The recruitment of tumor necrosis factor receptor–associated factors (TRAFs) 1, 2, 3, 5, and 6 to the CD40 cytoplasmic tail upon CD40 trimerization results in downstream signaling events that ultimately lead to CD40-dependent, thymus-dependent (TD) humoral immune responses. Previously, we have shown signaling through the C-terminal tail of CD40 in the absence of canonical TRAF-binding sites is capable of signaling through an alternative TRAF2-binding site. Here, we demonstrate that B cells from mice harboring CD40 with only the C-terminal tail can activate both canonical and noncanonical NFκB signaling pathways. Moreover, while lacking germinal center formation, several hallmarks of humoral immune responses including clonal B-cell activation/expansion, antibody isotype switching, and affinity maturation remain normal. This study demonstrates a new functional domain in CD40 that controls critical aspects of B-cell immunity in an in vivo setting.
Introduction
The seminal events that constitute the development of thymus-dependent (TD) humoral immunity include clonal B-cell expansion, germinal center (GC) formation, production of memory B cells, isotype switching, affinity maturation, and generation of long-lived plasma cells. All of these activities depend upon efficient CD40 signaling in B cells.1 Engagement of CD40 by its ligand (CD40L, CD154), expressed on activated helper T cells, leads to receptor multimerization and is followed by the recruitment of several different TNFR-associated factors (TRAFs) to the CD40 cytoplasmic tail.2,,,–6 Similar to other TNF receptor superfamily (TNFRSF) members, the recruitment of those adaptor proteins mediates the activation of multiple signaling pathways including NFκB, c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein (MAP) kinase, which in turn phosphorylate and activate downstream transcription factors.5,,,,,–11
Previously, to investigate the molecular mechanisms of CD40 signaling and how downstream signaling events control CD40-mediated immune responses, several approaches both in vitro and in vivo have been undertaken. In those studies, different TRAF-binding sites within the CD40 cytoplasmic domain have been identified.12,,,,–17 Mutagenesis of those defined TRAF-binding sites elucidated specific CD40-TRAF associations in regulating downstream signal transduction as well as controlling defined aspects of humoral immunity.18,19 Via TRAF6, CD40 activates the canonical NFκB pathway, while activation of both canonical and noncanonical NFκB, JNK, and p38 kinases is dependent on the binding of TRAF2/3.18,–20 In mice, mutation of the TRAF6 site on the CD40 cytoplasmic domain reduces antigen-induced affinity maturation and the generation of long-lived plasma cells.18 On the other hand, the recruitment of TRAF2/3 to CD40 plays a more important role in regulating isotype switching.19 In addition, other molecules besides TRAFs have been implicated in mediating CD40 activation. For example, Jak3 as well as a nuclear DNA-binding protein, Ku, have been shown to bind to membrane proximal regions of CD40.21,22 Moreover, the fact that early B-cell activities in response to CD40 signaling remain after the ablation of all known TRAF-binding sites further suggested the existence of TRAF-independent CD40 signaling pathways.11,18 A possible resolution to the TRAF-independent activities found in the CD40 cytoplasmic domain was reported to be due to a newly identified, “alternative” TRAF2-binding site within the highly conserved C-terminus of the CD40 cytoplasmic tail. The fact that this domain is functional was shown by site-directed mutagenesis of the second TRAF2-binding site with the loss of biologic activity in this domain.23
In this study, we have generated transgenic mice that expressed the h/m chimeric CD40 receptor containing the alternative TRAF2-binding site (Δ260). By testing its ability to propagate CD40 signals as well as develop humoral responses upon immunization, we have shown that B cells from Tg mice are able to activate both canonical and noncanonical NFκB signaling pathways. Moreover, CD40-induced proliferation and activation of B cells remains intact. The molecular signature of B cells from Δ260 mice also reveals a normal plasmacytic phenotype. In addition, comparable amounts of IgM were produced and those cells are also capable of isotype switching. More interestingly, despite an inability to produce germinal centers, Δ260 mice are able to manifest immunoglobulin (Ig) affinity maturation upon immunization. Hence, we have found that the C-terminus of the CD40 cytoplasmic tail is functionally important both ex vivo and in vivo.
Materials and methods
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth College (Lebanon, NH).
Generation of transgenic mice with chimeric CD40 receptors
All the chimeric CD40 transgenic mice were generated as described previously.18 In brief, different fragments of murine CD40 cytoplasmic tail were polymerase chain reaction (PCR) amplified, fused to the extracellular domain of human CD40, and subcloned into vector pDOI-5 via the EcoRI site for expression under MHC class II promoter. For constructing Δ260, a SalI site was introduced for making the fusion between the transmembrane domain and the conserved C-terminal domain.23 The engineered chimeric Δ260 CD40 transgenic construct was sequence verified and injected into pronuclei of fertilized oocyte from C57BL/6 mice by the transgenic facility at the University of Kentucky (Lexington, KY). Positive founders were then backcrossed with CD40-deficient mice and maintained in a pathogen-free facility at Dartmouth Medical School.
Antibodies and reagents
Monoclonal antibody against Ser32-phosphorylated IκBα was from Cell Signaling Technology (Beverly, MA). Monoclonal antibody S2C6 to human CD40 was a kind gift of S. Paulie (Stockholm University, Sweden). Staining antibodies against CD4, CD8, CD19, B220, CD23, CD80, MHC class II, and GL7 were purchased by BD Pharmingen (San Diego, CA). Biotinylated PNA and horseradish peroxidase (HRP)–conjugated goat antirabbit Ig secondary antibody were from Vector Labs (Burlingame, CA). Antibody sets for IgM, IgG, and IgG1 enzyme-linked immunosorbent assays (ELISAs) were from Southern Biotechnology (Birmingham, AL). Endogenous murine p100/p52 was detected by anti-p100N antisera, a kind gift from Stephen C. Ley (National Institute for Medical Research, London, United Kingdom). SAM68 (sc-333) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–β actin (AC 15) antibody was from Sigma-Aldrich (St Louis, MO). Recombinant hCD40L was from Immunex (Seattle, WA).
NFκB activation and immunoblotting
Splenic CD19+ B cells were purified by magnetic separation with MACS (Miltenyi Biotec, Germany) according to the manufacturer's instructions. For canonical NFκB activation, 2 × 106 B cells were cultured in 24-well plates and stimulated with 1 μg/mL recombinant hCD40L for various time points. Cells were then harvested and lysed in lysis buffer. For noncanonical NFκB activation, 2 × 106 B cells were cultured in 24-well plates and stimulated with 1 μg/mL recombinant hCD40L for 16 hours. Cells were then harvested and cytoplasmic and nuclear fractions were isolated as described.24 Protein samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes. A chemiluminescent substrate (Pierce, Rockford, IL) was used to detect HRP-labeled secondary antibodies on Western blots.
Proliferation and up-regulation of B-cell surface molecules
For proliferation assays, 1 × 105 purified B cells were cultured in 96-well flat-bottom plates for 72 hours at 37°C with or without 400 ng/mL recombinant hCD40L and 10 ng/mL IL-4. Cultures were pulsed with [3H]TdR for the last 8 hours of culture. For the induction of surface markers, B cells were cultured at 2 × 106 per well in 24-well plates for 48 hours with or without hCD40L (400 ng/mL). Cells were harvested and stained for CD23 and CD80 followed by fluorescence-activated cell sorting (FACS) analysis.
Real-time analysis
Purified B cells (2 × 106/well) were cultured in 24-well plates with or without 400 ng/mL hCD40L and 10 ng/mL IL-4. Cells were then harvested at 24 hours and 5 days. Total RNA was isolated using the RNeasy system (Qiagen, Valencia, CA). cDNA was then prepared and applied to real-time analysis (SYBR green; Bio-Rad, Hercules, CA). Relative expression of various gene targets normalized to β-actin was calculated as (2−(experimental CT − β-actin CT)) × 1000, where CT is the cycle threshold of signal detection.
GC formation
Mice were injected intraperitoneally with sheep erythrocytes without adjuvant to induce GC formation. Ten days after immunization, mice were killed and spleens were harvested for confocal microscopic analysis or FACS analysis. GCs were characterized as GL7+PNA+CD19+ B-cell clusters surrounded by CD4+ and CD8+ T-cell zones in the cryosection. FACS analysis was performed on B220+, T-cell–depleted splenocytes.
ELISA analysis
For in vitro analysis of Ig secretion and isotype switching, purified B cells (2 × 106/well) were cultured in 24-well plates with or without 400 ng/mL hCD40L and 10 ng/mL IL-4 for 5 days. Supernatants were collected and IgM and IgG production were quantified according to the manufacturer's instructions (Southern Biotechnology). For in vivo analysis of affinity maturation, mice were given intraperitoneal immunization of 100 μg NP30-KLH emulsified in CFA. Serum was collected from peripheral blood at indicated time points after immunization. Circulating antibodies were measured by an isotype- and antigen-specific ELISA. NP30-BSA and NP4-BSA were used to capture total and high-affinity Igs, respectively. Captured antibodies were then detected with enzyme-conjugated rabbit anti-IgG1.
Statistical analysis
Analysis of proliferation assays between the various treatments was analyzed by 2-tailed, paired Student t test. Values of P < .05 were considered significant.
Results
Generation of mice expressing h/m CD40 with only the C-terminus of the CD40 cytoplasmic tail
Previously, to define the role of TRAF molecules in CD40-mediated humoral immunity, we generated a cohort of transgenic mice that expressed mutant CD40 receptors in which different canonical TRAF-binding sites were disrupted.18 In brief, we engineered a chimeric CD40 transgene in which the murine transmembrane and cytoplasmic domains were fused to the extracellular domain of human CD40 and expressed under the control of the MHC class II (I-Eα) promoter. As described previously, this human-murine chimeric CD40 molecule allowed us in vitro to selectively activate the chimeric transgenic receptor in the presence of human CD40L protein while ensuring the intact species-specific cytoplasmic protein interactions. Moreover, because murine CD40L is able to activate human CD40, by backcrossing to a CD40−/− background, we could therefore examine the functional importance of those chimeric CD40 transgenic receptors in vivo.
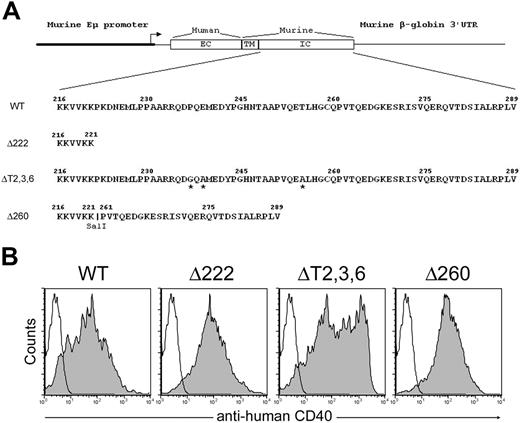
By applying the same idea, we produced a transgenic strain of mice that expressed a CD40 receptor that contains the alternative TRAF2-binding site (Δ260).23 As a negative control, a strain of mice that expressed a CD40 receptor with deletion of cytoplasmic domain (Δ222) was also generated as described previously (Figure 1A). Expression of different chimeric CD40 transgenes on B cells and other MHC class II–bearing cells was confirmed by FACS (Figure 1B and data not shown).
Generation of chimeric CD40 transgenic mice. (A) Schematic representation of the constructs used to generate transgenic mice expressing chimeric CD40 molecules consisted of the extracellular domain of the human CD40 and the transmembrane and varied cytoplasmic domains of murine CD40 under the MHC class II promoter control. Point substitutions are labeled by asterisks. Restriction enzyme site SalI has been inserted to connect 2 CD40 cytoplasmic tail fragments into the pDOI-5 vector. (B) Chimeric CD40 transgenic receptor expression on B220+ B cells were confirmed by FACS analysis. Filled histograms represented antihuman CD40 staining of the transgenic receptor; open histograms, the isotype control.
Generation of chimeric CD40 transgenic mice. (A) Schematic representation of the constructs used to generate transgenic mice expressing chimeric CD40 molecules consisted of the extracellular domain of the human CD40 and the transmembrane and varied cytoplasmic domains of murine CD40 under the MHC class II promoter control. Point substitutions are labeled by asterisks. Restriction enzyme site SalI has been inserted to connect 2 CD40 cytoplasmic tail fragments into the pDOI-5 vector. (B) Chimeric CD40 transgenic receptor expression on B220+ B cells were confirmed by FACS analysis. Filled histograms represented antihuman CD40 staining of the transgenic receptor; open histograms, the isotype control.
Signaling through the CD40 C-terminus tail activates both canonical and noncanonical NFκB signaling pathways
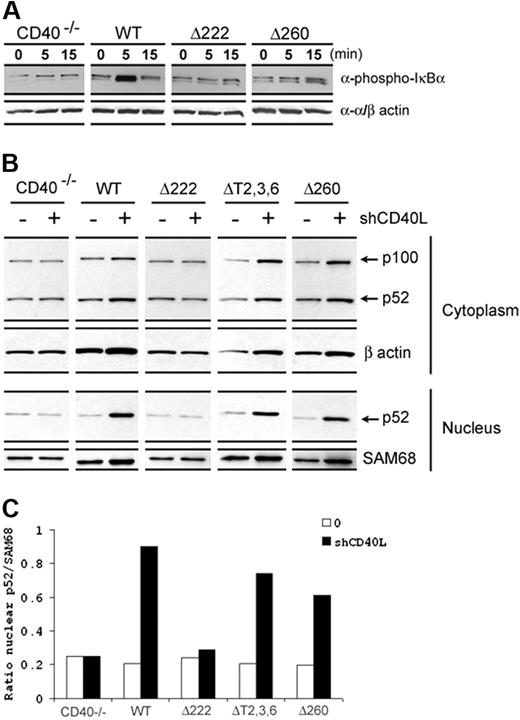
The role of different TRAFs in CD40-mediated activation of different downstream signaling cascades has been described in our earlier study in which we have shown the disruption of known TRAF-binding sites resulted in the loss of both CD40-driven phosphorylation of JNK and p38, while the phosphorylation of IκBα, an indication of NFκB activation, was still observed albeit with delayed and diminished responses.18 Based on the current knowledge of the presence of the second TRAF2 site, we thus re-evaluated whether the CD40 C-terminus containing the alternative TRAF2 site was adequate to activate NFκB signaling pathways. As shown in Figure 2A, a delayed and diminished IκBα phosphorylation was apparent in Δ260 mice that was comparable with that observed in ΔT2,3,6 mice as described in our previous study.18
CD40 induced NFκB activation. (A) CD19+ splenic B cells purified from different CD40 transgenic mice were cultured in vitro with hCD40L (1 μg/mL) stimulation for indicated time points. Canonical NFκB activation was measured by phosphorylated IκBα blotting. Anti-α/β actin antibody was used for loading control. (B) For noncanonical NFκB activation, B cells were cultured in 24-well plates and stimulated with 1 μg/mL hCD40L for 16 hours. Cells were then harvested and cytoplasmic and nuclear fractions were isolated. The level of noncanonical NFκB activation was detected by p52 blotting in the nuclear fraction. Anti-SAM68 antibody was used for loading control. Data are representative of more than 3 independent experiments. (C) The ratio of intensity values of p52 and SAM68 signals from the nucleus fraction was presented as quantitative analysis for measuring noncanonical NFκB activation.
CD40 induced NFκB activation. (A) CD19+ splenic B cells purified from different CD40 transgenic mice were cultured in vitro with hCD40L (1 μg/mL) stimulation for indicated time points. Canonical NFκB activation was measured by phosphorylated IκBα blotting. Anti-α/β actin antibody was used for loading control. (B) For noncanonical NFκB activation, B cells were cultured in 24-well plates and stimulated with 1 μg/mL hCD40L for 16 hours. Cells were then harvested and cytoplasmic and nuclear fractions were isolated. The level of noncanonical NFκB activation was detected by p52 blotting in the nuclear fraction. Anti-SAM68 antibody was used for loading control. Data are representative of more than 3 independent experiments. (C) The ratio of intensity values of p52 and SAM68 signals from the nucleus fraction was presented as quantitative analysis for measuring noncanonical NFκB activation.
Recent works illustrate the presence of 2 NFκB signaling pathways. In addition to a canonical pathway that involves the processing of precursor protein, NFκB1 (p105) to p50, a noncanonical pathway also exists. This pathway is mediated by the processing of a precursor protein, NFκB2 (p100) to p52, that translocates to the nucleus and mediates transcriptional activation.25 A previous report demonstrated that TRAF2/3 recruitment upon CD40 engagement activated both canonical and noncanonical NFκB pathways.20 Hence, the role of the C-terminus of the CD40 cytoplasmic tail in regulating noncanonical NFκB signaling was sought. As expected, Δ222 mice displayed no p52 translocation into the nucleus. In contrast, p52 induction in the nucleus was easily observed in Δ260 as well as ΔT2,3,6 mice upon CD40 activation (Figure 2B-C). Together, these results indicated that signaling through the C-terminus of the CD40 cytoplasmic tail containing the alternative TRAF2-binding site is sufficient to induce both canonical and noncanonical NFκB activation.
Signaling through the C-terminus of the CD40 cytoplasmic tail is capable of mediating B-cell proliferation and up-regulation of B-cell activation molecules
We next examined whether the CD40 C-terminus was able to induce CD40-mediated B-cell proliferation and up-regulation of surface molecules. As shown in Figure 3, deletion of the CD40 cytoplasmic tail (Δ222) prohibited both CD40-driven B-cell proliferation and up-regulation of the B-cell activation markers CD23 and CD80. In agreement with our previous studies,18 these defects were fully rescued in ΔT2,3,6 mice in which it was believed that all TRAF sites had been disrupted. However, we now show that B cells isolated from Δ260 mice are sufficient to expand and up-regulate activation markers in response to CD40 stimulation (Figure 3A-B). Hence, these data show that the C-terminus of the CD40 cytoplasmic tail is fully capable of driving early B-cell responses. Notably, the higher proliferation is consistently observed in B cells from Δ260 mice in response to CD40 stimulation compared with either ΔT2,3,6 or even WT mice (Figure 3A; *P = .005 and **P = .008, respectively).
CD40-mediated B-cell proliferation and up-regulation of surface markers. (A) Proliferation was measured in splenic B cells treated with IL-4 either with or without hCD40L for 3 days. Cultures were pulsed with [3H]TdR for the last 8 hours of culture (*P = .005; **P = .008). (B) For the induction of surface markers, splenic B cells were cultured in vitro for 48 hours with or without hCD40L (400 ng/mL). Cells were harvested and stained for CD23 and CD80 followed by FACS analysis. Data are representative of more than 3 independent experiments as the average ± standard deviation (SD).
CD40-mediated B-cell proliferation and up-regulation of surface markers. (A) Proliferation was measured in splenic B cells treated with IL-4 either with or without hCD40L for 3 days. Cultures were pulsed with [3H]TdR for the last 8 hours of culture (*P = .005; **P = .008). (B) For the induction of surface markers, splenic B cells were cultured in vitro for 48 hours with or without hCD40L (400 ng/mL). Cells were harvested and stained for CD23 and CD80 followed by FACS analysis. Data are representative of more than 3 independent experiments as the average ± standard deviation (SD).
B cells that express mutant CD40 with only the C-terminus in the cytoplasmic tail are capable of undergoing differentiation to Ig production and isotype switching
Upon CD40 activation, B cells are able to differentiate into plasma cells. Several transcription factors have been shown to be critical in regulating plasma cell differentiation, a phenomenon that includes a radical restructuring process that allows them to secrete large quantities of immunoglobulin.26,,–29 Among them, Bcl-6 and Blimp have been studied most thoroughly. Blimp is a transcriptional repressor that is highly up-regulated in plasma cells and plays a central role in plasmacytic differentiation. Blimp expression is directly regulated by Bcl-6. During the differentiation process of B cells to plasma cells, Bcl-6 levels decrease followed by the concomitant up-regulation of Blimp.28 Of interest, similar to ΔT2,3,6 mice, B cells from Δ260 mice are able to down-regulate Bcl-6 in 24 hours after CD40 engagement (Figure 4A). After 5 days of culture, the induction of Blimp is easily detected and indistinguishable between Δ260 and WT control mice, while no alteration of gene expression in Δ222 mice could be observed (Figure 4A-B).
Plasmacytic differentiation and Ig secretion.(A-B) Real-time analysis of Bcl-6 and Blimp expression from splenic B cells cultured in vitro with IL-4 either with or without hCD40L stimulation for 24 hours and 5 days, respectively. Relative expression of various gene targets normalized to β-actin was calculated as (2−(experimental CT − β-actin CT)) × 1000, where CT is the cycle threshold of signal detection. (C-D) IgM and IgG secretion from B cells was accessed by ELISA analysis using supernatant collected from 5 days of in vitro culture of splenic B cells with or without 400 ng/mL hCD40L and 10 ng/mL IL-4 (*P = .004; **P = .001). Data are representative of 3 independent experiments (mean ± SD).
Plasmacytic differentiation and Ig secretion.(A-B) Real-time analysis of Bcl-6 and Blimp expression from splenic B cells cultured in vitro with IL-4 either with or without hCD40L stimulation for 24 hours and 5 days, respectively. Relative expression of various gene targets normalized to β-actin was calculated as (2−(experimental CT − β-actin CT)) × 1000, where CT is the cycle threshold of signal detection. (C-D) IgM and IgG secretion from B cells was accessed by ELISA analysis using supernatant collected from 5 days of in vitro culture of splenic B cells with or without 400 ng/mL hCD40L and 10 ng/mL IL-4 (*P = .004; **P = .001). Data are representative of 3 independent experiments (mean ± SD).
Heightened levels of Ig secretion are achieved after 4 days of culture of WT B cells with CD40 stimulation plus IL-4, as described previously.18 Both IgM and IgG are detected. As shown in Figure 4C-D, B cells from any of the CD40 Tg mice produced similar levels of IgM and IgG upon activation. In fact, the Δ260 mice routinely demonstrated statistically significantly higher IgM responses compared with others (Figure 4C; *P = .004 and **P = .001, respectively). Taken together, the C-terminus of CD40 cytoplasmic tail can control gene expression essential for plasma cell differentiation, and this site is sufficient to initiate Ig production and isotype switching.
The role of the C-terminus of the CD40 cytoplasmic tail in GC formation
GC formation is dependent upon CD40 signaling and is a hallmark of TD humoral immune responses. In most cases, GC formation is essential for affinity maturation and the generation of B-cell memory.30 Mice were immunized with sheep erythrocytes as described previously.18 Ten days after immunization, GC formation was assessed by either fluorescence microscopy or FACS analysis. As shown in Figure 5A, while PNA+ (peanut agglutinin), GL7+, and B220+ GC cells could be easily observed in WT mice, GC formation was absent in the Δ222, ΔT2,3,6, and Δ260 mice. Similar results were obtained by immunohistologic analysis (Figure 5B). Previously, it has been shown that CD40 signaling through either TRAF6 or the canonical TRAF2/3-binding sites was sufficient to induce GC formation.18 However, the data presented herein demonstrated that the CD40 C-terminus containing the alternative TRAF2 site is not sufficient for GC formation.
CD40-mediated GC formation. (A) FACS analysis of GC formation was performed from mice 10 days after sheep erythrocyte immunization. Splenocytes were isolated and stained with B220, PNA, and GL7. Fluorescence was quantified by flow cytometry and profiles were gated on B220+ cells. (B) Immunohistologic analysis of GC formation was performed from the spleen treated similarly as mentioned in “The role of the C-terminus of the CD40 cytoplasmic tail in GC formation” section. In brief, spleens from sheep erythrocyte–immunized mice were cryocut and stained with B220 (green), PNA (red), and CD4 (blue). GC is indicated by white arrow. Data are representative of 8 to 12 mice from 2 independent experiments.
CD40-mediated GC formation. (A) FACS analysis of GC formation was performed from mice 10 days after sheep erythrocyte immunization. Splenocytes were isolated and stained with B220, PNA, and GL7. Fluorescence was quantified by flow cytometry and profiles were gated on B220+ cells. (B) Immunohistologic analysis of GC formation was performed from the spleen treated similarly as mentioned in “The role of the C-terminus of the CD40 cytoplasmic tail in GC formation” section. In brief, spleens from sheep erythrocyte–immunized mice were cryocut and stained with B220 (green), PNA (red), and CD4 (blue). GC is indicated by white arrow. Data are representative of 8 to 12 mice from 2 independent experiments.
Δ260 mice demonstrate Ig affinity maturation in the absence of GC formation
Despite a lack of GC formation and impaired Ig affinity maturation, our earlier work demonstrated that isotype switching and the early IgG1 response during immunization could be readily observed in the ΔT2,3,6 mice.18 We next sought to investigate whether the alternative TRAF2 site on CD40 is sufficient for the generation of humoral immune response in vivo. Mice were immunized with nitrophenol-conjugated keyhole limpet hemocyanin (NP-KLH) in complete Freund adjuvant (CFA). As previously described, immunization with NP-KLH allows the detection of antibody production in response to the hapten, NP, as well as the ability to assess the affinity maturation by quantitatively measuring the development of high-affinity Ig responses. NP-specific IgG1 titers (NP30 for total Ig) are readily detected in serum after 14 days of immunization in the WT, ΔT2,3,6, and Δ260 mice but not in CD40-deficient mice or Δ222 mice (Figure 6A). This result suggests that the C-terminus of the CD40 cytoplasmic tail is sufficient for inducing humoral immune responses. Of interest, while the response in ΔT2,3,6 mice is reduced as described previously, the response in Δ260 mice is almost indistinguishable from WT mice at early time points (Figure 6A). More intriguingly, while the production of high-affinity NP-specific IgG1 (NP4) was significantly reduced in ΔT2,3,6 mice, as demonstrated in our previous work, high-affinity NP-specific IgG1 could be easily detected in Δ260 mice over time (Figure 6A-B). At day 56, almost all the IgG1 against NP antigen became high affinity in Δ260 mice (Figure 6B). These results suggest that the C-terminus of the CD40 cytoplasmic tail containing the alternative TRAF2 site is able to manifest strong humoral immune responses and induce Ig affinity maturation in the absence of GC formation. Together, these data implicated the existence of 2 active TRAF2-binding sites on CD40 with distinct functions in vivo.
Humoral immune responses in chimeric CD40 transgenic mice. Mice were immunized with 100 μg NP30-KLH emulsified in CFA. Circulating antibodies were measured by an NP-specific, isotype-specific ELISA. (A) Both total NP-specific IgG1 (NP30) and high-affinity NP-specific IgG1 (NP4) responses from different chimeric CD40 transgenic mice were followed over time (days 14, 28, 42, and 56) (*P < .05 by analysis of variance versus values for CD40−/− at indicated time points). (B) The alterations of IgG1 responses (total versus high affinity) to NP antigen in Δ260 mice over time were shown. Data represent 2 independent experiments (mean ± SD).
Humoral immune responses in chimeric CD40 transgenic mice. Mice were immunized with 100 μg NP30-KLH emulsified in CFA. Circulating antibodies were measured by an NP-specific, isotype-specific ELISA. (A) Both total NP-specific IgG1 (NP30) and high-affinity NP-specific IgG1 (NP4) responses from different chimeric CD40 transgenic mice were followed over time (days 14, 28, 42, and 56) (*P < .05 by analysis of variance versus values for CD40−/− at indicated time points). (B) The alterations of IgG1 responses (total versus high affinity) to NP antigen in Δ260 mice over time were shown. Data represent 2 independent experiments (mean ± SD).
Discussion
Since their discovery more than 10 years ago, the role of TRAFs in CD40 signaling has been studied extensively. From those studies, individual TRAF-binding motifs have been mapped and their role in controlling CD40-mediated thymus-dependent (TD) humoral immunity (including B-cell proliferation/activation, germinal center [GC] formation, production of memory B cells, isotype switching, affinity maturation, and generation of long-lived plasma cells) has been elucidated.1 In this study, we exploited the use of chimeric CD40 transgenic molecules with deletion of all known TRAF-binding motifs to further examine the role of a new functional domain within the cytoplasmic tail of CD40. The data presented herein suggest that this new functional domain is important and sufficient in regulating humoral immune responses.
Previously, TRAF-independent pathways in CD40 downstream signaling have been speculated based on the fact that several earlier B-cell responses were retained in the absence of all TRAF recruitment.11,18 In fact, other than TRAF proteins, several molecules have been shown to directly associate with CD40. For example, a molecule termed p23 has been shown to interact with CD40 through the extracellular domain.31 Two other molecules, Jak3 and Ku, which both bind to the proximal region of the CD40 cytoplasmic tail, have been implicated in participating in CD40-mediated gene regulation.21,22 However, all the molecules mentioned previously have been studied mostly in vitro and their roles in vivo have yet to be demonstrated. Previously, to identify non-TRAF intermediaries in CD40 signaling, both genetic and biochemical approaches were used. We found a new functional domain within the cytoplasmic tail of CD40 that contains an alternative TRAF2 site.23 Here, we demonstrate the in vivo role of the C-terminus of the CD40 tail devoid of all other identified TRAF or non–TRAF-binding motifs. Although the role of other TRAF and non-TRAF molecules could not be completely excluded, it strongly suggests that the previously identified alternative TRAF2 site was sufficient to replicate all of the earlier B-cell responses such as activating NFκB pathways, inducing B-cell activation/proliferation as well as Ig secretion and isotype switching previously seen in CD40 transgenic mice.18 Of interest, a second C-terminal TRAF2-binding site in TNFR2 has also been identified by Grech et al where they showed the second TRAF2 site did not activate but attenuated signaling from canonical site.32 In addition, the same group has shown TRAF2 may serve as a negative regulator to noncanonical NFκB activation.33 The discrepancy of these findings with our study could be explained by the fact that the second TRAF2 site in the C-terminus of TNFR2 does not conform to any TRAF2 consensus binding motifs and appears to bind TRAF2 indirectly. Thus, other factors may be involved in recruiting TRAF2 to this site and contribute to the dominant-negative effect. Moreover, the negative effect on the noncanonical NFκB cascade from TRAF2 was shown in mice with TRAF2 deficiency in B cells where multiple TNFR member signaling pathways could be affected. If only the CD40 receptor system is considered, then it is clear that TRAF2 has been repeatedly shown to be important in activating both canonical and noncanonical NFκB pathways.20,34
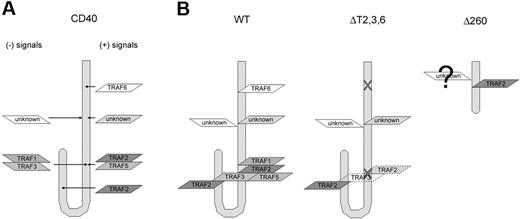
One of the most intriguing findings presented is the observation of Ig affinity maturation in Δ260 mice but not ΔT2,3,6 mice in response to NP immunization in the absence of GC formation (Figures 5-6). Together, these results raise several interesting questions. First, what are the differences between ΔT2,3,6 and Δ260 mice? Although both strains shared similar CD40 activities in vitro, functional differences in vivo clearly indicate reduced responsiveness associated with mice expressing the ΔT2,3,6 CD40 compared with the Δ260 cytoplasmic tail. Similarly, both B-cell proliferation and IgM secretion were greater in Δ260 mice compared with either WT or ΔT2,3,6 mice in vitro. The strength of signal transduced through CD40 is probably the result of positive regulators (TRAF 2,5,6) and negative regulators (TRAF1,3) (Figure 7A). During CD40 engagement, TRAF1, 2, 3, and 5 are all recruited to the canonical TRAF2-binding site, because the residues in the binding motif are highly conserved, as described previously (Figure 7B: WT).17 Theoretically, the single mutation of T254 to A introduced in ΔT2,3,6 mutant ablates the binding of all 4 TRAFs10 ; however, our studies, as well as the work of others have shown that this mutation only reduces, but not completely eliminates, TRAF binding to CD40 (Figure 7B: ΔT2,3,6).20,23 Thus, it is possible, if not likely, that residual TRAF3 binding is sustained in the ΔT2,3,6 mutant mice. Crystallographic studies suggests that the C-terminal domain of the cytoplasmic tail loops back and places the canonical TRAF2,3 site proximal to the alternative TRAF2 site.35 Hence, residual TRAF3 binding to the canonical TRAF2/3 site could negatively regulate the function of the alternative TRAF2 site in the ΔT2,3,6 mutant mice (Figure 7B: ΔT2,3,6). Previously, both TRAF1 and TRAF3 have been indicated as negative regulators for CD40 and other TNFR signaling events.20,36,,–39 Our previous works have shown that only TRAF2 could be recruited to this second TRAF2 site by yeast 2-hybrid assays.23 Although it does not exclude the possibility that some residual TRAF3 binding might still occur, we have further shown while TRAF2 degradation could be readily observed in Δ260 mice, TRAF3 degradation was absent upon CD40 stimulation (unpublished results, January, 2006). This CD40-mediated TRAF degradation phenomenon has been described previously.40 In that report, the investigators showed that the degradation of TRAF molecules occurs after association with CD40. Together, these results suggest that increased responses in Δ260 mice could be due to the lack of functional TRAF1/TRAF3 involvement (Figure 7B: Δ260). We are still in the midst of more definitively determining the full extent of TRAF recruitment to this C-terminal site in primary B cells. However, the abundance of TRAF recruitment to CD40 in primary cells is extremely low and is at the limits of detection.
Schematic representation of the potential interaction between CD40 and its adaptor molecules. (A) CD40-mediated signaling results from a combination of both positive (right) and negative (left) signals. (B) WT CD40 (left) recruits TRAF6 in its membrane-proximal domain; TRAF1, TRAF2, TRAF3, and TRAF5 through oligomerization in the canonical TRAF2-binding site; and another TRAF2 in the C-terminus of the CD40 cytoplasmic tail. Site-directed mutagenesis resulted in the disruption of TRAF6 recruitment, much reduced TRAF2, TRAF3 binding in the canonical TRAF2 site but left the second TRAF2 site intact in ΔT2,3,6 (middle). Δ260 (right) recruits TRAF2 to the alternative site and while less likely it may also recruit other TRAF and non-TRAF molecules.
Schematic representation of the potential interaction between CD40 and its adaptor molecules. (A) CD40-mediated signaling results from a combination of both positive (right) and negative (left) signals. (B) WT CD40 (left) recruits TRAF6 in its membrane-proximal domain; TRAF1, TRAF2, TRAF3, and TRAF5 through oligomerization in the canonical TRAF2-binding site; and another TRAF2 in the C-terminus of the CD40 cytoplasmic tail. Site-directed mutagenesis resulted in the disruption of TRAF6 recruitment, much reduced TRAF2, TRAF3 binding in the canonical TRAF2 site but left the second TRAF2 site intact in ΔT2,3,6 (middle). Δ260 (right) recruits TRAF2 to the alternative site and while less likely it may also recruit other TRAF and non-TRAF molecules.
Ig affinity maturation in the absence of GC formation is unusual and has been reported only in a few examples. Previously, it has been shown that mice deficient in lymphotoxin-alpha (LTα), which have no lymph nodes or Peyer patches and fail to form GC in the spleen, can manifest a high-affinity anti-NP IgG1 response similarly to WT mice upon high-dose immunization.41 Likewise, immunization of the Δ260 mice results in Ig affinity maturation in the absence of GC formation. The data suggest that residual TRAF1 or TRAF3 binding to the canonical TRAF2 site may impair terminal B-cell differentiation. However, with complete deletion of previously characterized TRAF-binding sites, release of the negative regulation from TRAF1/3 leads to heightened CD40-induced activities from TRAF2 binding to the alternative site. While the signal from the second TRAF2 site is not enough to induce GC formation, it is sufficient to induce Ig affinity maturation and manifest humoral immune responses. However, it is also possible that the canonical and alternative TRAF2 site on CD40 may exhibit distinct functions with different regulation machineries or the existence of additive or synergistic effects between 2 TRAF2 sites. Thus, for some CD40 functions, such as GC formation, the presence of both TRAF2 sites may have to work cooperatively. The findings from both the previous study in LTA-deficient mice and this work are rather descriptive and fail to provide direct molecular mechanisms for GC-independent affinity maturation; nonetheless, the data herein provide a useful tool and the first step to dissect different components required for each cellular event in B-cell maturation and differentiation.
Moreover, our previous study has shown the disruption of TRAF6-binding site results in a loss of affinity maturation and the generation of long-lived plasma cells indicating the essential role of the CD40-TRAF6 axis in controlling humoral immunity.18 However, the work presented herein has suggested the second TRAF2 site in the absence of potential inhibitory signals is sufficient to manifest those humoral immune responses. The discrepancy of these studies together with the finding of 2 TRAF2 sites have led us to question whether the functional differences between each TRAF site observed from our laboratory and others were due to the different qualitative nature of individual TRAF molecule–mediated downstream signaling cascades or simply due to the differences of quantitative strength from a combination of both positive and inhibitory signals. Recently, Kadono et al have shown that enhanced CD40 mediated TRAF6 signal strength by introducing additional TRAF6-binding sites into the CD40 cytoplasmic tail, enabling CD40 to induce osteoclastogenesis, which normally only TRANCE-R can do; in contrast, WT CD40 failed to induce osteoclastogenesis.42 That study has suggested that it is quantitatively but not qualitatively different TRAF-binding sites in the cytoplasmic tail that decide the osteoclastogenic potential between CD40 and TRANCE-R. Hence, it is possible that the lack of TRAF6 signaling in Δ260 mice could be compensated by the deletion of inhibitory sites, which results in a sufficient amount of signal strength for Ig affinity maturation. Although this finding may not be fully applicable to our work, nonetheless, this work has opened a whole new avenue of study with regard to the role of TRAFs, especially TRAF2, in CD40-mediated humoral immune responses. Ongoing work will address the questions raised with the generation of mice expressing CD40 that lack only the alternative TRAF2 site.
Finally, the possibility will always remain that CD40Δ260 mediates its effect via a non–TRAF2-dependent pathway (Figure 7B: Δ260). This could be ascertained by using TRAF2-deficient cells. However, as mentioned earlier, TRAF2 deficiency results in a CD40-independent noncanonical NFκB activation,33 hence preventing using this approach. Moreover, a human/mouse chimeric CD40 transgene may not exactly reflect the behavior of a homologous CD40 counterpart despite its ability to bind and signal through mouse CD40L stimulation normally.18,23 Nonetheless, together with previous work, both in vitro and in vivo,18,23 these results strongly suggested an important role of the C-terminus of the CD40 cytoplasmic tail in mediating B-cell responses.
In summary, we have shown an in vivo functional role of the C-terminus of the CD40 cytoplasmic tail. Signaling through the CD40 C-terminus is capable of activating both canonical and noncanonical NFκB pathways, inducing B-cell activation/proliferation, regulating gene expression required for plasma cell differentiation, Ig secretion, isotype switching, as well as Ig affinity maturation. Thus, the results herein provide an in vivo functional relevance in understanding the complex network of CD40 signaling in controlling humoral immunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by NIH grant (AI52211) to R.J.N.
National Institutes of Health
Authorship
Contribution: L.-F.L. designed and performed research, contributed vital new reagents, collected data, analyzed data, and wrote the paper; C.L.A. designed and performed research, contributed vital new reagents, collected data, and analyzed data; E.F.L. performed research and collected data; V.S.R. performed research and collected data; W.J.C. contributed vital new reagents; L.-L.L. contributed vital new reagents; R.J.N. designed research. L.-F.L. and C.L.A. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randolph J. Noelle, Dept of Microbiology & Immunology, Norris Cotton Cancer Center, Dartmouth Hitchcock Medical Center, Dartmouth Medical School, 1 Medical Center Drive, Lebanon, NH, 03756; e-mail: rjn@dartmouth.edu.

![Figure 3. CD40-mediated B-cell proliferation and up-regulation of surface markers. (A) Proliferation was measured in splenic B cells treated with IL-4 either with or without hCD40L for 3 days. Cultures were pulsed with [3H]TdR for the last 8 hours of culture (*P = .005; **P = .008). (B) For the induction of surface markers, splenic B cells were cultured in vitro for 48 hours with or without hCD40L (400 ng/mL). Cells were harvested and stained for CD23 and CD80 followed by FACS analysis. Data are representative of more than 3 independent experiments as the average ± standard deviation (SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2006-07-038414/2/m_zh80130702820003.jpeg?Expires=1767717095&Signature=t5i-nCCgNs~qAPLHyEmsTWIY0m8SMqCL1k5IkJHO7PtZBW5Bh9Hyr8qdnyU3A53TBjAtEtOiWLtFj~Ta-GILvXyXsdbsA~J2O0ZfZOy-inhQ2AN95f5EvEPKCInn9H~PGWFr84EM5SmNPG3VfuTd2~MEXyUQdOOMHmAbRFlSLNXqdaz2eg-Rrt3KLpRLMdjJNAGD6IvB6sdK6ygjkJQjAnwiOrFYblJCTe2VSu2agxihykiNhKfi0ALs0sn~ZmEoDuwrRl5InPr57K81ZJ~RBkxbGBbgYmOvV9zdt94KGL8z1Rvm3uXohbGi8M2GGWdmyl2heyx2YCpe-UNBeEmwQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal