of myeloid or erythroid cell predominance. Lastly, patients expressing pSTAT-5 (score 1+ to 3+) had clinical and hematologic features similar to those of patients with score 0.
Myelofibrosis
pSTAT-3 and pSTAT-5 expression was evaluated in 11 patients with IM at diagnosis (11 patients) or during the follow-up (1 patient). We found that both pSTAT-3 and pSTAT-5 were expressed at very low level in the majority of evaluated patients. In particular, 9 patients were classified as score 0 for pSTAT-3 and 11 patients were classified as score 0 for pSTAT-5 (Figure 3C). Of interest, while low pSTAT-5 expression was commonly detected in the majority of ET patients as well, the reduced pSTAT-3 expression was typical of IM. Actually, this finding appeared significantly more frequently in IM compared to healthy controls (P > .001, Figure 3C), to ET (P > .001, data not shown), and to PV (P > .001, data not shown).
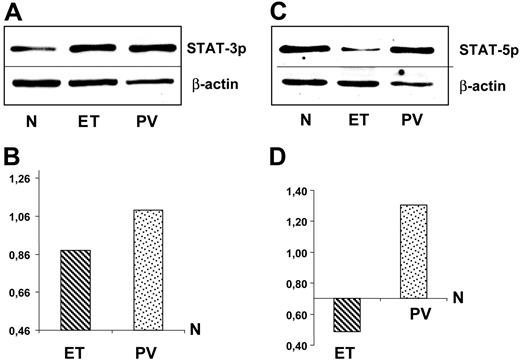
Western blot analysis of pSTAT-3 and pSTAT-5. Immunoblotting (upper panels) and densitometric analysis (lower panel) of pSTAT-3 (A) and pSTAT-5 (B) expression. Mononuclear cells were obtained from bone marrow of 5 healthy controls, 5 patients with PV, and 5 patients with ET. Immunoblots refer to a representative experiment. Values of densitometric analysis in PV and ET are normalized for results obtained in healthy controls (mean values of 5 experiments for each set of samples). SD are less than 10% and have been omitted.
Western blot analysis of pSTAT-3 and pSTAT-5. Immunoblotting (upper panels) and densitometric analysis (lower panel) of pSTAT-3 (A) and pSTAT-5 (B) expression. Mononuclear cells were obtained from bone marrow of 5 healthy controls, 5 patients with PV, and 5 patients with ET. Immunoblots refer to a representative experiment. Values of densitometric analysis in PV and ET are normalized for results obtained in healthy controls (mean values of 5 experiments for each set of samples). SD are less than 10% and have been omitted.
pSTAT-5 and pSTAT-3 expression were also evaluated in 4 patients with bone marrow fibrosis following a pre-existing diagnosis of PV (2 cases) and ET (2 cases). One of them, with post-PV myelofibrosis, showed the same pattern of IM, while the other 3 cases (2 ET and 1 PV) had a high expression of both pSTAT-3 and pSTAT-5. Thus, in contrast to IM, we did not find in secondary myelofibrosis a typical pattern of pSTAT-3 and pSTAT-5 expression.
Western blot analysis of pSTAT-3 and pSTAT-5
The pSTAT-3 and pSTAT-5 levels were assessed by Western blot analysis in bone marrow mononuclear cells isolated from 10 patients (5 PV and 5 ET) and from 5 healthy individuals. Results are shown in Figure 3. They confirmed the characteristic pattern of expression detected by immunohistochemistry (Figure 3A-B). In particular, the densitometric analysis of bands revealed that PV samples exhibited higher levels of both pSTAT-3 and pSTAT-5 compared to healthy individuals and ET patients (Figure 3C-D). The densitometric analysis of pSTAT-3 expression in ET showed higher levels than in healthy controls (Figure 3C). This finding was not in contrast with immunohistologic data, considering that among bone marrow biopsies obtained from 44 ET patients, we found a subgroup of 7 patients with high pSTAT-3 (Figure 2B). Lastly, pSTAT-5 expression in ET appeared significantly lower than normal (Figure 3D).
pSTAT-3 and pSTAT5 expression and myeloproliferative markers
We investigated if the specific pSTAT-3 and pSTAT-5 staining patterns were associated with the presence of biologic markers commonly detected in Ph-negative MPDs, and in particular to the presence of the V617F JAK2 mutation. The expression of myeloproliferative markers in our series of patients is shown in Table 2. We found that most of PV, ET, and IM patients shared the presence of EEC growth and of the impaired expression of c-mpl. Moreover, the overexpression of PRV-1 RNA was found in the majority of PV and ET cases, but not in IM. Lastly, the incidence of V617F JAK2 mutation was 81% in PV, 44% in ET, and 60% of IM. All these findings are in agreement with those reported in the literature1,,,–5,11,–13,15,16 and with our previous observations.14,17,20 We analyzed the different expression of myeloproliferative markers in patients with PV and ET, grouped according to the presence/absence of the typical pSTAT-3/pSTAT-5 expression pattern. No significant differences were detectable between the 2 groups of patients for all assays evaluated, neither in PV nor in ET.
Discussion
According to the classical criteria of the Polycythemia Vera Study Group,18 the diagnosis of Ph-negative MPD is usually performed by excluding secondary forms of thrombocytosis and polyglobulia. Over the years, several MPD biologic markers have been investigated in the diagnostic setting of these diseases, such as the EEC growth,11 the granulocyte PRV-1 RNA content,13,14 the reduced expression of c-mpl,15,–17 and, recently, the V617F JAK2 mutation.1,,,–5 Although the discovery of these markers significantly contributed to the knowledge of the MPD pathogenesis, their detection does not permit to distinguish among different allied myeloproliferative forms.
Previous studies demonstrated that STAT-3 and STAT-5 are constitutively activated in PV patients.4,9,10 Moreover, in a recent study on IM, Mesa et al demonstrated by immunoblotting high amounts of phosphorylated STAT-3 in neutrophils carrying the V617F JAK2 mutation, but not in cells with wild-type JAK2.21 In this study, we focused our attention on the bone marrow expression of pSTAT-3 and pSTAT-5, assuming that the constitutive activation of the Epo or Tpo receptors, due to the V617F JAK2 mutation, induces an increased phosphorylation of these signals transducers. We extensively evaluated by immunohistochemistry the expression of pSTAT-3 and pSTAT-5 in bone marrow biopsies obtained from 114 patients with MPD, and results were further confirmed by immunoblot assay. Our observations allowed 2 major conclusions: (a) pSTAT-3 and pSTAT-5 are moderately expressed in normal hematopoiesis and their phosphorylation is slightly increased in response to Epo and Tpo receptor stimulation (as can be argued from pSTAT-3 and pSTAT-5 patterns observed in secondary forms of erythrocytosis and thrombocytosis). (b) In MPDs, pSTAT-3 and pSTAT-5 expression is deeply altered, and PV, ET, and IM are specifically associated with 3 distinct abnormal patterns of pSTAT-3/pSTAT-5: uniformly increased expression of pSTAT-3 and pSTAT-5 in PV, slightly increased expression of pSTAT-3 and reduced pSTAT-5 expression in ET, and uniformly reduced expression of pSTAT-3 and pSTAT-5 in IM. The main implication of our observations is that, being that these patterns are highly specific for each MPD, pSTAT-3/pSTAT-5 staining could represent a useful and innovative diagnostic tool to distinguish the different MPDs. Actually, although the specificity of various pSTAT-3/pSTAT-5 patterns is only relative and not absolute, it might contribute to a more precise subtyping of disorders showing similar hematologic findings. In particular, high platelet counts, even higher than 1000 × 109/L, can be encountered in all MPDs.22 Of interest, among ET patients, we found one case carrying the JAK2 mutation, overexpressing PRV-1, showing EEC growth and exhibiting a high pSTAT-5 expression: this finding could suggest special care in following the possible evolution in PV of this case.23 On the other hand, in the same group of patients, 4 patients presented very low expression of pSTAT-3, which in our experience was typically associated with IM. In 3 of these patients, bone marrow histology was compatible with a diagnosis of prefibrotic/early stages of IM.24 Of importance, although these forms are indistinguishable from ET on the basis of hematologic parameters, they show a higher risk of myelofibrotic transformation, with a significant loss of life expectancy, in comparison to true ET.24
The first issue we attempted to address was if these histologic patterns could anticipate the presence of the V617F JAK2 mutation. In our series of patients, the prevalence of the V617F JAK2 mutation was 81% in PV, 44% in ET, and 60% in IM, in agreement with the literature data.1,,,–5 Nevertheless, we failed to detect any significant difference in pSTAT-5 or pSTAT-3 staining pattern between mutated and wild-type patients, in all MPDs evaluated. On the other hand, the pSTAT-5/pSTAT-3 patterns are significantly different among the various MPDs, while the V617F mutation is one single molecular defect detectable in all these diseases. Regarding this matter, our study highlights the importance of additional4,25,26 or alternative26,27 molecular alterations in the pathogenesis of MPDs.
The different pSTAT-5/pSTAT-3 patterns could reflect biologic peculiarities of PV, ET, and IM. Actually, the high pSTAT-5 expression in PV can be explained by its ability to up-regulate and cooperate with Bcl-xL,6 (the antiapoptotic gene overexpressed in PV erythroid progenitor cells28 ) in inducing erythroid differentiation.29,30 On the contrary, STAT-3 is an important regulator of megakaryocytopoiesis in vivo,31 explaining the increased phosphorylation of this signal transducer but not of STAT-5 in ET. Of interest, according to our histologic findings on bone marrow, Heller et al failed to detect pSTAT-5 in platelets isolated from patients with V617F JAK2–positive ET.32 Furthermore, other evidence indicates that STAT-3 is an anti-inflammatory response mediator.33,34 Thus, its impaired activation in IM could have a role in facilitating bone marrow fibrosis induced by the cytokine release.35
In conclusion, our observations confirm the importance of bone marrow histology in the diagnostic approach to Ph-negative MPDs and suggest an attractive role for pSTAT-3/pSTAT-5 expression in subtyping these diseases. The prospective observation of MPD patients will clarify whether the staining overlap among PV, ET, and IM reflects the presence of a fraction of ET patients in whom thrombocytosis is an early sign of PV or IM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maria Paola Marino, MD, for the skillful assistance in performing Western blot analysis; and Sara Capodimonti and Pier Paolo Occhilupo for their technical contribution.
This work was supported in part by grants of Università Cattolica of Rome (Fondi d'Ateneo, Progetti D1 2005–2006) and PRIN 2006, Ministero Università e Ricerca Scientifica, Roma.
Authorship
Contribution: L.T. contributed to the study design, enrolled patients in the study, analyzed data, and wrote the paper; M.M. performed the immunohistologic analysis; T.C. and F.G. performed cell cultures and molecular analysis; G.P. performed the immunostaining of bone marrow biopsies; L.T. and S.S. enrolled patients in the study and recorded clinical data; G.L. critically reviewed the paper; and L.M.L. designed the study, carried out the immunohistologic analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi M. Larocca, Istituto di Anatomia Patologica, Università Cattolica, Largo Gemelli 8, 00168 Roma, Italy; e-mail: llarocca@rm.unicatt.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal