Abstract
HOX genes, MEIS1, and FLT3 are frequently up-regulated in human myeloid leukemias. Meis1 cooperates with Hox genes to induce leukemias in mice, hypothetically the consequence of Meis1-induced Flt3 overexpression. To test this, we compared the properties of Flt3−/− and Flt3+/+ progenitors transduced with Hoxa9 or Hoxa9/Meis1. In a myeloid clonogenic assay, Meis1 greatly enhanced the proliferation of Hoxa9-expressing cells, massively up-regulating Flt3 protein. However, the transforming potential of Hoxa9/Meis1 was unaltered in Flt3−/− cells. All mice that received Hoxa9/Meis1-transduced progenitors succumbed to rapid acute myeloid leukemias regardless of Flt3 genotype. Flt3 expression levels in leukemic blasts did not correlate with parameters reflecting their proliferative rate or their impaired differentiation. Furthermore, analysis of c-Myb expression levels in Hoxa9/Meis1-transformed cells showed that the up-regulation of this critical downstream effector was independent of Flt3. Altogether, our findings demonstrate that Flt3 is dispensable to the oncogenic cooperation of Meis1 with Hoxa9.
Introduction
HOX proteins are evolutionarily conserved homeodomain transcription factors that are involved in the regulation of hematopoietic differentiation and self-renewal. HOX genes are frequently up-regulated in leukemias and, clinically, those with the highest expression have been associated with a bad prognosis.1,2 The oncogenic activity of several HOX genes has been demonstrated in murine leukemia models; however, these leukemias develop with long latencies, suggesting that additional oncogenic events cooperate toward leukemogenesis. The HOX cofactor MEIS1 cooperates with HOX genes to accelerate the onset of acute myeloid leukemias (AMLs) in mouse models (reviewed in Abramovich and Humphries3 ). Interestingly, MEIS1 is frequently found up-regulated along with HOX genes in human leukemias and this is associated with particularly high levels of FLT3 mRNA.4 FLT3 is a tyrosine kinase receptor that is transiently expressed during hematopoietic stem cell maturation but whose expression is persistently up-regulated in AMLs. FLT3 signaling triggers cellular proliferation, and mutations that constitutively activate FLT3 are found in approximately 30% of AMLs. Activation of FLT3 signaling can also be triggered by the overexpression of wild-type FLT3, possibly through autocrine or paracrine stimulatory loops in leukemic cells secreting FLT3 ligand (FL) or independently of FL.5 Recently, Wang et al6 proposed a mechanism by which MEIS1 cooperates with HOXA9 to induce leukemias. Using mouse progenitors immortalized by Hoxa9, they showed that Meis1 coexpression induced the expression of Flt3 and the ability to proliferate in response to FL stimulation, suggesting that the acquisition of leukemogenic potential conferred by Meis1 could be mediated by Flt3 up-regulation.6 In another recent study, retroviral transduction of FLT3 was shown to cooperate with NUP98-HOX fusion genes in accelerating the onset of leukemias and, to some extent, recapitulate the leukemogenic cooperative effect of Meis1.7 To test whether the proleukemic effect of Meis1 was mediated by the up-regulation of Flt3, we studied the transforming potential of Meis1 in combination with Hoxa9 in the context of Flt3-deficient cells.
Materials and methods
Retroviral transduction of murine BM progenitors
Murine Hoxa9 was cloned in the MIE vector (MSCV-IRES-eGFP)8 or into the MSCVneo vector.9 Meis1a was cloned in the MSCVneo vector. The MSCV-Hoxa9-IRES-Meis1 vector and homozygous Flt3−/− mice were kindly provided by Guy Sauvageau and Ihor Lemischka,10 respectively. Lin−/low bone marrow (BM) cells were spinoculated as previously described.11
Recipient mice and characterization of leukemias
Lethally irradiated (9 Gy) 129/Sv mice received 60 × 103 to 70 × 103 infected progenitors and 105 129/Sv BM cells for radioprotection. Sick mice were analyzed as previously described.12 Tumor transplantability was assessed by injecting 105 leukemic BM cells into 2 to 3 C57Bl/6–Rag-2−/− mice.
Results and discussion
Meis1 synergizes with Hoxa9 to transform myeloid progenitors in culture independently of Flt3
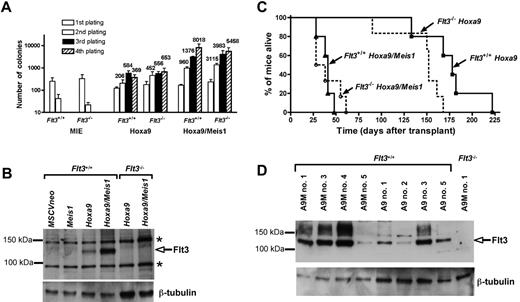
To determine whether Flt3 is essential for the cooperative oncogenicity of Meis1 with Hoxa9, we infected BM progenitors from Flt3−/− or control 129/Sv Flt3+/+ cells with retroviral vectors coding for Hoxa9 or Hoxa9 and Meis1. Transduced cells were serially replated in a myeloid colony-forming assay to compare their proliferative potential (Figure 1A). While Flt3−/− or Flt3+/+ cells infected with either Meis1 alone or an empty (MIE) vector ceased proliferating after 2 rounds of plating (data not shown), cells infected with Hoxa9 sustained high colony-forming ability and generated more than 500 quaternary colonies (per 104 cells seeded) regardless of Flt3 genotype. A drastic synergistic effect of Meis1 with Hoxa9 was observed generating more than 5000 quaternary colonies (per 104 cells) in both Flt3−/− and Flt3+/+ cells. This demonstrates that the ability of Meis1 to synergize with Hoxa9 in conferring to cells a very high clonogenic potential (> 50%) does not require Flt3.
Meis1 transforms myeloid progenitors in synergy with Hoxa9 independently of Flt3 expression. (A) Myeloid clonogenic assay performed on Flt3−/− or Flt3+/+ progenitors infected with the MIE control vector (MSCV-IRES-eGFP), Hoxa9 (MSCV-hoxa9-IRES-eGFP), or Hoxa9/Meis1 (MSCV-Hoxa9-IRES-Meis1). The number of colonies are shown per 103 (primary passage) or 104 seeded cells. Results are the mean ± SEM of 3 independent experiments. The colony assay was performed as previously described.11 (B) Flt3 expression in cell extracts from pooled primary colonies. Flt3+/+ cells infected with MSCVneo, Meis1 (MSCVneo:Meis1), or Hoxa9 (MSCVneo-Hoxa9) were seeded in selective media containing G418, whereas Flt3+/+ cells infected with Hoxa9/Meis1 (MSCV-Hoxa9-IRES-Meis1) or infected Flt3−/− cells were grown without antibiotic. The blot was stained with FLT3 (rabbit polyclonal SC-340; Santa Cruz Biotechnology, Santa Cruz, CA) or β-tubulin (mouse monoclonal; SIGMA, Saint Louis, MO) antibodies. Bands indicated * are nonspecific. (C) Survival curves showing the fraction of Hoxa9/Meis1 Flt3−/− (n = 6), Hoxa9/Meis1 Flt3+/+ (n = 5), Hoxa9 Flt3−/− (n = 6), and Hoxa9 Flt3+/+ (n = 5) mice alive following transplantation. (D) Leukemias induced by Hoxa9 and Hoxa9/Meis1 express variable amounts of Flt3. Western-blot analysis of BM from several Hoxa9/Meis1 (A9M) and Hoxa9 (A9) leukemic mice hybridized with the antibodies directed against FLT3 or β-tubulin. BM from mice A9M#1 engrafted with Hoxa9/Meis1-transduced Flt3−/− cells is shown as a negative control for Flt3 expression.
Meis1 transforms myeloid progenitors in synergy with Hoxa9 independently of Flt3 expression. (A) Myeloid clonogenic assay performed on Flt3−/− or Flt3+/+ progenitors infected with the MIE control vector (MSCV-IRES-eGFP), Hoxa9 (MSCV-hoxa9-IRES-eGFP), or Hoxa9/Meis1 (MSCV-Hoxa9-IRES-Meis1). The number of colonies are shown per 103 (primary passage) or 104 seeded cells. Results are the mean ± SEM of 3 independent experiments. The colony assay was performed as previously described.11 (B) Flt3 expression in cell extracts from pooled primary colonies. Flt3+/+ cells infected with MSCVneo, Meis1 (MSCVneo:Meis1), or Hoxa9 (MSCVneo-Hoxa9) were seeded in selective media containing G418, whereas Flt3+/+ cells infected with Hoxa9/Meis1 (MSCV-Hoxa9-IRES-Meis1) or infected Flt3−/− cells were grown without antibiotic. The blot was stained with FLT3 (rabbit polyclonal SC-340; Santa Cruz Biotechnology, Santa Cruz, CA) or β-tubulin (mouse monoclonal; SIGMA, Saint Louis, MO) antibodies. Bands indicated * are nonspecific. (C) Survival curves showing the fraction of Hoxa9/Meis1 Flt3−/− (n = 6), Hoxa9/Meis1 Flt3+/+ (n = 5), Hoxa9 Flt3−/− (n = 6), and Hoxa9 Flt3+/+ (n = 5) mice alive following transplantation. (D) Leukemias induced by Hoxa9 and Hoxa9/Meis1 express variable amounts of Flt3. Western-blot analysis of BM from several Hoxa9/Meis1 (A9M) and Hoxa9 (A9) leukemic mice hybridized with the antibodies directed against FLT3 or β-tubulin. BM from mice A9M#1 engrafted with Hoxa9/Meis1-transduced Flt3−/− cells is shown as a negative control for Flt3 expression.
To examine the effect of Meis1 on the level of Flt3 protein in the transformed progenitors, we performed Western-blot analyses of pooled primary colonies (Figure 1B). Compared with cells infected with Hoxa9, cells infected with Hoxa9/Meis1 expressed much greater amounts of Flt3, whereas no Flt3 was detected in cells infected with Meis1 alone. Thus, Meis1 acts in synergy with Hoxa9 to up-regulate Flt3.
To analyze the molecular pathways possibly underlying the Hoxa9/Meis1 oncogenic synergy, we studied the protooncogene c-Myb recently shown to be an important downstream target of Hoxa9 and Meis1.13 To explore whether c-Myb expression was affected by Flt3, we analyzed its level in pooled primary colonies (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Regardless of Flt3 genotype, we found that Hoxa9 cells expressed c-Myb very moderately, whereas a much stronger expression was seen in Hoxa9/Meis1 cells. This further demonstrates that mechanisms underlying leukemic transformation are independent of Flt3.
The acceleration of leukemia onset induced by Meis1 is independent of Flt3
To analyze whether Flt3 plays a role in Meis1's collaborative induction of leukemias with Hoxa9,14 progenitors from Flt3−/− or Flt3+/+ mice transduced with either Hoxa9 or Hoxa9/Meis1 were transplanted into irradiated histocompatible 129/Sv recipients. All mice that received Flt3−/− or Flt3+/+Hoxa9/Meis1-transduced cells rapidly developed aggressive AMLs with a mean latency of 40 days, whereas the latency of AMLs seen in Hoxa9 mice was longer than 150 days (Figure 1C). The absence of Flt3 did not delay the onset of either Hoxa9 or Hoxa9/Meis1 leukemias. The features of the AMLs are summarized in Table 1and in Figure S1 and Table S1. Whereas important interindividual variations were seen, there was no consistent trend between Flt3−/− and Flt3+/+ mice with regards to leukemic blast morphology. Interestingly, there was a large range of Flt3 expression among Hoxa9/Meis1 Flt3+/+ leukemias (Figure 1D; Table S1). However, we could not see any correlation between the frequency of Flt3-expressing cells, ranging from 16% to 81%, and aggressiveness of the disease, further indicating that Flt3 plays little role in the pathogenesis. Moreover, Flt3 genotype did not influence the transplantability of Hoxa9/Meis1 tumors, as injection of Flt3−/− or Flt3+/+ leukemic BM cells induced similar AMLs in all secondary recipients (data not shown).
Features of AMLs induced by Flt3+/+ or Flt3−/− BM infected by Hoxa9/Meis1 or Hoxa9 alone
| . | Survival, d . | WBC, ×109/L . | PLT, ×109/L . | Spleen weight, mg . |
|---|---|---|---|---|
| Flt3+/+Hoxa9/Meis1 | 39 ± 7 | 235 ± 144* | 283 ± 102 | 307 ± 127 |
| Flt3−/−Hoxa9/Meis1 | 40 ± 15 | 106 ± 36* | 231 ± 87 | 268 ± 121 |
| Flt3+/+Hoxa9 | 177 ± 32 | 91 ± 132 | 234 ± 48 | 494 ± 207 |
| Flt3−/−Hoxa9 | 150 ± 18 | 63 ± 42 | 262 ± 274 | 392 ± 128 |
| . | Survival, d . | WBC, ×109/L . | PLT, ×109/L . | Spleen weight, mg . |
|---|---|---|---|---|
| Flt3+/+Hoxa9/Meis1 | 39 ± 7 | 235 ± 144* | 283 ± 102 | 307 ± 127 |
| Flt3−/−Hoxa9/Meis1 | 40 ± 15 | 106 ± 36* | 231 ± 87 | 268 ± 121 |
| Flt3+/+Hoxa9 | 177 ± 32 | 91 ± 132 | 234 ± 48 | 494 ± 207 |
| Flt3−/−Hoxa9 | 150 ± 18 | 63 ± 42 | 262 ± 274 | 392 ± 128 |
Values shown are mean ± SD.
WBC indicates white blood cell count; and PLT, platelet count.
The incidence of Flt3 genotype on leukocytosis could not be confirmed by comparing the WBC of secondary recipients that received Flt3−/− and Flt3+/+Hoxa9/Meis1 (data not shown).
All mice that received Hoxa9-transduced BM cells eventually developed AMLs (Figure 1C). Once established, the Hoxa9 AMLs displayed similar phenotypic characteristics to those induced by Hoxa9/Meis1 and again were not influenced by Flt3 genotype. Interestingly, some Flt3 expression was detected in the leukemic BM of most Flt3+/+Hoxa9 mice, possibly resulting from somatic mutations that occurred to trigger the onset of AMLs in collaboration with Hoxa9.
Altogether, our results demonstrate that Flt3 is dispensable for the induction of aggressive leukemias by the concomitant expression of Hoxa9 and Meis1. Although we cannot rule out that certain signaling pathways possibly downstream of another fms-related kinase may be activated to compensate for the absence of Flt3 and may mediate Hoxa9/Meis1-induced transformation in the Flt3−/− mice, our findings nonetheless provide grounds to question the relevance of therapeutically targeting FLT3 to fight myeloid leukemias associated with an up-regulation of HOXA9 and MEIS1.
Authorship
Contribution: E.M. performed research and analyzed data; S.A. performed research; and C.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Lavau, CNRS UMR 7151, Hôpital Saint-Louis, 1 Ave Claude Vellefaux 75010 Paris, France; e-mail: catherine.lavau@paris7.jussieu.fr.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We are very grateful to Ihor Lemischka for the Flt3−/− mice, to Guy Sauvageau for the Hoxa9/Meis1 retroviral vector, and to Marika Pla and the Animal Experimentation Department of the Institut Universitaire d'Hematologie (IUH) for mouse husbandry.
This work was supported by grants from Groupement d'Intérêt Scientifique (GIS) maladies rares, Sang pour Cent La Vie, and the Ligue Comité de Paris. S.A. is a recipient of a Ministère de la Recherche fellowship; C.L. is an Institut National de la Santé et de la Recherche Médicale (INSERM) scientist.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal