Tissue factor (TF) is the cellular receptor for clotting factor VIIa (FVIIa), and the formation of TF-FVIIa complexes on cell surfaces triggers the activation of the coagulation cascade and the cell signaling. Our recent studies have shown that a majority of TF resides in various intracellular compartments, predominantly in the Golgi, and that FVIIa binding to cell surface TF induces TF endocytosis and mobilizes the Golgi TF pool to translocate it to the cell surface. This present study is aimed to elucidate the mechanisms involved in TF endocytosis and its mobilization from the Golgi. Activation of protease-activated receptor 1 (PAR1) and PAR2 by specific peptide agonists and proteases, independent of FVIIa, mobilized TF from the Golgi store and increased the cell surface expression of TF. Blocking PAR2 activation, but not PAR1, with neutralizing antibodies fully attenuated the FVIIa-induced TF mobilization. Consistent with these data, silencing the PAR2 receptor, and not PAR1, abrogated the FVIIa-mediated TF mobilization. In contrast to their effect on TF mobilization, PAR1 and PAR2 activation, in the absence of FVIIa, had no effect on TF endocytosis. However, PAR2 activation is found to be critical for the FVIIa-induced TF endocytosis. Overall the data herein provide novel insights into the role of PARs in regulating cell surface TF expression.
Introduction
Binding of clotting factor VIIa (FVIIa) to tissue factor (TF) on cell surfaces initiates the coagulation cascade by activating both factors IX and X, which, in turn, leads to thrombin generation, and subsequently platelet activation and fibrin clot formation.1 In addition to its role in coagulation, TF-FVIIa may also have nonhemostatic functions. TF-FVIIa and the coagulation proteases generated by TF-FVIIa (ie, factor Xa and thrombin) have been shown to initiate cell signaling via activation of protease-activated receptors (PARs). TF-dependent signaling pathways are thought to contribute to a variety of pathophysiological processes, including inflammation, atherosclerosis, angiogenesis, and tumor metastasis.2,–4 Therefore, proper regulation of TF expression at cell surfaces is critical not only for the maintenance of hemostatic balance but also health in general. Tissue factor expression on cell surfaces is regulated by multiple and tightly controlled regulatory mechanisms, including transcriptional regulation of the TF gene,5 control of the membrane phospholipid composition surrounding the TF receptor,6,7 and inhibition of TF-FVIIa proteolytic activity by specific plasma inhibitors.1,8 In addition to these established mechanisms, recent studies suggest that functional expression of TF-FVIIa on cell surfaces could also be regulated by other novel mechanisms,6,9,10 including endocytosis of TF.11,,–14
Tissue factor is present constitutively in many extravascular cell types, including fibroblasts, smooth muscle cells, pericytes in and surrounding blood vessel walls, and lung epithelial cells.15,16 Although it was thought that TF is entirely localized on cell surfaces,17,18 immunohistochemical studies with various cell types revealed that only a small fraction of the total cellular TF antigen is localized at the cell surface, with the majority in intracellular pools with a distinct perinuclear localization.19,,–22 Our recent studies on TF distribution in fibroblasts revealed that a substantial fraction of intracellular TF is localized in the Golgi, and that FVIIa binding to the cell surface TF both induced the endocytosis of surface TF and concomitantly mobilized intracellular TF from the Golgi pool to the cell surface.22 Of interest, the catalytic activity of FVIIa was essential for both TF endocytosis and the mobilization of TF from the Golgi.22 At present, the mechanism by which FVIIa mobilizes TF from the Golgi and whether this solely depends on TF-FVIIa protease activity at the cell surface or is influenced by TF-FVIIa endocytosis are unknown.
This study is designed to investigate possible mechanisms involved in TF internalization and mobilization from the Golgi pool. Since studies from our laboratory and others showed that TF-FVIIa could activate PAR-mediated cell signaling2,23,24 and FVIIa protease activity is required for FVIIa-dependent internalization and trafficking of TF,22 we focused on investigating the role of PAR1 and PAR2 activation on TF internalization and trafficking. The data presented in the paper show that activation of PAR1 or PAR2, independent of FVIIa binding to cell surface TF, induces TF mobilization from the Golgi pool. Our data also show that blocking PAR2 receptors by PAR2-specific antibodies or PAR2-specific siRNA completely attenuated FVIIa-mediated cell surface TF internalization and Golgi TF trafficking, providing direct evidence that FVIIa modulates TF internalization and trafficking through activation of PAR2.
Materials and methods
Reagents
Monospecific polyclonal antibodies against human TF were prepared as described earlier.25 TF monoclonal antibodies (TF9–10H10), polyclonal neutralizing antibodies to PAR2, and TF phosphospecific antibodies were obtained from Wolfram Ruf, Scripps Research Institute (La Jolla, CA). PITPβ antibodies were kindly provided by Bruce Hamilton, University of California (San Diego, CA). PAR1-specific monoclonal antibodies ATAP-2 and WEDE-15 were obtained from Beckman Coulter (Fullerton, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Antihuman golgin-97, secondary antibodies conjugated to Oregon Green or Rhodamine Red were from Molecular Probes (Eugene, OR). Recombinant human FVIIa26 and active site–inactivated FVIIa (FFR-FVIIa)27 were obtained from Novo Nordisk (Maaloev, Denmark). Thrombin, factors X, and factor Xa were either from Enzyme Research Laboratories (South Bend, IN) or Haematological Technologies (Essex Junction, VT). PAR agonist peptides (PAR1, TFLLRNPNDK; PAR2, SLIGRL; PAR3, TFRGAP; PAR4, AYPGKF) were custom synthesized (Biosynthesis, Lewisville, TX).
Cell culture
A human fibroblast cell line (WI-38), derived from normal embryonic lung tissue, was obtained from ATCC (Rockville, MD). Fibroblasts were maintained in DMEM with Glutamax and high-glucose medium supplemented with 1% penicillin/streptomycin and 10% FBS. The cells were cultured at 37°C and 5% CO2 in a humidified incubator.
Radiolabeling of proteins
Factor VIIa and TF mAB were labeled by using Iodo-Gen (Pierce Biotechnology, Rockford, IL)–coated tubes and Na125I according to the manufacturer's technical bulletin and as described previously.14 Free iodine was removed by extensive dialysis against 10 mM Hepes, pH 7.5, 150 mM NaCl. Our earlier studies13 established that the radiolabeled proteins were intact with no apparent degradation, and 125I-labeled FVIIa retained 80% or more of the functional activity of the unlabeled material.
Binding studies
Cell surface binding of 125I-FVIIa or 125I-TF mAB (TF9H10) was performed essentially as described previously.14
Measurement of TF-FVIIa activity on cell surfaces
Monolayers of WI-38 cells were incubated with FVIIa (10 nM) in buffer B28 (10 mM Hepes, 0.15 M NaCl, 4 mM KCl, 11 mM glucose, pH 7.5 buffer [buffer A] containing 5 mM CaCl2 and 1 mg/mL bovine serum albumin) for 5 minutes at 37°C, followed by the addition of substrate factor X (175 nM). Unless otherwise specified, an aliquot was removed at a specific time point (usually at 5 minutes) into stopping buffer (TBS containing 1 mg/mL BSA and 10 mM EDTA), and factor Xa in the sample was measured in a chromogenic assay as described earlier.29 Activation of factor X was linear for 20 minutes or more under these experimental conditions.
Determination of prothrombin activation on cell surfaces
Monolayers of WI-38 cells were incubated with FVa (10 nM) in buffer B followed by the addition of factor Xa (1 nM) for 5 minutes at 37°C. Then, prothrombin (1.4 μM) was added to the well and at the end of the 10-minute activation period, an aliquot was removed into stopping buffer (TBS containing 1 mg/mL BSA and 10 mM EDTA), and the thrombin generated was measured by chromogenic assay using Chromozym TH, as described earlier.30
Inhibition of TF, PAR1 receptor, and PAR2 receptor expression by siRNA transfection
PAR2 siRNA (F2RL1: 5′-CCU CAU AAC AUU AAA CAG GTT-3′) was purchased from Ambion (Austin, TX). A mixture of 2 different 18-nucleotide PAR1 siRNAs (F2RA: 5′-AGA UUA GUC UCC AUC AAU-3′ and F2RK: 5′-AGG CUA CUA UGC CUA CUA C-3′) was obtained from MWG Biotech (High Point, NC). When cells reached 70% confluency, they were washed once with serum-free medium, and then incubated with 250 nM siRNA + 4% SiPort amine (Ambion) in serum-free OptiMEM medium (Invitrogen, Carlsbad, CA). Six hours after transfection, the medium was changed to DMEM supplemented with 10% serum and antibiotics and cultured for 60 to 72 hours. To inhibit de novo synthesis of TF without depleting pre-existing TF protein, fibroblasts were transfected with TF siRNA (150 nM; 5′-GCG CUU CAG GCA CUA CAA ATT-3′; MWG Biotech) and the transfected cells were used for experiments 24 hours after transfection.
Measurement of cytosolic Ca2+ release
Fluorescence microscopy was used for the measurement of calcium influxes. Fibroblasts were seeded in 8-well chambered cover slips (Nunc, Rochester, NY) at a density of 10 000 cells/well. After 24 hours, cells were washed and incubated with 4 μM Fluo-4/AM Ester (Molecular Probes) for 45 minutes in buffer B in a humidified atmosphere of 37°C/5% CO2. The cells were then washed with buffer B, and the chambered cover slip was placed on the stage of Nikon Eclipse TE2000–5 (Tokyo, Japan) microscope equipped with a CCD camera. Live images of fluorescence were recorded by exciting the Fluo-4 probe with 488 nm light and monitoring 520 nm emission using Perkin Elmer Ultraview LC1 confocal microscopy system (Shelton, CT). After 30 seconds of image acquisition, the test compound was added to the cells and the recording was continued for a further 250 seconds. Results are represented as the change in fluorescence intensity over time.
FACS analysis
Monolayers of fibroblasts cultured in T-25 flask were washed with phosphate-buffered saline (PBS) and harvested using 0.5 mM EDTA (versene solution) for 3 minutes. Following 2 washes with fluorescence-activated cell sorting (FACS) buffer (PBS containing 1% bovine serum albumin and 0.05% sodium azide) to remove any residual EDTA, cells were placed in blocking buffer (5% goat serum in PBS) for 30 minutes on ice. The cells (∼ 1 × 105 cells for staining with each antibody) were washed again in FACS buffer and incubated with the desired antibody in the blocking buffer for 60 minutes on ice. Two additional washes were performed to remove any residual antibody and then incubated with an FITC-conjugated antibody for 30 minutes on ice in the dark. The cells were then washed, fixed in 2% paraformaldehyde, and analyzed using a FACSCalibur (Becton and Dickinson, San Jose, CA) flow cytometer.
Cell surface biotinylation and internalization of tissue factor
Cell surface biotinylation was performed as described earlier.22 Briefly, monolayers of fibroblasts cultured in T-25 flasks were washed twice with PBS and incubated for 30 minutes on ice in the presence of 0.5 mg/mL NHS-SS-biotin (Pierce Biotechnology) in PBS. The biotinylation reaction was terminated by removing the biotin and washing the monolayers twice with PBS, followed by a 10-minute incubation with 10 mM glycine on ice. Biotin-labeled cells were then treated with FVIIa or other reagents in buffer B for 60 minutes at 37°C to allow internalization. Following the treatment, the cells were washed 3 times with PBS and the cell surface biotin was cleaved by incubating them with a cell-impermeable reducing buffer (50 mM glutathione, 1 mM MgCl2, 1.0 mM EDTA, 0.2% BSA, and 75 mM NaCl, pH 8.0) for 10 minutes at 37°C. Cells were then washed with PBS and lysed in 300 μL lysis buffer (20 mM Tris-HCl, pH 7.4, containing 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, and a cocktail of protease inhibitors).
Immunoprecipitation and Western blotting
Cell lysates from biotinylated cells were incubated overnight at 4°C with affi-gel–coupled anti-TF beads (20 μL, 7 mg/mL anti-TF IgG/mL packed beads). Following the removal of the residual lysate, the beads were washed 3 times with 10 mM Hepes buffer to remove unbound material, and the bound material was eluted with 50 μL sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Eluted samples (15 μL) were subjected to SDS-PAGE, followed by Western blot analysis with streptavidin-HRP conjugate. To determine the extent of phosphorylation of the TF cytoplasmic domain, fibroblasts were treated with control vehicle, PMA (20 ng/mL), FVIIa or other proteases (10 nM), or PAR2 agonist peptide (50 μM) for 1 hour. The cells were lysed in lysis buffer as described earlier31 and the cell lysates, either directly or immunoprecipitated with anti-TF beads were subjected to SDS-PAGE for Western blot analysis with phosphospecific or total TF antibodies.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as reported earlier.22 Fibroblasts were cultured on 8-chamber glass slide system (Nunc). After one day of culture, cells were treated with FVIIa or other agents as indicated in “Results,” and then fixed for 1 hour at 4°C in PBS containing 4% paraformaldehyde and 0.1% glutaraldehyde. The fixed cells were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes and blocked with 3% goat serum in PBS for 1 hour at room temperature. The cells were stained overnight at 4°C with polyclonal rabbit anti-TF and monoclonal antihuman golgin-97 antibody as indicated in figure legends, followed by incubation with Oregon Green (excitation/emission wavelengths, 490/510 nm)–conjugated or Rhodamine Red (excitation/emission wavelength, 590/620 nm)–conjugated anti–rabbit or anti–mouse IgG for 60 minutes at the room temperature. For negative controls, cells were incubated with non–immune IgG or irrelevant antibody (anti–factor IX) in place of specific primary antibodies.
Image acquisition, scoring, and colocalization
The immunostained cells were viewed using a Nikon Eclipse TE2000–5 microscope at 60 × magnification (oil) at room temperature. Images were acquired of a field of view at 2-μm Z-axis increments using UltraVIEW LCI confocal system (Perkin Elmer) equipped with a digital CCD camera (Ultra Pix; Hamamatsu Photonics, Okayama City, Japan) with a resolution of 1344 × 1024 × 12. The laser setting wavelengths were as follows: channel 1, 488 ± 10 nm excitation and 525 ± 10 nm emission; channel 2, 568 ± 10 nm excitation and 600 ± 45 nm emission. Perkin-Elmer's ImagingSuite (version 5.2) Acquisition & Processing Software was used for the acquisition of images and determining the colocalization (overlap of the green and the red fluorescence). The scanned images were imported into Power Point (Office 2000 software; Microsoft, Redmond, WA) for compilation of figures. To determine percentage of cells with TF in the Golgi, immunofluorescence-stained cells from 8 to 10 different fields (5 to 10 cells/field) were examined through the eye piece and the presence of TF fluorescence staining in the perinuclear region was taken as TF in the Golgi. The scoring was done by the same investigator who performed the experiment. For colocalization analysis, an ROI (region of interest) was selected with a defined area of approximately 3000 μm2. After the background deduction (making gray level to zero from a noncellular region), the correlation coefficients of the RBG composite image were analyzed using the same ROI size. Ten to 15 randomly selected areas were chosen with the same ROI to calculate the overall average correlation coefficient (or pixel value in case of quantifying cell surface TF). Twenty-five to 50 cells were analyzed for each experimental condition within the experiment. Values from 3 or more independent experiments were used to determine mean correlation coefficient values and percentage of cells with a Golgi TF pool.
Statistical analysis
All experiments were repeated a minimum of 3 times. Statistical significance of differences between the groups were determined by t test, and the difference was considered significant when P < .05. GraphPad Prism (version 4; GraphPad Software, San Diego, CA) software was used for the statistical analysis.
Results
Activation of PAR1 and PAR2 enhances TF expression at the cell surface
Our recent studies showed that a substantial fraction of intracellular TF is localized in the Golgi, and FVIIa binding to the cell surface mobilizes intracellular TF from the Golgi and increases TF expression at the cell surface.22 To investigate the potential role of PAR-mediated cell signaling in FVIIa-induced TF trafficking, we first tested whether PAR activation up-regulates TF levels at the cell surface and mobilizes TF from the Golgi as with FVIIa.
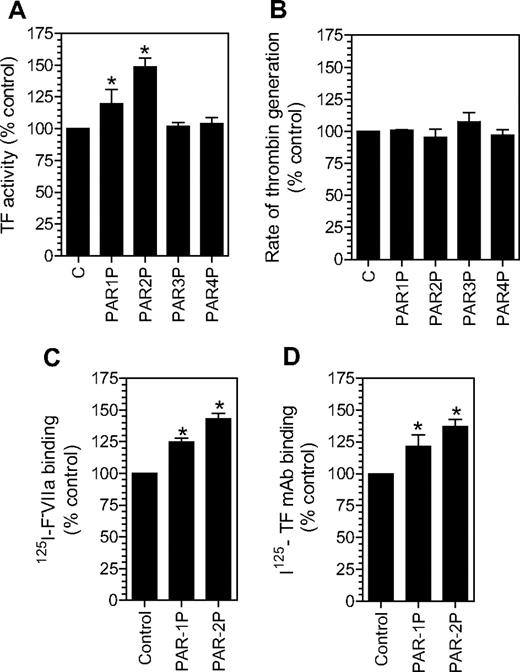
As shown in Figure 1A, treatment of fibroblasts with PAR1 or PAR2 activation agonist peptides increased the TF levels at the cell surface by 20% to 50%, whereas PAR3 and PAR4 agonist peptides had no effect. The increase in the cell surface TF activity associated with PAR1 and PAR2 activation is highly consistent and statistically significant. To address whether potential changes in negatively charged phospholipids at the cell surface following PAR1 or PAR2 agonist peptide treatment could be responsible for the increased TF activity at the cell surface, we measured prothrombinase activity in fibroblasts treated with PAR agonist peptides. No differences in the prothrombinase activity were observed between the control cells and cells treated with PAR agonist peptides (Figure 1B). The increased TF expression on the cell surface following PAR1 and PAR2 agonist peptide treatment was confirmed using other assays (ie, TF-specific FVIIa binding; Figure 1C) and TF mAB binding (Figure 1D) assays. In additional studies, thrombin and trypsin, the physiological activators of PAR1 and PAR2, respectively, were also found to increase TF expression at the cell surface to a similar extent as that of PAR1 and PAR2 agonist peptides (data not shown).
Activation of PAR1 or PAR2 increases tissue factor expression at the cell surface. Fibroblasts were incubated with a control buffer or a buffer containing different PAR agonist peptides (50 μM) for 2 hours at 37°C. The cell surface TF expression was evaluated in a TF functional activity assay (in factor X activation assay) (A), TF-specific 125I-FVIIa binding (C), and 125I-TF mAB binding (D) assays. Panel B depicts cell surface prothrombinase activity in control fibroblasts and fibroblasts treated with different agonist peptides as in panel A. * denotes that the value significantly differs from the value obtained with fibroblasts treated with a control vehicle (n = 3, mean ± SEM).
Activation of PAR1 or PAR2 increases tissue factor expression at the cell surface. Fibroblasts were incubated with a control buffer or a buffer containing different PAR agonist peptides (50 μM) for 2 hours at 37°C. The cell surface TF expression was evaluated in a TF functional activity assay (in factor X activation assay) (A), TF-specific 125I-FVIIa binding (C), and 125I-TF mAB binding (D) assays. Panel B depicts cell surface prothrombinase activity in control fibroblasts and fibroblasts treated with different agonist peptides as in panel A. * denotes that the value significantly differs from the value obtained with fibroblasts treated with a control vehicle (n = 3, mean ± SEM).
PAR1 and PAR2 activation mobilizes TF from the Golgi
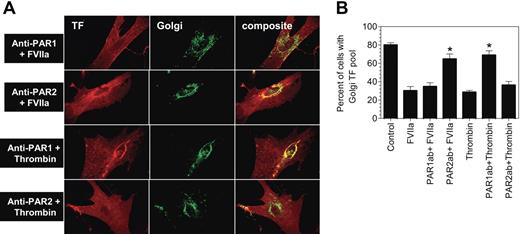
To evaluate the influence of PAR activation on TF mobilization from the Golgi, fibroblasts were treated with various coagulation proteases that activate PAR1 or PAR2, or specific PAR agonist peptides for 2 hours, and the distribution of TF was analyzed by immunofluorescence confocal microscopy after staining the cells with antibodies specific to TF and the Golgi marker protein, golgin-97 (Figure 2). As observed in our earlier study,22 TF is localized throughout the cell with a major pool in the nuclear peripheral region as revealed by intense bright staining around the perinuclear region. TF in the perinuclear region is colocalized with golgin-97, indicating the localization of TF in the Golgi. Treatment of fibroblasts with FVIIa, thrombin, trypsin, PAR1 AP, and PAR2 AP resulted in mobilization of TF pool from the Golgi, resulting in a substantially reduced colocalization of TF and golgin-97. The reduction in TF levels in the Golgi following PAR1 and PAR2 activation is associated with increased TF levels at the cell surface (Table 1). In contrast, no detectable differences on either TF levels in the Golgi or at the cell surface were found in control cells or cells treated with FFR-FVIIa, PAR3 AP, or PAR4 AP. Factor Xa treatment also resulted in TF mobilization from the Golgi, but FXa was not as effective as FVIIa (Table 1). Fibroblasts treated with FVIIa + FX (or FXa) behaved the same as cells treated with FVIIa alone (data not shown). Brefeldin A treatment, which causes disintegration of the Golgi, abolished the perinuclear staining of TF, and also attenuated the FVIIa and PAR2-mediated increase in TF activity at the cell surface (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Overall, these data suggest that activation of PAR1 or PAR2, independent of FVIIa binding to cell surface TF, modulates TF trafficking in fibroblasts, and externalization of TF from the Golgi might be responsible for increased TF activity at the cell surface in cells stimulated with FVIIa or PAR agonists.
Effect of PAR agonists on intracellular tissue factor mobilization. Fibroblasts were exposed to control vehicle, FVIIa (10 nM), FFR-FVIIa (10 nM), PAR agonist peptides (50 μM), thrombin (10 nM), or trypsin (10 nM) for 2 hours at 37°C. Cells were fixed, permeabilized, and immunostained with rabbit polyclonal antihuman TF and monoclonal antihuman golgin-97 antibodies, followed by Rhodamine Red–labeled antirabbit and Oregon Green–labeled antimouse antibodies as secondary reporter antibodies. Left panel images represent TF staining, middle panel images represent the Golgi staining, and right panel images represent the overlay of TF and golgin-97 staining (colocalization). Inserts show a magnified view of TF localization in the Golgi.
Effect of PAR agonists on intracellular tissue factor mobilization. Fibroblasts were exposed to control vehicle, FVIIa (10 nM), FFR-FVIIa (10 nM), PAR agonist peptides (50 μM), thrombin (10 nM), or trypsin (10 nM) for 2 hours at 37°C. Cells were fixed, permeabilized, and immunostained with rabbit polyclonal antihuman TF and monoclonal antihuman golgin-97 antibodies, followed by Rhodamine Red–labeled antirabbit and Oregon Green–labeled antimouse antibodies as secondary reporter antibodies. Left panel images represent TF staining, middle panel images represent the Golgi staining, and right panel images represent the overlay of TF and golgin-97 staining (colocalization). Inserts show a magnified view of TF localization in the Golgi.
Effect of coagulation proteases and PAR peptide agonists on Golgi TF trafficking
| Proteases/peptides . | Percent of cells with Golgi TF pool, mean ± SD . | Colocalization of TF and golgin-97, correlation coefficient, mean ± SD . | TF expression at cell surface, pixel value by image analysis, mean ± SD . |
|---|---|---|---|
| Untreated control | 83.8 ± 4.50 | 0.85 ± 0.017 | 15.9 ± 1.41 |
| FVIIa | 23.3 ± 6.12* | 0.48 ± 0.053* | 25.1 ± 1.15* |
| FFR-FVIIa | 82.9 ± 3.83 | 0.84 ± 0.022 | 16.5 ± 1.84 |
| Thrombin | 26.0 ± 2.90* | 0.42 ± 0.046* | 21.6 ± 3.05* |
| Trypsin | 21.0 ± 5.60* | 0.48 ± 0.036* | 23.5 ± 2.88* |
| FXa | 54.0 ± 8.48* | 0.62 ± 0.063* | 19.1 ± 3.48* |
| PAR1 AP | 22.2 ± 1.16* | 0.44 ± 0.051* | 22.3 ± 2.35* |
| PAR2 AP | 25.4 ± 1.56* | 0.47 ± 0.027* | 22.7 ± 2.59* |
| PAR3 AP | 79.2 ± 3.22 | 0.78 ± 0.085 | 16.9 ± 1.04 |
| PAR4 AP | 80.5 ±5.15 | 0.85 ± 0.050 | 15.8 ± 1.53 |
| Proteases/peptides . | Percent of cells with Golgi TF pool, mean ± SD . | Colocalization of TF and golgin-97, correlation coefficient, mean ± SD . | TF expression at cell surface, pixel value by image analysis, mean ± SD . |
|---|---|---|---|
| Untreated control | 83.8 ± 4.50 | 0.85 ± 0.017 | 15.9 ± 1.41 |
| FVIIa | 23.3 ± 6.12* | 0.48 ± 0.053* | 25.1 ± 1.15* |
| FFR-FVIIa | 82.9 ± 3.83 | 0.84 ± 0.022 | 16.5 ± 1.84 |
| Thrombin | 26.0 ± 2.90* | 0.42 ± 0.046* | 21.6 ± 3.05* |
| Trypsin | 21.0 ± 5.60* | 0.48 ± 0.036* | 23.5 ± 2.88* |
| FXa | 54.0 ± 8.48* | 0.62 ± 0.063* | 19.1 ± 3.48* |
| PAR1 AP | 22.2 ± 1.16* | 0.44 ± 0.051* | 22.3 ± 2.35* |
| PAR2 AP | 25.4 ± 1.56* | 0.47 ± 0.027* | 22.7 ± 2.59* |
| PAR3 AP | 79.2 ± 3.22 | 0.78 ± 0.085 | 16.9 ± 1.04 |
| PAR4 AP | 80.5 ±5.15 | 0.85 ± 0.050 | 15.8 ± 1.53 |
Values are based on 3 to 7 independent experiments.
Significantly differs from the value obtained with untreated control (P < .01).
To examine whether the effect of FVIIa-mediated PAR-dependent signaling is specific to TF mobilization from the Golgi or is a more generalized effect, we examined the localization of phosphatidylinositol transfer protein β-isoform (PITPβ), a protein that has been shown to be dynamically associated with the Golgi,32 in control fibroblasts and fibroblasts exposed to FVIIa or PAR2 AP. As expected, PITPβ was associated with the Golgi. FVIIa or PAR2 AP treatment had no effect on PITPβ localization in the Golgi (Figure S2).
Next, we examined whether the Golgi TF pool is newly synthesized TF that is in an active constitutive pathway destined to undergo exocytosis as it gets synthesized or is stored in the Golgi to undergo regulated secretion. For these studies, cells were first treated with cycloheximide (10 μg/mL) to inhibit protein synthesis and TF localization in the Golgi, and TF expression at the cell surface was analyzed. No TF was found in the Golgi in cells treated with cycloheximide for 1 hour (Figure S3) or longer. TF activity at the cell surface in cycloheximide-treated cells was increased by about 25% at 1 hour. Even prolonged treatment (24 hours) of fibroblasts with cycloheximide failed to diminish TF activity significantly at the cell surface (data not shown). Since cycloheximide completely shuts off all protein synthesis, it is difficult to conclude whether the absence of TF in the Golgi in cycloheximide-treated cells indicates that TF in the Golgi is newly synthesized or that TF residence in the Golgi requires the presence of an additional protein that is constitutively synthesized. Therefore, in additional experiments we selectively reduced TF mRNA levels by either inhibiting gene transcription by serum deprivation7 or by using TF-specific siRNA. Northern blot analysis revealed no TF mRNA transcripts in fibroblasts that were serum deprived overnight or 12 hours after siRNA transfection (data not shown). Analysis of TF localization in these cells revealed, in contrast to cycloheximide-treated cells, the presence of TF in the Golgi apparatus (Figure S3).
FVIIa-induced TF mobilization from the Golgi is mediated via PAR2
Although FVIIa binding to TF at the cell surface is known to transduce cell signaling predominantly via PAR2, TF-FVIIa has also been shown to activate PAR123 (U.P., unpublished data, November 2005). Further, FVIIa binding to TF, independent of PARs, has also been found to influence various cellular processes.33,,–36 To determine whether FVIIa-induced TF mobilization is mediated via TF-FVIIa activation of PARs, fibroblasts were first treated with specific neutralizing antibodies against PAR1 or PAR2 before they were exposed to FVIIa. A combination of PAR1 antibodies, ATAP-2 and WEDE-15, failed to inhibit FVIIa-induced TF mobilization (Figure 3A-B), In control experiments, the antibodies were shown to inhibit thrombin-induced TF mobilization. In contrast to PAR1-specific antibodies, treatment of fibroblasts with PAR2 antibodies completely attenuated FVIIa-induced TF mobilization. PAR2 antibodies, as expected, had no effect on thrombin-induced TF mobilization (Figure 3A-B).
Inhibition of PAR2 impairs FVIIa-induced tissue factor mobilization. Fibroblasts were treated with a combination of PAR1 monoclonal antibodies (ATAP-2, 10 μg/mL; WEDE-15, 25 μg/mL) or rabbit antihuman PAR2 IgG (200 μg/mL) for 30 minutes at the room temperature. Then, the cells were exposed to FVIIa (10 nM) or thrombin (10 nM) for 2 hours at 37°C. Following the treatment, the cells were fixed, permeabilized, and immunostained for TF and the Golgi marker, golgin-97, as described in Figure 2. Panel A depicts representative images of immunostaining. Left panel images represent TF staining, middle panel images represent golgin-97 staining, and right panel images represent the overlay of TF and the Golgi marker staining (colocalization). Panel B shows percentage of total number of cells with TF pool intact in the Golgi (mean ± SEM from 3 to 4 experiments).
Inhibition of PAR2 impairs FVIIa-induced tissue factor mobilization. Fibroblasts were treated with a combination of PAR1 monoclonal antibodies (ATAP-2, 10 μg/mL; WEDE-15, 25 μg/mL) or rabbit antihuman PAR2 IgG (200 μg/mL) for 30 minutes at the room temperature. Then, the cells were exposed to FVIIa (10 nM) or thrombin (10 nM) for 2 hours at 37°C. Following the treatment, the cells were fixed, permeabilized, and immunostained for TF and the Golgi marker, golgin-97, as described in Figure 2. Panel A depicts representative images of immunostaining. Left panel images represent TF staining, middle panel images represent golgin-97 staining, and right panel images represent the overlay of TF and the Golgi marker staining (colocalization). Panel B shows percentage of total number of cells with TF pool intact in the Golgi (mean ± SEM from 3 to 4 experiments).
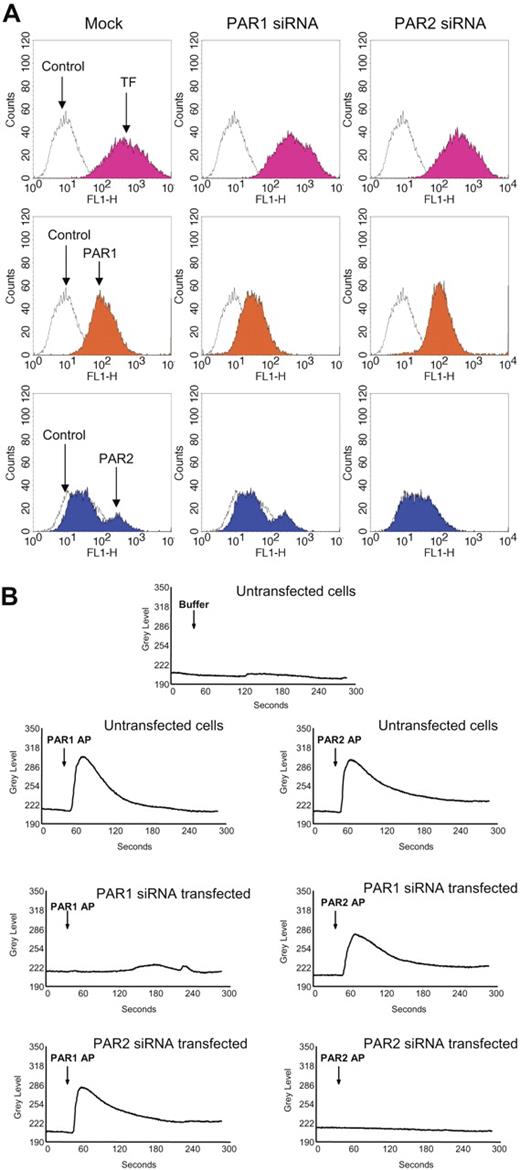
To further confirm these findings, we used siRNAs to selectively knock down PAR1 or PAR2 expression in fibroblasts prior to their exposure to FVIIa or thrombin. Fibroblasts transfected with PAR1 siRNA or PAR2 siRNA were shown to express reduced levels of PAR1 or PAR2, respectively, on their cell surfaces (Figure 4A). Expression of the other relevant PAR (PAR2 in PAR1 knocked-down cells and PAR1 in PAR2 knocked-down cells) and TF remained unaltered in PAR1 siRNA– or PAR2 siRNA–transfected cells. Loss of PAR1 and PAR2 receptor functional expression in PAR1 siRNA–transfected and PAR2 siRNA–transfected, respectively, cells was evaluated in calcium signaling measurements. Mock-transfected fibroblasts showed an increase in intracellular Ca2+ signaling in response to PAR1 and PAR2 peptide agonists (Figure 4B). PAR2AP, but not PAR1AP, elicited an increase in intracellular Ca2+ in PAR1-silenced cells, and vice versa with PAR2-silenced cells (Figure 4B). These data provide strong evidence that the siRNA-targeted inhibition of PAR1 and PAR2 is very specific.
Evidence for selective PAR1 and PAR2 silencing in PAR1 or PAR2 siRNA–transfected cells. Fibroblasts were transfected with PAR1 or PAR2 siRNA as described in “Materials and methods.” (A) Sixty hours after the transfection, the cells were removed from the dish using EDTA solution (no trypsin), and the cell suspension was divided into 4 aliquots, and stained with TF mAB (TF9H10, 10 μg/mL), PAR1 mAB (ATAP2, 10 μg/mL), anti–rabbit PAR2 IgG (100 μg/mL), and appropriate control IgGs. The stained cells were subjected to FACS analysis. (B) Mock- and siRNA-transfected cells cultured in glass-chambered slides were loaded with Fluo-4 AM as described in “Materials and methods” and mounted on a microscope stage. After obtaining live fluorescence images for 30 to 45 seconds, control vehicle, or PAR1 or PAR2 agonist peptide (25 μM) was added to the cells and the imaging was continued for 5 minutes. The plots represent intracellular Ca2+ levels.
Evidence for selective PAR1 and PAR2 silencing in PAR1 or PAR2 siRNA–transfected cells. Fibroblasts were transfected with PAR1 or PAR2 siRNA as described in “Materials and methods.” (A) Sixty hours after the transfection, the cells were removed from the dish using EDTA solution (no trypsin), and the cell suspension was divided into 4 aliquots, and stained with TF mAB (TF9H10, 10 μg/mL), PAR1 mAB (ATAP2, 10 μg/mL), anti–rabbit PAR2 IgG (100 μg/mL), and appropriate control IgGs. The stained cells were subjected to FACS analysis. (B) Mock- and siRNA-transfected cells cultured in glass-chambered slides were loaded with Fluo-4 AM as described in “Materials and methods” and mounted on a microscope stage. After obtaining live fluorescence images for 30 to 45 seconds, control vehicle, or PAR1 or PAR2 agonist peptide (25 μM) was added to the cells and the imaging was continued for 5 minutes. The plots represent intracellular Ca2+ levels.
Next, we evaluated whether the loss of PAR1 or PAR2 expression affected FVIIa-induced TF mobilization. As shown in Figure 5A-B, loss of PAR1 expression had no effect on FVIIa-induced TF mobilization. As a control, PAR1 siRNA transfection was shown to block thrombin-induced TF mobilization. In contrast, inhibition of PAR2 expression impaired FVIIa-induced TF mobilization with no significant effect on thrombin-induced response. These data, coupled with the data obtained with antibodies that are described above, provide strong evidence that FVIIa-induced TF mobilization is mediated via PAR2 activation.
PAR2, and not PAR1, silencing attenuates FVIIa-mediated TF mobilization from the Golgi. Mock-transfected, or PAR1 or PAR2 siRNA–transfected cells were exposed to FVIIa (10 nM) or thrombin (10 nM) for 2 hours at 37°C. Thereafter, the cells were fixed, permeabilized, and immunostained for the TF and the golgin-97. Panel A depicts representative images of immunostaining and panel B shows quantified data (mean ± SEM from 3 to 4 experiments).
PAR2, and not PAR1, silencing attenuates FVIIa-mediated TF mobilization from the Golgi. Mock-transfected, or PAR1 or PAR2 siRNA–transfected cells were exposed to FVIIa (10 nM) or thrombin (10 nM) for 2 hours at 37°C. Thereafter, the cells were fixed, permeabilized, and immunostained for the TF and the golgin-97. Panel A depicts representative images of immunostaining and panel B shows quantified data (mean ± SEM from 3 to 4 experiments).
Both FVIIa and PAR2 are required for TF endocytosis
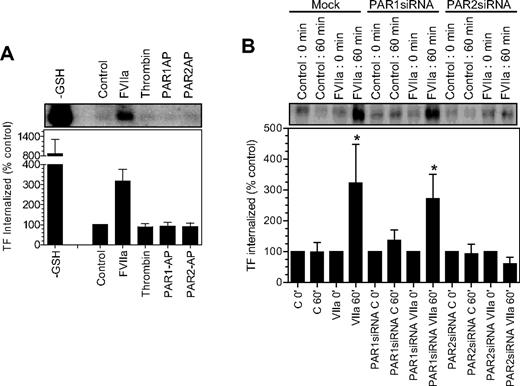
Our earlier findings22 showed that FVIIa binding to cell surface TF induces the endocytosis of TF. It is unclear whether FVIIa-induced TF endocytosis requires the activation of PAR1 or PAR2, and whether activation of PAR1 or PAR2, in the absence of FVIIa, can lead to TF endocytosis. To investigate whether PAR1 or PAR2 activation leads to TF endocytosis, as they mobilize TF from the intracellular pool, we evaluated the endocytosis of cell surface TF in fibroblasts exposed to control vehicle, FVIIa, thrombin, PAR1 AP, and PAR2 AP by labeling cell surface proteins with sulfo-NHS-S-S-biotin and then monitoring the levels of endocytosed biotin-labeled TF.22 Analysis of TF immunoprecipitates from fibroblasts showed only traces of biotinylated TF in control vehicle-, thrombin-, PAR1 AP–, and PAR2 AP–treated cells, whereas a significant amount of biotinylated TF in fibroblasts was exposed to FVIIa (Figure 6A). These data suggest that PAR1 or PAR2 activation alone is insufficient to induce TF endocytosis. Next, we investigated the role of PAR1 and PAR2 on FVIIa-induced TF endocytosis using mock-transfected fibroblasts and fibroblasts transfected with either PAR1 siRNA or PAR2 siRNA. As shown in Figure 6B, an identical pattern of TF endocytosis was observed with both mock-transfected cells and cells transfected with PAR1 siRNA (ie, traces of TF endocytosis in the absence of FVIIa and a 3- to 5-fold increase in TF endocytosis in cells exposed to FVIIa), suggesting that PAR1 is not involved in FVIIa-induced TF endocytosis. In contrast to this data, PAR2 silencing completely attenuated FVIIa-induced TF endocytosis. These data clearly illustrate that not only FVIIa binding to TF but the subsequent activation of PAR2 is essential for TF endocytosis.
Effect of PAR activation on tissue factor endocytosis. (A) Fibroblasts were surface-labeled with sulfo NHS-SS-biotin at 4°C and then incubated at 37°C for 1 hour with a control buffer or the buffer containing FVIIa (10 nM), thrombin (10 nM), PAR1 agonist peptide (50 μM), and PAR2 agonist peptide (50 μM). The cells were then treated with glutathione (50 mM) to remove the surface biotin, lysed, and immunoprecipitated with anti-TF beads. The immunoprecipitated samples were analyzed for the biotin to identify the internalized TF. TF biotin signal detected in cells that were treated with the reducing agent immediately following the biotinylation was taken as 100%. To show the extent of cell surface TF biotinylation, immunoprecipitates of cells that were not treated with the reducing agent were analyzed for the biotin label (labeled as −GSH). The top panel shows a representative blot and the bottom panel shows quantified data obtained by measuring the band intensity by densitometry (n = 3). (B) Mock, PAR1 siRNA–, or PAR2 siRNA–transfected cells were labeled with sulfo NHS-SS-biotin at 4°C and treated with a control vehicle or FVIIa (10 nM) for 1 hour at 37°C, and the cells were processed as in panel A. * denotes the values significantly differ (P < .05) from the TF internalized in the absence of FVIIa. The data shown in the figure represent mean ± SEM (n = 3 to 4 experiments).
Effect of PAR activation on tissue factor endocytosis. (A) Fibroblasts were surface-labeled with sulfo NHS-SS-biotin at 4°C and then incubated at 37°C for 1 hour with a control buffer or the buffer containing FVIIa (10 nM), thrombin (10 nM), PAR1 agonist peptide (50 μM), and PAR2 agonist peptide (50 μM). The cells were then treated with glutathione (50 mM) to remove the surface biotin, lysed, and immunoprecipitated with anti-TF beads. The immunoprecipitated samples were analyzed for the biotin to identify the internalized TF. TF biotin signal detected in cells that were treated with the reducing agent immediately following the biotinylation was taken as 100%. To show the extent of cell surface TF biotinylation, immunoprecipitates of cells that were not treated with the reducing agent were analyzed for the biotin label (labeled as −GSH). The top panel shows a representative blot and the bottom panel shows quantified data obtained by measuring the band intensity by densitometry (n = 3). (B) Mock, PAR1 siRNA–, or PAR2 siRNA–transfected cells were labeled with sulfo NHS-SS-biotin at 4°C and treated with a control vehicle or FVIIa (10 nM) for 1 hour at 37°C, and the cells were processed as in panel A. * denotes the values significantly differ (P < .05) from the TF internalized in the absence of FVIIa. The data shown in the figure represent mean ± SEM (n = 3 to 4 experiments).
Role of different signaling pathway inhibitors on the Golgi-TF trafficking
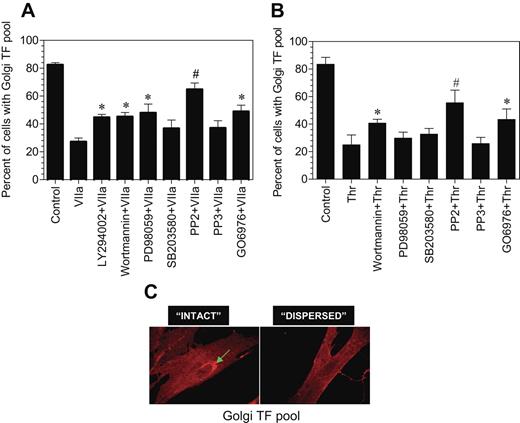
To ascertain the signaling events involved in transducing FVIIa-induced TF endocytosis and TF mobilization from the intracellular pool, we investigated the effect of various specific intracellular signaling inhibitors on both FVIIa-induced TF mobilization and endocytosis. Fibroblasts were first treated with the various inhibitors for 45 minutes and then exposed to FVIIa for 2 hours (for evaluating TF mobilization) or 1 hour (for TF endocytosis). PP2, a specific Src-kinase inhibitor, completely inhibited FVIIa-induced TF mobilization, whereas PP3, an inactive form of PP2, had no significant effect (Figure 7A). Wortmannin and LY294002, the cell permeable inhibitors of phosphatidylinositol 3-kinase, partly blocked the FVIIa-induced effect. PD98059, a specific inhibitor for p42/44 MAP kinase also partly inhibited the FVIIa-induced TF mobilization, whereas p38 MAP kinase inhibitor SB203580 had no significant effect. The PKC phosphorylation-specific inhibitor, Go6976, partly (but significantly) attenuated the FVIIa-induced Golgi TF trafficking. None of the inhibitors had any noticeable effect on the morphology of the Golgi or golgin-97 staining. We also investigated the effect of these signaling inhibitors on thrombin-induced TF mobilization. The inhibition profile was similar to that observed with FVIIa-treated cells except that p44/42 MAPK inhibitor partially reversed FVIIa-induced TF mobilization, whereas they had no significant effect on thrombin-induced TF mobilization (Figure 7B). We found no significant effect of any of these inhibitors on FVIIa-induced TF endocytosis (data not shown).
Effect of various intracellular signaling inhibitors on FVIIa and thrombin-induced TF mobilization. Fibroblasts were preincubated with inhibitors for 1 hour prior to the addition of FVIIa (A) or thrombin (B) (10 nM). Following FVIIa or thrombin treatment for 2 hours at 37°C, the cells were fixed, permeabilized, and immunostained for TF and the Golgi. Number of total cells and cells with intense TF staining in the Golgi were counted in multiple fields of 3 to 4 independent experiments, and the data were shown as percentage of cells with TF in the Golgi. Presence of intense TF staining in the perinuclear region (C, arrow mark) was taken as TF in the Golgi, and the absence of visible perinuclear staining was taken as no TF in the Golgi. In most of the observed cells, TF staining in perinuclear region was either intense or unnoticeable. The concentration of the inhibitors used are as follows: LY29042, 10 μM; wortmannin, 0.1 μM; PD98059, 50 μM; SB203580, 25 μM; PP2, 10 μM; PP3, 10 μM; and Go6976, 1.0 μM. * and # denote that these values differ from the values obtained with no inhibitor but treated with FVIIa with a P value of < .05 and < .01, respectively.
Effect of various intracellular signaling inhibitors on FVIIa and thrombin-induced TF mobilization. Fibroblasts were preincubated with inhibitors for 1 hour prior to the addition of FVIIa (A) or thrombin (B) (10 nM). Following FVIIa or thrombin treatment for 2 hours at 37°C, the cells were fixed, permeabilized, and immunostained for TF and the Golgi. Number of total cells and cells with intense TF staining in the Golgi were counted in multiple fields of 3 to 4 independent experiments, and the data were shown as percentage of cells with TF in the Golgi. Presence of intense TF staining in the perinuclear region (C, arrow mark) was taken as TF in the Golgi, and the absence of visible perinuclear staining was taken as no TF in the Golgi. In most of the observed cells, TF staining in perinuclear region was either intense or unnoticeable. The concentration of the inhibitors used are as follows: LY29042, 10 μM; wortmannin, 0.1 μM; PD98059, 50 μM; SB203580, 25 μM; PP2, 10 μM; PP3, 10 μM; and Go6976, 1.0 μM. * and # denote that these values differ from the values obtained with no inhibitor but treated with FVIIa with a P value of < .05 and < .01, respectively.
Discussion
In a recent study, we characterized the localization of TF in fibroblasts and defined specific changes in the cellular distribution of TF in response to FVIIa. These studies revealed that FVIIa binding to cell surface TF induced the endocytosis of TF but also mobilized intracellular TF from the Golgi store. In this study, we provide evidence that PAR2-mediated cell signaling plays a critical role in mediating both FVIIa-induced TF endocytosis and in the mobilization of intracellular TF from the Golgi to the cell surface. The data presented herein also show that activation of PAR1 or PAR2, independent of FVIIa binding to TF, mobilizes TF from the intracellular pool and thereby enhances TF expression at the cell surface. These observations suggest a novel role for PAR-medicated cell signaling in regulating TF expression on cell surfaces.
It is well known that agonist binding induces marked alterations in the subcellular distribution of G-protein–coupled receptors (GPCRs), including PAR1 and PAR2.37,38 However, little is known about whether or how activation of GPCRs modulates trafficking of other receptors. Our recent observations22 revealed marked differences in TF trafficking following its occupation with FVIIa or proteolytically inactive FVIIa. This, coupled with the knowledge that TF-FVIIa has been shown to induce cell signaling via activation of PARs,2 raised the possibility that PAR activation may regulate TF trafficking. Consistent with this notion, we found in this study that exposure of fibroblasts to PAR1 or PAR2 peptide agonists or proteases (eg, thrombin and trypsin) mobilized TF from the Golgi apparatus, and consequently increased the surface expression of TF. The data also show that mobilization of TF from the Golgi store is not linked to TF internalization since neither PAR1 nor PAR2 activation had any effect on TF endocytosis.
At present, there is no information on how PAR1 or PAR2 activation modulates trafficking of other receptors. It is interesting to note that both PAR1 and PAR2 also localize intracellularly in the Golgi apparatus in certain cell types.39,–41 Their activation at the cell surface by agonists not only induces the rapid endocytosis of the receptor but also mobilizes the receptor from the Golgi store to replenish the surface receptors.39,–41 Although the mechanisms that control and mediate trafficking of PARs from the Golgi to the plasma membrane remains to be determined, rab11a is thought to mediate agonist-induced trafficking of PAR2 since the PAR2 agonists stimulated redistribution of rab11a into vesicles containing PAR2 that migrated to cell surface.42 Rab11a is known also to mediate insulin-stimulated transport of GLUT4 from storage pools to the cell surface43 and to transport vesicular stomatitis virus G protein from the Golgi apparatus to the plasma membrane.44 These data suggest that rab11a may play a general role in mediating the transport of the storage proteins to the plasma membrane. Therefore, it is possible that the transport of TF from the Golgi store to the plasma membrane in response to FVIIa or PAR1/2 agonists is also mediated by rab11. Although data obtained with cycloheximide-treated cell is at some variance, other data suggest that TF localized in the Golgi represents stored TF and not newly synthesized TF. If so, TF appears to undergo regulated secretion in response to PAR1 or PAR2 activation. However, further proof is needed to establish this beyond doubt.
Extensive studies will be required to identify and characterize the signaling pathway/components responsible for TF mobilization. Nonetheless, our initial experiments described above using various signaling pathway inhibitors suggest that TF-VIIa–induced Src activation plays a key role in TF mobilization since the inhibitor of Src kinase, PP2, is most effective in attenuating the FVIIa-induced TF mobilization. Downstream to Src activation, activation of the p42/44 MAP kinase pathway may also play a role in TF mobilization, as suggested by the observation that the specific inhibitor for p42/44 MAPK impaired partly, but significantly, the FVIIa-induced response. These data are consistent with earlier findings that showed FVIIa-TF–induced cell signaling involves the activation of Src-like kinase45 and p44/42 MAPK pathway.46
PAR1 activation has been shown to induce the endocytosis of endoglin and type II TGF-β receptor in endothelial cells.47 TGF-β binding to the receptor is not required for the PAR1-induced endocytosis. In contrast to this, activation of PAR1 had no effect on TF endocytosis, either in the presence or absence of FVIIa. This suggests that PAR1 activation affects the endocytotic process of cell receptors in a selective fashion. Although PAR2 activation, similar to PAR1 activation, had no effect on TF endocytosis in the absence of FVIIa, FVIIa-induced TF endocytosis requires the activation of PAR2 since silencing the PAR2 gene using siRNA completely abrogated the FVIIa-induced TF endocytosis. This could explain why we failed in our earlier studies to detect internalized TF in fibroblasts exposed to inactive FVIIa (FFR-FVIIa),22 which binds to TF as effective as FVIIa and undergoes TF-dependent internalization13,14 but cannot activate PAR2.
Protein phosphorylation has been implicated as playing an important role in the regulation of endocytosis, and many receptors that undergo the process have been identified to be phosphorylated.48 Recent studies by Ahamed and Ruf49 showed that activation of PAR2, but not PAR1, induced the phosphorylation of the cytoplasmic domain of TF. Of more importance, the TF cytoplasmic domain phosphorylation is induced only by TF-dependent signaling but not by other coagulation factors. This may explain why PAR2 silencing and not PAR1 silencing impaired the FVIIa-induced TF endocytosis in the present study. However, we found no evidence for PAR2-depedent TF phosphorylation in fibroblasts. We were unable to detect, using TF phosphospecific antibodies provided by Ruf, phosphorylated TF in fibroblasts treated with FVIIa or with PMA, which was used as a positive control (data not shown). Moreover, the PKC inhibitor Go6976, which was shown to block TF cytoplasmic domain phosphorylation in earlier studies,49 had no effect on TF endocytosis. Therefore, it appears that PAR2-dependent TF phosphorylation, if it does occur, is not required for FVIIa-induced TF endocytosis. In this context, it may be pertinent to point out that recent studies showed that the c-tail of PAR2, which contains multiple phosphorylation sites and is an important determinant for arrestin interaction, was not required for the internalization of activated PAR2.50
It is also interesting to note that, as observed in our earlier study,22 only a fraction of total cell surface TF was endocytosed upon exposure to FVIIa. Here, it may be pertinent to note that FVIIa can bind to all available TF sites on the cell surface.51 It is unclear why only a few and not all TF sites occupied by FVIIa are endocytosed. One possible explanation is that few TF molecules are associated with PAR2, whose interaction is critical for inducing TF endocytosis, at the cell surface. An alternative explanation, which does not negate the above possibility, is that the specific localization of TF at the cell surface may be critical. Ultrastructural localization of TF in fibroblasts52 and smooth muscle cells (SMCs)53 showed that about 15% to 20% of TF in these cells was associated with caveolae. It is possible that TF localized in caveolae, but not at other cellular domains, is endocytosed upon binding to FVIIa. This explanation fits with our earlier observation that suggested FVIIa bound to TF in the absence of TFPI was internalized via LRP-independent, noncoated pit pathway.14
Externalization of TF from intracellular pools in response to stimuli could play an important role in hemostasis and thrombosis. FVIIa binding to TF or traces of thrombin formed at injury would increase TF concentration at the cell surface where and when it is needed. The ability of thrombin and other proteases, independent of FVIIa, to regulate externalization of TF via PAR-mediated cell signaling may play an important role in cells that express little or no TF at the cell surface but might have TF in intracellular storage compartments. In this regard, it may be pertinent to note that recent studies suggest that unstimulated monocytes20 and endothelial cells54 contain low levels of TF antigen in intracellular compartments, which are yet to be characterized. In addition to PAR-mediated cell signaling, activation of other signaling pathways may also modulate TF exocytosis. Recent studies showed that LPS stimulation of monocytes from high responders not only induced TF synthesis but also redistributed TF antigen from intracellular compartments onto the cell surface.20 High expression of TF activity in monocytes and other cells was thought to contribute to the pathogenesis of atherosclerosis and to acute coronary syndrome.55,56 Exocytosis of TF in response to pathological stimuli could further enhance aberrant expression of TF activity on the cell surface of these cells.
Overall the data we present here provide mechanistic insights into FVIIa-induced TF endocytosis and transport of intracellular TF to the cell surface. The data establish that TF mobilization from the Golgi store is independent of TF endocytosis and is mediated by FVIIa or other PAR-activating proteases. In contrast to TF exocytosis, TF endocytosis requires its occupancy by FVIIa as well as PAR2-mediated cell signaling. Thus, TF expression at cell surfaces at any given time may be controlled by many factors, including TF synthesis, TF endocytosis, and exocytosis of TF.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health HL58869 and HL 65550. S.K.M. is a recipient of a postdoctoral fellowship award from the American Heart Association, Texas Affiliate.
The authors thank Mark Atkinson for critically reading the paper.
National Institutes of Health
Authorship
Contribution: S.K.M. performed all the experiments reported herein, analyzed the data, and prepared a preliminary draft of the paper; U.R.P. participated in experimental design, data interpretation, and the preparation of the paper; and L.V.M.R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Biomedical Research, The University of Texas Health Center at Tyler, 11937 US HWY 271, Tyler, TX 75708; e-mail: vijay.rao@uthct.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal