To the editor:
Among the more than 100 published cases of Philadelphia chromosome–positive chronic myelogenous leukemia (Ph+ CML) investigated for the 1849G > T mutation of the Janus kinase 2 (JAK2V617F),1 no mutated cases have been found so far.2,3 Selected patients with Ph+ CML with marked thrombocytosis also proved to be negative for the JAK2V617F mutation.3 We report for the first time a case of coexisting JAK2V617F mutation in Ph+ CML evolving to myelofibrosis during imatinib treatment.
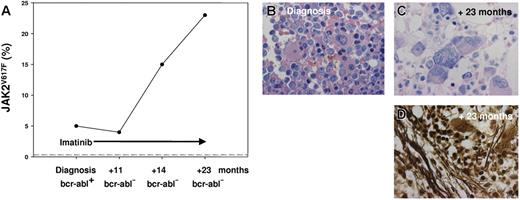
The 55-year-old white man presented with splenomegaly, leukocytosis (white blood cell [WBC] count of 163 × 109/L), a low hemoglobin (Hb) level (115 g/L), and an elevated lactate dehydrogenase (LDH) level (1337 U/L). A total of 14% blast cells in peripheral blood indicated acceleration. Peripheral blood smears revealed typical features of a CML, and molecular analysis showed a rare variant of bcr-abl fusion gene (e19a2). Imatinib therapy induced normalization of the patient's blood parameters within 11 months (WBC count, 5 × 109/L; Hb, 138 g/L; LDH, 232 U/L; and 0% blast cells), including cytogenetic and molecular remission, with no detectable BCR-ABL fusion transcripts in the bone marrow. Furthermore, splenomegaly was no longer present. Follow-up biopsies of the bone marrow 11, 14, and 23 months after initial diagnosis and onset of treatment revealed an increasing accumulation of megakaryoctes and focal deposition of argyrophilic fibers (Figure 1). Whereas histomorphology suggested persistence or relapse of the Ph+ CML clone, no BCR-ABL fusion transcripts could be detected in the bone marrow. Consequently, a JAK2V617F mutation was analyzed with a highly sensitive pyrosequencer assay,2,4 and in the initial biopsy, 5% mutated alleles were found, which increased to 15% and 23% after 14 and 23 months, respectively (Figure 1). During the first 23 months of follow-up red blood cell counts, Hb, platelets, and WBC counts remained stable and within normal range. When the initial pretherapeutic trephine was re-evaluated, minor focal fibrosis was obvious, but besides a BCR-ABL fusion, there was evidence for a small JAK2V617F-mutated clone encompassing 5% of alleles. In rare cases, JAK2V617F can occur simultaneously with the recently discovered thrombopoietin receptor point mutation in the oncogene of myeloproliferative leukemia (MPL 1544G > T/W515L),5,6 but pyrosequencing and direct sequencing revealed a MPL wild-type status in all biopsies.
JAK2VG17F allele frequency and bone marrow histology. (A) The percentage of mutated JAK2 alleles increased from 5% (prior to imatinib therapy) to 23% (after 23 months of imatinib therapy), while the initial BCR-ABL e19a2 fusion transcript became undetectable after 11 months of treatment. The dotted line indicates the JAK2 wild-type status of 125 control volunteers (87 bone marrow biopsies from patients with Ph+ CML and peripheral blood from 38 healthy blood donors). For the Pyrosequencer assay (Biotage, Uppsala, Sweden), 25 ng of formalin-fixed and paraffin-embedded (FFPE) bone marrow biopsy–derived DNA was used to generate a 102-bp JAK2 polymerase chain reaction (PCR) product (JAK2 forward, 5′-TATGATGAGCAAGCTTTCTCACAAG-3′; JAK2 reverse, 5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′; GenBank accession no. AL161450) and a 130-bp MPL product (MPL forward, 5′-ATCTCCTTGGTGACCGCTCTG-3′; MPL reverse, 5′-TGGTCCACCGCCAGTCTG-3′; GenBank accession no. U68161). The Pyrosequencer detects the pyrophosphate release per wild-type G or mutated T incorporation at JAK2 position 1849 or at MPL position 1544, respectively, by a connected enzyme cascade with a luciferase-induced light signal. The light signal intensity is proportional to the amount of G or T and allows a quantification of the mutated T allele (positive/negative controls: JAK2 mutant cell line HEL, MPL mutated patient sample, and JAK2/MPL wild-type cell line HL-60). (B) Pretherapy bone marrow trephine revealed increased cellularity with a prominent promyelocytic proliferation and typical micromegakaryocytes (Giemsa stain; original magnification, ×1000). (C) Posttherapy bone marrow trephine showed predominant megakaryocytic proliferation with large and bizarre megakaryocytes arranged in clusters (Giemsa stain; original magnification, ×1000). (D) Megakaryocytic proliferation was associated with a marked increase of argyrophilic fibers (silver stain; original magnification, ×1000). Images in panels B-D were produced with a BX51 microscope equipped with a 40×/0.8 Vplan/Apo objective, a DP50 digital camera, and DP3.1 software, all from Olympus (Hamburg, Germany).
JAK2VG17F allele frequency and bone marrow histology. (A) The percentage of mutated JAK2 alleles increased from 5% (prior to imatinib therapy) to 23% (after 23 months of imatinib therapy), while the initial BCR-ABL e19a2 fusion transcript became undetectable after 11 months of treatment. The dotted line indicates the JAK2 wild-type status of 125 control volunteers (87 bone marrow biopsies from patients with Ph+ CML and peripheral blood from 38 healthy blood donors). For the Pyrosequencer assay (Biotage, Uppsala, Sweden), 25 ng of formalin-fixed and paraffin-embedded (FFPE) bone marrow biopsy–derived DNA was used to generate a 102-bp JAK2 polymerase chain reaction (PCR) product (JAK2 forward, 5′-TATGATGAGCAAGCTTTCTCACAAG-3′; JAK2 reverse, 5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′; GenBank accession no. AL161450) and a 130-bp MPL product (MPL forward, 5′-ATCTCCTTGGTGACCGCTCTG-3′; MPL reverse, 5′-TGGTCCACCGCCAGTCTG-3′; GenBank accession no. U68161). The Pyrosequencer detects the pyrophosphate release per wild-type G or mutated T incorporation at JAK2 position 1849 or at MPL position 1544, respectively, by a connected enzyme cascade with a luciferase-induced light signal. The light signal intensity is proportional to the amount of G or T and allows a quantification of the mutated T allele (positive/negative controls: JAK2 mutant cell line HEL, MPL mutated patient sample, and JAK2/MPL wild-type cell line HL-60). (B) Pretherapy bone marrow trephine revealed increased cellularity with a prominent promyelocytic proliferation and typical micromegakaryocytes (Giemsa stain; original magnification, ×1000). (C) Posttherapy bone marrow trephine showed predominant megakaryocytic proliferation with large and bizarre megakaryocytes arranged in clusters (Giemsa stain; original magnification, ×1000). (D) Megakaryocytic proliferation was associated with a marked increase of argyrophilic fibers (silver stain; original magnification, ×1000). Images in panels B-D were produced with a BX51 microscope equipped with a 40×/0.8 Vplan/Apo objective, a DP50 digital camera, and DP3.1 software, all from Olympus (Hamburg, Germany).
This case demonstrates for the first time that BCR-ABL translocation and JAK2 mutation may be concomitantly detectable in hematopoietic cells of a single patient. The suppression of the Ph+ CML clone beyond the level of detection by imatinib therapy while the JAK2V617F-mutated alleles steadily increased argues in favor of 2 independently growing aberrant stem cell clones in this patient. The clinical and histopathologic diversity of Ph− chronic myeloproliferative disease (CMPD), encompassing polycythemia vera, essential thrombocythemia, and chronic idiopathic myelofibrosis, all of which share the JAK2V617F mutation, is still unexplained. Recently, it has been hypothesized by Kralovics et al7 that a varying combination of different molecular defects in 1 pathologic stem cell might be responsible for the phenotypic heterogeneity,7 but this case indicates that at least in a subfraction of patients, heterogeneity might also be caused by independently coexisting abnormal hematopoietic stem cell clones. Furthermore, persistent anemia or evolving myelofibrosis during imatinib treatment of Ph+ CML despite molecular suppression might be caused by a coexisting Ph− CMPD clone.
Authorship
Correspondence: Hans Kreipe, Institute of Pathology, Medizinische Hochschule Hannover, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany; e-mail: kreipe.hans@mh-hannover.de.
Supported by Deutsche Krebshilfe, Dr Mildred Scheel Stiftung 10-2191 (O.B., H.K.) and Deutsche Forschungsgemeinschaft—DFG BO 1954/1-1 (O.B., H.K.)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal