Abstract
Foreign CpG-DNA from viruses and bacteria can activate memory B cells through binding to toll-like receptor 9, and this pathway has been hypothesized to be involved in the continuous activation of memory B cells ensuring life-long humoral immunity. In this study, we demonstrate that retinoic acid (RA) is a potent coactivator of this pathway in human B cells. RA enhanced the CpG-mediated proliferation of CD27+ memory B cells, and the proliferative response was accompanied by increased immunoglobulin (Ig) secretion indicative of plasma-cell formation. The RA-induced proliferation was preceded by enhanced expression of cyclin D3, and both the expression of cyclin D3 and the induced Ig secretion were found to be dependent on IL-10. Of importance, RA increased the CpG-induced phosphorylation of ERK1/2, p38MAPK, and IκB as early as 30 minutes after stimulation. By using specific inhibitors, all the RA-mediated events, including proliferation, cyclin D3 expression, IL-10 secretion, and Ig secretion, were shown to be dependent on p38MAPK. Hence, we propose that RA can strengthen humoral immunity by promoting CpG-mediated stimulation of CD27+ B cells via activation of p38MAPK resulting in increased proliferation and differentiation to Ig-secreting plasma cells.
Introduction
Vitamin A is important for an optimal functioning of the immune system, but the mechanisms involved are not fully understood. A part of the increased resistance to infection has been attributed to improved epithelial integrity, but a direct effect on cells of the immune system is also established.1–3 We and others have shown that all-trans retinoic acid has a stimulatory role in T lymphocytes.4–11 However, there has been some controversy regarding the importance of vitamin A for B-cell activity. Some reports document that retinoids are required for B-cell activity12 and have the potential to enhance differentiation and antibody responses of B cells,13–16 and that B-cell responses are impaired in vitamin A–deficient rats.17 We have previously shown that RA inhibits the proliferation of human and murine B-cell precursors18 as well as peripheral-blood B cells stimulated via B-cell receptor (BcR).19 We also demonstrated that the inhibition of B-cell proliferation was accompanied by inhibition of the cell-cycle machinery driving the cells from G1 to S phase.20
In the primary immune response, naive B cells proliferate and differentiate into plasma cells that secrete antibodies mainly of the immunoglobulin-M (IgM) class. During this process, somatic hypermutation and isotype switching take place, producing high-affinity antibodies of the IgG class.21 Some B cells embark on a different program to become long-lived memory B cells, which are characterized by lower threshold for activation and differentiation.22 In the past 2 decades, it has become clear that B cells can be activated by DNA from bacteria and viruses.23 The discrimination between human and bacterial/viral DNA is based on the fact that CpG dinucleotides are underrepresented and generally methylated in vertebrate DNA, while they are present at expected frequency and are unmethylated in bacteria and viruses.23,24 Unmethylated CpG DNA is recognized by toll-like receptor 9 (TLR9), which is primarily expressed in memory B cells and plasmacytoid dendritic cells.25–29 After binding of CpG to TLR9, a signaling cascade involving MyD88 is initiated that leads to phosphorylation and activation of the MAP kinases p38 and JNK1/2 followed by activation of AP-1 complexes.30,31 This pathway also conveys signals from CpG via TLR9 that lead to phosphorylation and inactivation of IκB, a protein that sequesters NF-κB proteins in the cytoplasm.30,31 In addition, a MyD88-independent pathway downstream of TLR9 has been proposed resulting in phosphorylation and activation of ERK1/2.30,31 Through continuous exposure of B cells to polyclonal activators such as CpG, the TLR9 signaling pathway has been implicated in the constant stimulation of memory B cells to ensure an adequate level of circulating antibodies long after infection or vaccination.32,33
In the present study, we demonstrate that RA has differential effect on proliferation of peripheral-blood B cells, depending on the pathway engaged in stimulating the cells. Thus, in accordance with our previous results,19 RA inhibited the proliferation of B cells stimulated via Igs, whereas the proliferation of CpG-stimulated memory B cells was enhanced even at low concentrations of RA, concomitant with differentiation into Ig-secreting plasma cells. We also provide a mechanistic explanation for how RA may promote CpG-mediated activation of memory B cells.
Material and methods
Reagents and antibodies
Modified CpG oligodeoxynucleotide phophorothionat (PS) 2006-5′TCGT-CGTTTTGTCGTTTTGTCGT3′ was purchased from MWG (Ebersberg, Germany) and dissolved in pyrogen-free water. Staphylococcus aureus Cowan I (SAC) was obtained from Calbiochem (La Jolla, CA). All-trans RA, TTNPB, Am580, and propidium iodide (PI) were from Sigma (St Louis, MO). Ro 41-5253 was kindly provided by Dr M Klaus (Hoffman-La Roche, Basel, Switzerland), and SR11217 was kindly provided by Dr M. I. Dawson (Burnham Institute, La Jolla, CA). Retinoids were dissolved in ethanol or DMSO, flushed with argon, and stored in lightproof containers at −20°C. Experiments with retinoids were performed in subdued light. Anti-pRB antibody (G3-254) was from BD Biosciences (San Jose, CA). Antibodies against p27Kip1 (C-19), cyclin D2 (C-17), cyclin E (HE12), cyclin A (C-19), and cdk6 (C-21) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–cyclin D3 (DCS-22) was purchased from Medical and Biological Laboratories (Woburn, MA), anti–α-tubulin (T9062) was from Sigma, and antiactin (C-2) was from Santa Cruz Biotechnology. Antibodies recognizing phospho-ERK1/2, ERK1/2, phospho-p38MAPK, p38MAPK, phospho-IκB, IκB, and phospo-JNK1/2 were obtained from Cell Signaling Technology (Beverly, MA). Blocking anti–IL-10 antibody and control Ig were purchased from R&D Systems (Minneapolis, MN). U0126, SB202190, and Bay11-7082 were purchased from Calbiochem and were dissolved in DMSO.
B-cell isolation
Resting human B lymphocytes were isolated from buffy coats of human blood donors obtained from the Blood Bank (Ullevål Hospital, Oslo, Norway) using anti-CD19 antibody-coated Dynabeads (Dynal Biotech, Oslo, Norway) as previously described.20 To detach the beads from the cells, rosetted cells were incubated with an equal volume of CD19 Detachabead (Dynal Biotech) for 1 hour at room temperature. CD27+ B cells (memory B cells) and CD27− cells (naive B cells) were isolated by positive and negative selection using CD27 Microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) and MS and LD columns (Miltenyi Biotech), respectively, according to the manufacturer.
Cell culturing
B cells were cultured in RPMI 1640 (Life Technologies, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum (FBS; Life Technologies), 2 mM glutamine, 125 U/mL penicillin, and 125 μg/mL streptomycin at 37°C in a humidified incubator with 5% CO2.
Measurement of DNA synthesis and cell division
Cells were cultured in flat-bottomed microtiter plates in triplicates at an initial density of 0.5 × 106/mL. After pulsing the cells with 1.25 μCi (0.046 MBq) [3H]thymidine (TRA120; Amersham, Buckinghamshire, United Kingdom) for the last 20 hours of a 72-hour incubation, incorporation was determined as previously described.4 CFSE labeling for determination of cell division was performed as previously described,5 except that a concentration of 0.15 μM CFSE was used.
Western blot analysis
Western blot analysis was performed as previously described.34 Briefly, B cells were lysed in radioimmunoprecipitation assay (RIPA) buffer for 30 minutes on ice, and protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA). Immunoprobing was carried out by use of standard techniques, and immunoreactive proteins were visualized with the enhanced chemiluminescence detection system (Amersham).
Immunofluorescent staining
Cells (1 × 105) were incubated with FITC-conjugated anti-IgD (DAKO, Glostrup, Denmark), FITC-conjugated anti-IgM (DAKO), or PE-conjugated anti-CD27 (BD Biosciences), or isotype-matched control antibody. After 15 minutes at room temperature in the dark, cells were washed twice with PBS/0.5% FBS, and 10 000 cells were analyzed by flow cytometry using a FACSCalibur flow cytometer and Cell Quest software (BD Biosciences).
ELISA
The level of IgG, IgM, and IL-10 in cell-free culture supernatants was determined in duplicate by enzyme-linked immunosorbent assays (ELISAs) specific for IgG and IgM (Bethyl Laboratories, Montgomery, TX) or IL-10 (R&D Systems) according to the manufacturer's recommendation. The absorbance at 450 nM was determined in an ELISA reader. The range of detection was 7.8 to 500 ng/mL for IgG and IgM and 7.8 to 500 pg/mL for IL-10.
Statistical analysis
SPSS13.0 for windows (SPSS, Chicago, IL) was used to perform the Wilcoxon signed-rank test and paired sample t test as indicated.
Results
RA stimulates cell-cycle progression and cell division of memory B cells
We have previously shown that human peripheral-blood B lymphocytes are inhibited by RA when stimulated through BcR.19,20 In the present study, we examined whether RA influences the signaling pathway in B cells activated through TLR9. Due to rapid degradation of phosphodiester DNA in culture, synthetic DNA with phosphorotionat backbone has been used in order to mimic the activation of B cells by bacterial and viral DNA.23,35 B cells from peripheral blood of human blood donors were cultured with CpG (1 μg/mL) in the presence or absence of RA (100 nM) for 3 days. For comparison, B cells were stimulated via BcR by SAC 0.005%. In accordance with our previous findings,19 RA inhibited SAC-mediated incorporation of thymidine. In contrast, RA significantly enhanced the thymidine uptake in cells stimulated with CpG (Figure 1A). Thus, the incorporation of [3H]thymidine was increased from 8861 ± 1461 cpm (mean ± SEM) to 25 769 ± 4556 cpm (mean ± SEM) (P < .01, n = 7) in the presence of RA. Cells stimulated with RA alone did not show enhanced DNA synthesis above control (data not shown). The optimal dose of RA on CpG-induced proliferation of B cells was then assessed. As shown in Figure 1B, we observed a steady rise in thymidine incorporation with increasing concentrations of RA reaching a plateau level at 1 to 1000 nM. In the subsequent experiments, RA was used at a concentration of 100 nM.
RA potentiates CpG-mediated DNA synthesis and proliferation of human memory B cells. (A) Peripheral-blood B cells (0.5 × 106/mL) were cultured in triplicates with medium alone or with CpG (1 μg/mL) or SAC (0.005%) in the presence or absence of RA (100 nM). [3H]thymidine was added for the last 20 hours of a 72-hour period, and thymidine incorporation was determined. Data represent mean cpm ± SEM of the median of triplicates (**P < .01, paired sample t test; n = 7). (B) B cells were cultured with CpG (1 μg/mL) in the presence or absence of increasing concentrations of RA, and thymidine incorporation was determined. The results are presented as mean cpm ± SD of triplicates from 1 representative experiment of 3. (C) Peripheral-blood B cells were fractionated into CD27+ or CD27− cells as described in “Materials and methods,” and cells of the fractionated and unfractionated populations were analyzed for the expression of CD27, IgD, and IgM as indicated. Isotype-matched control antibodies were included as negative controls (dotted lines), and the flow cytometry histograms represent 1 of 3 reproducible experiments. (D) CD27+ and CD27− B cells were cultured in medium alone or with CpG or SAC in the presence or absence of RA as described in panel A, and thymidine incorporation was determined. The results are presented as mean cpm ± SEM of the median of triplicates (*P < .05, Wilcoxon signed-rank test; **P = .01, paired sample t test; n = 7). (E) CD27+ B cells (0.5 × 106/mL) were labeled with CFSE, and cultured in medium alone or with CpG (1 μg/mL) with or without RA (100 nM). Cell division was analyzed by flow cytometry on successive days as indicated. During the acquisition, events were obtained for a fixed time so that the amplitude of the peak is proportional to the cell yield. The data were gated for living cells based on forward- and side-scatter distribution, and the results are from 1 representative experiment of 3.
RA potentiates CpG-mediated DNA synthesis and proliferation of human memory B cells. (A) Peripheral-blood B cells (0.5 × 106/mL) were cultured in triplicates with medium alone or with CpG (1 μg/mL) or SAC (0.005%) in the presence or absence of RA (100 nM). [3H]thymidine was added for the last 20 hours of a 72-hour period, and thymidine incorporation was determined. Data represent mean cpm ± SEM of the median of triplicates (**P < .01, paired sample t test; n = 7). (B) B cells were cultured with CpG (1 μg/mL) in the presence or absence of increasing concentrations of RA, and thymidine incorporation was determined. The results are presented as mean cpm ± SD of triplicates from 1 representative experiment of 3. (C) Peripheral-blood B cells were fractionated into CD27+ or CD27− cells as described in “Materials and methods,” and cells of the fractionated and unfractionated populations were analyzed for the expression of CD27, IgD, and IgM as indicated. Isotype-matched control antibodies were included as negative controls (dotted lines), and the flow cytometry histograms represent 1 of 3 reproducible experiments. (D) CD27+ and CD27− B cells were cultured in medium alone or with CpG or SAC in the presence or absence of RA as described in panel A, and thymidine incorporation was determined. The results are presented as mean cpm ± SEM of the median of triplicates (*P < .05, Wilcoxon signed-rank test; **P = .01, paired sample t test; n = 7). (E) CD27+ B cells (0.5 × 106/mL) were labeled with CFSE, and cultured in medium alone or with CpG (1 μg/mL) with or without RA (100 nM). Cell division was analyzed by flow cytometry on successive days as indicated. During the acquisition, events were obtained for a fixed time so that the amplitude of the peak is proportional to the cell yield. The data were gated for living cells based on forward- and side-scatter distribution, and the results are from 1 representative experiment of 3.
Peripheral-blood B cells consist of naive and memory cells, and these B-cell subgroups can vary considerably in their response to different stimuli.22,36–38 The effect of RA on these 2 B-cell subpopulations was therefore studied. B cells were fractionated based on the expression of CD27, a marker of memory B cells.39 As shown in Figure 1C, unfractionated cells (upper middle panel) consisted of 2 populations, one CD27 negative (CD27−) and one CD27 positive (CD27+). The percentage of CD27+ cells typically varied between 20% and 50% of the total B-cell population (data not shown). The CD27+ cells (Figure 1C right panels) were a mixture of IgD− and IgDlow cells, and they demonstrated variable expression of IgM. In contrast, the CD27− cells (Figure 1C left panels) were all IgDhigh and IgMlow. This pattern of expression is consistent with previous reports on surface markers of memory versus naive B cells.37,40 CD27− (naive) and CD27+ (memory) cells were stimulated with CpG or SAC in the presence or absence of RA. Both B-cell subpopulations responded to CpG with increased thymidine incorporation (Figure 1D), and as expected, memory B cells showed a stronger response than did the naive B cells, consistent with their higher expression of TLR9.41,42 Of more importance, RA markedly enhanced the CpG-mediated DNA synthesis of both B-cell populations (Figure 1D). Thus, RA more than doubled the thymidine incorporation of CpG-treated memory B cells from 22 954 ± 5437 cpm (mean ± SEM) to 51 658 ± 12 508 cpm (mean ± SEM) (P = .01, n = 7). In both cell populations, RA inhibited SAC-mediated proliferation, and of interest, the inhibition of B-cell proliferation was more pronounced in the naive cells.
The cell-division–tracking dye CFSE was then used to assess the amount of cell division. CpG increased the amount of cells that had undergone CFSE dilution, indicative of cell division (Figure 1E), and as evident from day 3, costimulation with RA markedly enhanced the number of cells that had divided (Figure 1E).
To confirm that the enhanced proliferation and division were not merely due to reduced cell death, the uptake of propidium iodide (PI) into dead cells was measured. In agreement with previous reports,43,44 CpG repressed spontaneous B-cell death, but RA did not induce any further inhibition of cell death (data not shown).
RA drives memory B cells into Ig-secreting plasma cells
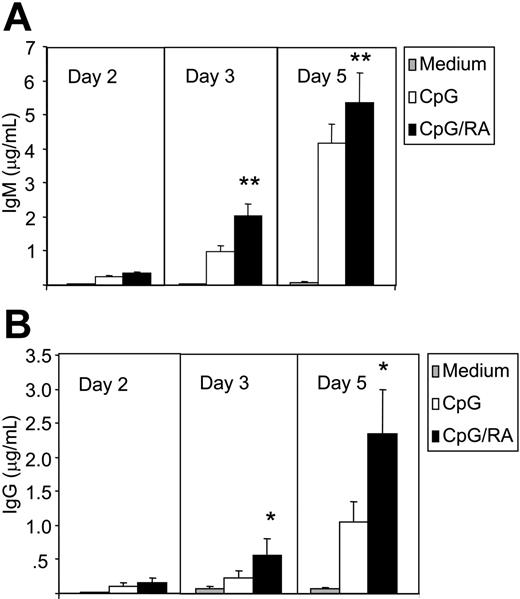
Next, we asked whether RA induces differentiation of memory B cells toward Ig-secreting plasma cells. As shown in Figure 2A-B, CpG stimulation of CD27+ B cells induced both IgM and IgG secretion. When stimulated with RA both IgM and, to a greater extent, IgG secretion was markedly enhanced after 3 and 5 days of culture (Figure 2A-B). Thus, RA enhanced the secretion of IgG from 1.1 ± 0.3 μg/mL to 2.3 ± 0.6 μg/mL (mean ± SEM) (P < .05, n = 7) at day 5. Increased IgG secretion was noted at concentrations as low as 1 nM (data not shown). Since the concentration of RA in plasma is about 5 to 10 nM,45 we concluded that physiologic concentrations of RA have the ability to enhance IgG secretion in CpG-stimulated B cells.
RA induces Ig secretion. CD27+ B cells (0.5 × 106/mL) were cultured in the presence or absence of CpG (1 μg/mL) and RA (100 nM). At consecutive days, cell supernatants from 100 000 cells were harvested and subjected to ELISA for the determination of IgM (A) and IgG (B). The data represent mean ± SEM of duplicate determinations (*P < .05, paired sample t test; **P ≤ .01, paired sample t test; n = 7).
RA induces Ig secretion. CD27+ B cells (0.5 × 106/mL) were cultured in the presence or absence of CpG (1 μg/mL) and RA (100 nM). At consecutive days, cell supernatants from 100 000 cells were harvested and subjected to ELISA for the determination of IgM (A) and IgG (B). The data represent mean ± SEM of duplicate determinations (*P < .05, paired sample t test; **P ≤ .01, paired sample t test; n = 7).
RA enhances the secretion of IL-10
Upon CpG stimulation of B cells, secretion of IL-6 and IL-10 is induced within a few hours.46–48 Since both cytokines are known to be involved in B-cell proliferation and differentiation,49 we asked whether RA could act by altering the level of these cytokines. Whereas IL-6 was rapidly up-regulated by CpG in CD27+ B cells, we could not detect any further increase upon treatment with RA (data not shown). CpG stimulation also induced the secretion of IL-10, and on day 3, RA significantly enhanced IL-10 protein secretion from 271 ± 59 pg/mL to 503 ± 139 pg/mL (mean ± SEM) (P < .05, n = 7) (Figure 3A). To test whether IL-10 production was essential for the RA-mediated induction of B-cell differentiation, we used a blocking anti–IL-10 antibody. Culturing the cells in the presence of the anti–IL-10 antibody clearly inhibited the increase in RA-mediated IgG secretion (Figure 3B).
RA enhances the secretion of IL-10 from CD27+ B cells. (A) CD27+ B cells (0.5 × 106/mL) were cultured with CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated days, cell supernatants were harvested and subjected to ELISA for determination of IL-10. The results represent mean ± SEM. (*P < .05, paired sample t test; n = 7). (B) CD27+ B cells were cultured with CpG (1 μg/mL) with or without RA (100 nM) in the presence or absence of anti–IL-10 antibody (0.5 μg/mL, α–IL-10) or control Ig antibody (0.5 μg/mL, Ig). Supernatants were harvested at day 3 and subjected to ELISA specific for IgG. The results represent mean ± SEM of 4 independent experiments.
RA enhances the secretion of IL-10 from CD27+ B cells. (A) CD27+ B cells (0.5 × 106/mL) were cultured with CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated days, cell supernatants were harvested and subjected to ELISA for determination of IL-10. The results represent mean ± SEM. (*P < .05, paired sample t test; n = 7). (B) CD27+ B cells were cultured with CpG (1 μg/mL) with or without RA (100 nM) in the presence or absence of anti–IL-10 antibody (0.5 μg/mL, α–IL-10) or control Ig antibody (0.5 μg/mL, Ig). Supernatants were harvested at day 3 and subjected to ELISA specific for IgG. The results represent mean ± SEM of 4 independent experiments.
The RA-mediated induction of cyclin D3 is dependent on IL-10
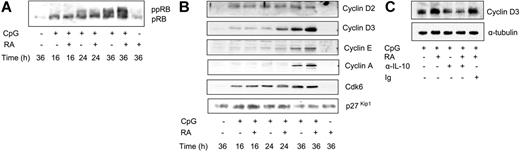
Phosphorylation of pRB is one of the key events in regulating transition of cells from G1 to S phase.50 pRb phosphorylation is catalyzed by cyclin-dependent kinases (cdks), which in turn are regulated by a fine-tuned balance between cyclins and cdk inhibitors (ckis).51 Previously, we found that addition of RA to SAC-stimulated peripheral-blood B cells led to a rapid increase in the expression of the cki p21Cip1, accompanied by reduced activity of cdk2, reduced phosphorylation of pRB, and cell-cycle arrest.20 This effect was assumed to be a direct effect of RA via a known RARE in the p21Cip1 promoter. Here, we demonstrate that CpG treatment of CD27+ cells increases the phosphorylation of pRB commencing at 24 hours (Figure 4A), and that RA further enhanced this phosphorylation (Figure 4A). As shown in Figure 4B, CpG treatment alone induced the expression of cyclin D2, cyclin D3, cyclin E, cyclin A, and cdk6, whereas the level of p27kip1 was only marginally affected. Of more importance, costimulation with RA induced a marked up-regulation of cyclin D3 expression (Figure 4B). The induction was observed after 24 hours of stimulation, and remained elevated at 36 hours of treatment. A slight increase in the expression of cyclin E and cyclin A was also observed after 36 hours, whereas the level of p27Kip1 was unchanged (Figure 4B). The protein level of p21Cip1 was not regulated by either CpG or RA (data not shown).
RA enhances phosphorylation of pRB and increases the expression of cyclin D3. (A-B) CD27+ B cells (1 × 106/mL) were stimulated with or without CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated time points, total-cell extract was prepared, and protein expression was determined by Western blot analysis. One representative experiment of 3 is shown. (C) CD27+ B cells (1 × 106/mL) were cultured as in panels A-B for 24 hours prior to harvesting. Total -cell extracts were subjected to Western blot analysis and examined for the expression of cyclin D3.
RA enhances phosphorylation of pRB and increases the expression of cyclin D3. (A-B) CD27+ B cells (1 × 106/mL) were stimulated with or without CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated time points, total-cell extract was prepared, and protein expression was determined by Western blot analysis. One representative experiment of 3 is shown. (C) CD27+ B cells (1 × 106/mL) were cultured as in panels A-B for 24 hours prior to harvesting. Total -cell extracts were subjected to Western blot analysis and examined for the expression of cyclin D3.
To test whether IL-10 was involved in the RA-mediated increase in cyclin D3, we cultured CD27+ B cells in the presence or absence of a blocking anti–IL-10 antibody. We noted a small reduction in CpG-mediated cyclin D3 expression in the presence of the anti–IL-10 antibody, whereas the potentiating effect of RA on cyclin D3 was completely abolished (Figure 4C). Thus, RA appears to enhance CpG-mediated expression of cyclin D3 via induction of IL-10.
RA enhances the early activation of the CpG stimulatory pathway
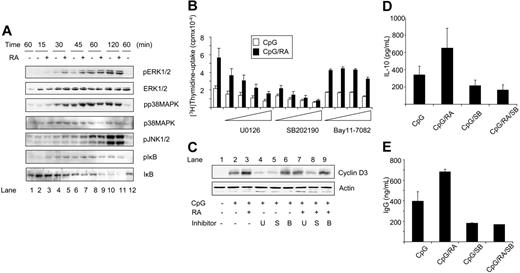
After binding of CpG to TLR9, signaling cascades are initiated involving activation of p38MAPK, JNK1/2, and ERK1/2 as well as phosphorylation and degradation of IκB.30,31 To test whether one or more of these proteins was activated by RA, Western blot analysis was performed using phosphospecific antibodies. CD27+ cells treated with CpG displayed increased phosphorylation of ERK1/2, p38MAPK, JNK1/2, and IκB as early as 30 minutes after stimulation (Figure 5A, compare lanes 1 and 4). RA enhanced the phosphorylation of both ERK1/2 and p38MAPK and to a smaller extent the phosphorylation and degradation of IκB (Figure 5A, compare lanes 4 and 5, and lanes 6 and 7). The effects of RA were transient, being most notable between 30 and 45 minutes. The phosphorylation of JNK1/2 was not regulated by RA (Figure 5A), and treatment with RA alone had no effect on any of the proteins (Figure 5A, lane 12).
RA modulates the activity of early signaling pathways in CpG-stimulated CD27+ B cells. (A) CD27+ B cells were treated with CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated time points, total-cell extracts were prepared, and protein levels were determined by Western blot analysis. Lane 1 indicates medium control, and for lane 12, cells were treated with RA alone. (B) CD27+ B cells (0.5 × 106/mL) were pretreated with U0126 (1, 3, 10, or 20 μM), SB202190 (0.5, 1, 2.5, or 5 μM), or Bay11-7082 (0.5, 1, 2, or 4 μM) for 60 minutes prior to stimulation with CpG (1 μg/mL) in the presence or absence of RA (100 nM), and thymidine incorporation was determined. The data represent mean cpm ± SEM of 5 independent experiments. (C) CD27+ B cells (1 × 106/mL) were pretreated with U0126 (U, 10 μM), SB202190 (S, 0.5 μM), or Bay11-7082 (B, 2 μM) for 60 minutes prior to stimulation with CpG (1 μg/mL) for 24 hours in the presence or absence of RA (100 nM). Total-cell extracts were subjected to Western blot analysis and examined for cyclin D3 expression. One representative experiment is shown. (D) CD27+ B cells (0.5 × 106/mL) were pretreated with SB202190 (0.5 μM) and cultured as described in panel B. Cell supernatants were collected at day 3 and subjected to ELISA specific for IL-10. The results represent mean IL-10 secretion ± SEM of 4 independent experiments. (E) CD27+ B cells were treated as in panel D, and supernatants were harvested at day 5 and subjected to ELISA specific for IgG. The data represent mean IgG secretion ± SD of 2 independent experiments. S indicates SB202190.
RA modulates the activity of early signaling pathways in CpG-stimulated CD27+ B cells. (A) CD27+ B cells were treated with CpG (1 μg/mL) in the presence or absence of RA (100 nM). At indicated time points, total-cell extracts were prepared, and protein levels were determined by Western blot analysis. Lane 1 indicates medium control, and for lane 12, cells were treated with RA alone. (B) CD27+ B cells (0.5 × 106/mL) were pretreated with U0126 (1, 3, 10, or 20 μM), SB202190 (0.5, 1, 2.5, or 5 μM), or Bay11-7082 (0.5, 1, 2, or 4 μM) for 60 minutes prior to stimulation with CpG (1 μg/mL) in the presence or absence of RA (100 nM), and thymidine incorporation was determined. The data represent mean cpm ± SEM of 5 independent experiments. (C) CD27+ B cells (1 × 106/mL) were pretreated with U0126 (U, 10 μM), SB202190 (S, 0.5 μM), or Bay11-7082 (B, 2 μM) for 60 minutes prior to stimulation with CpG (1 μg/mL) for 24 hours in the presence or absence of RA (100 nM). Total-cell extracts were subjected to Western blot analysis and examined for cyclin D3 expression. One representative experiment is shown. (D) CD27+ B cells (0.5 × 106/mL) were pretreated with SB202190 (0.5 μM) and cultured as described in panel B. Cell supernatants were collected at day 3 and subjected to ELISA specific for IL-10. The results represent mean IL-10 secretion ± SEM of 4 independent experiments. (E) CD27+ B cells were treated as in panel D, and supernatants were harvested at day 5 and subjected to ELISA specific for IgG. The data represent mean IgG secretion ± SD of 2 independent experiments. S indicates SB202190.
The RA-mediated induction of proliferation, cyclin D3 expression, and secretion of IL-10 and IgG are dependent on p38MAPK
To determine whether the induced phosphorylation of ERK1/2, p38MAPK, and IκB was required for the proliferative and the differentiating effect of RA, we used specific inhibitors of ERK1/2 (U0126), p38MAPK (SB202190), and NF-κB (Bay11-7082). Examined at day 3, the ERK1/2 inhibitor and the p38MAPK inhibitor decreased the DNA synthesis in a dose-dependent manner in cells stimulated with CpG alone (Figure 5B), without affecting cell viability (data not shown). The inhibitor of NF-κB affected CpG-mediated DNA synthesis only at a very high concentration (Figure 5B). There were, however, striking differences in the ability of the inhibitors to inhibit the RA-mediated induction of DNA synthesis. Whereas RA induced a 2.6-fold increase in DNA synthesis in the absence of inhibitors, the induction was reduced by 50% in the presence of the highest nontoxic concentration of the p38MAPK inhibitor (Figure 5B). The highest nontoxic concentration of the ERK1/2 inhibitor reduced the RA-mediated induction by only 20% (Figure 5B), whereas the NF-κB inhibitor had no effect on RA-mediated induction of DNA synthesis (Figure 5B). We next investigated whether the phosphorylation of ERK1/2, p38MAPK, or IκB was required for the increased cyclin D3 expression. As shown in Figure 5C, the ERK1/2 inhibitor decreased the CpG-mediated expression of cyclin D3, but did not prevent the ability of RA to enhance the cyclin D3 expression (Figure 5C lanes 4 and 7). The NF-κB inhibitor had only minor effect on the cyclin D3 levels (Figure 5C lanes 6 and 9), whereas the p38MAPK inhibitor markedly prevented the ability of RA to enhance CpG-mediated expression of cyclin D3 (Figure 5C lanes 5 and 8). Taken together, these data indicate that RA-mediated stimulation of the cell-cycle machinery and proliferation of CpG-stimulated CD27+ B cells are highly dependent on p38MAPK and to a lesser extent on ERK1/2, and that the effects of RA are independent of NF-κB.
To examine whether p38MAPK activation was required for the induction of IL-10, we measured IL-10 secretion in cells pretreated with the p38MAPK inhibitor SB202190. After 3 days of culture, SB202190 slightly reduced CpG-mediated induction of IL-10 (Figure 5D). Even more striking was the almost complete inhibition of IL-10 production in CD27+ B cells stimulated with CpG and RA in the presence of the p38MAPK inhibitor (Figure 5D). This indicates that RA-mediated IL-10 production in memory B cells is strictly dependent on p38MAPK. Then, we examined the effects of the p38MAPK inhibitor on IgG secretion. As shown in Figure 5E, RA markedly enhanced the secretion of IgG at day 5 from 396 ± 94 ng/mL (mean ± SD) to 683 ± 25 ng/mL (mean ± SD), whereas the p38MAPK inhibitor reduced the IgG secretion to 180 ± 6 ng/mL and 168 ng/mL in CpG- and CpG/RA-treated cells, respectively, indicating that p38MAPK also is required for RA-mediated induction of IgG.
The stimulatory effect of RA on memory B cells is mediated via RARs
The main function of RA is to regulate gene expression by binding to RARs and RXRs.1 To elucidate the involvement of RARs in the processes under study, we used various agonists of RARs and RXRs. As shown in Figure 6A, the pan-RAR agonist TTNPB and the RARα-specific agonist Am580 were as potent as RA in enhancing CpG-induced DNA synthesis. In contrast, the pan-RXR agonist SR11217 only marginally enhanced CpG-induced thymidine incorporation (Figure 6A). The involvement of RARs in RA-mediated proliferation of CD27+ B cells was confirmed by treating the cells with the RAR antagonist Ro 41-5253. As shown in Figure 6B-D, the antagonist almost completely blocked RA-mediated induction of DNA synthesis, cyclin D3 expression, and IgG secretion, strongly indicating that RARs are involved in RA-mediated induction of proliferation and differentiation.
RA stimulates memory B cells via RARs. (A) CD27+ B cells (0.5 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA, Am580 (AM), TTNPB (TT), or SR-11217 (SR) (all 100 nM) for 72 hours, and thymidine incorporation was determined. The data represent mean cpm ± SD of triplicates from 1 representative experiment of 3. (B) CD27+ B cells (0.5 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA (1 nM). Where indicated, cells were pretreated with Ro 41-5253 (500 nM) for 30 minutes prior to stimulation, and thymidine incorporation was determined after 3 days. The data represent mean cpm ± SD of triplicates from 1 representative experiment of 3. (C) CD27+ B cells (1 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA (1 nM or 100 nM) for 24 hours. Where indicated, cells were pretreated with Ro 41-5253 (500 nM) for 30 minutes prior to stimulation. Total-cell extracts were subjected to Western blot analysis and examined for cyclin D3 expression. One representative experiment is shown. (D) Cells were cultured as in panel B with or without RA (1 or 100 nM). At day 3, cell supernatants were harvested and subjected to ELISA for determination of IgG. The results represent mean IgG ± SD of duplicates of 1 representative experiment. Ro indicates Ro 41-5253.
RA stimulates memory B cells via RARs. (A) CD27+ B cells (0.5 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA, Am580 (AM), TTNPB (TT), or SR-11217 (SR) (all 100 nM) for 72 hours, and thymidine incorporation was determined. The data represent mean cpm ± SD of triplicates from 1 representative experiment of 3. (B) CD27+ B cells (0.5 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA (1 nM). Where indicated, cells were pretreated with Ro 41-5253 (500 nM) for 30 minutes prior to stimulation, and thymidine incorporation was determined after 3 days. The data represent mean cpm ± SD of triplicates from 1 representative experiment of 3. (C) CD27+ B cells (1 × 106/mL) were stimulated with CpG (1 μg/mL) in the presence or absence of RA (1 nM or 100 nM) for 24 hours. Where indicated, cells were pretreated with Ro 41-5253 (500 nM) for 30 minutes prior to stimulation. Total-cell extracts were subjected to Western blot analysis and examined for cyclin D3 expression. One representative experiment is shown. (D) Cells were cultured as in panel B with or without RA (1 or 100 nM). At day 3, cell supernatants were harvested and subjected to ELISA for determination of IgG. The results represent mean IgG ± SD of duplicates of 1 representative experiment. Ro indicates Ro 41-5253.
Discussion
Bacterial and viral products activate the cells of the immune system by binding to pattern-recognizing receptors such as TLRs.52,53 Human memory B cells possess a distinct expression profile of TLRs with high levels of TLR9, which binds CpG resulting in B-cell activation, proliferation, and differentiation.41 In the present study, we show that physiologic concentrations of RA enhance the proliferation and differentiation of human CD27+ peripheral-blood B cells stimulated by CpG. Furthermore, we demonstrate that RA-mediated early activation of p38MAPK is required for induction of IL-10 secretion, which in turn is necessary for the enhanced differentiation into Ig-secreting plasma cells.
The important role of retinoids for development of a competent immune system has been underscored by numerous studies in humans as well as in animal models,2,3,54 and the stimulatory function of RA for human T lymphocytes has been established by our own studies4,5,11 as well as by others.6–10 However, the role of retinoids for the function of B lymphocytes has been more difficult to reveal, and most in vitro studies have reported inhibition of proliferation.19,55–57 In the majority of these studies, including our own,19 B cells were stimulated via cell-surface Ig receptors or via CD40 or CD38. It was therefore surprising to find that memory B cells stimulated by CpG were not inhibited by RA. On the contrary, in these cells RA induced both proliferation and differentiation into Ig-secreting plasma cells.
One of the key findings in the present study was that RA induced secretion of IL-10 in the CpG-stimulated memory B cells, and that blocking the downstream effects of IL-10 inhibited both RA-mediated cyclin D3 expression and Ig production. The IL-10 gene has no consensus RAR- or RXR-binding elements in its promoter, indicating that its induction by RA is indirect. The early activation of p38MAPK was of particular interest in this respect, since specific inhibition of this kinase completely prevented RA-mediated induction of IL-10 and IgG secretion and markedly reduced cyclin D3 expression and cell proliferation. Rapid activation of p38MAPK by RA has previously been reported in keratinocytes, but in these cells RA alone was able to activate the kinase.58 In our experiments, RA enhanced the phosphorylation of p38MAPK only in combination with CpG. This indicates that RA has a more costimulatory role in B cells. We also found that RA enhanced the CpG-induced phosphorylation of ERK1/2, as well as the phosphorylation and degradation of IκB. However, the specific inhibition of either the ERK1/2 or NF-κB pathways had only minor effect on RA-driven cyclin D3 expression and proliferation of the memory B cells, again pinpointing the key role of p38MAPK in the process under study.
More than 100 genes have been reported to be directly regulated by RA, and several hundred genes are indirectly regulated by crosstalk between RARs/RXRs and other nuclear receptors.59 Still, there is increasing evidence that RA may also have transcription-independent functions. Early activation of PKC60,61 and Akt62 has been found to be regulated by RA, but it remains unclear whether RARs and/or RXRs are involved in these processes. Although RARs are predominantly localized in the nucleus,1 certain isoforms of RARs have been reported to have cytoplasmatic localization,63 which might explain how RA can regulate enzyme activation in a transcription-independent manner. It should also be noted that RAR/RXR-independent functions of RA have been reported.64 In the present study, we used specific RAR agonists and antagonists to demonstrate that the effects of RA on cyclin D3 expression and proliferation and IgG secretion in CpG-treated memory B cells are all mediated via RARs. We cannot yet conclude, however, that RARs are involved in the early activation of p38MAPK. In a series of experiments, we observed a slight increase in p38MAPK phosphorylation when CpG-stimulated cells were cotreated with the RAR agonist TTNPB and Am580, and the RAR antagonist Ro 41-5253 caused a small, but reproducible inhibition of RA-mediated induction of p38MAPK phosphorylation (data not shown). The latter experiments are made more difficult to interpret since the antagonist has to be used with low concentrations of RA (1-10 nM) due to its toxicity. The exact mechanism for how RA activates p38MAPK in CpG-treated memory B cells is currently under study in our laboratory. However, the rapid (within 15-30 minutes) effect of RA on phosphorylation of p38MAPK makes it unlikely that RAR-mediated transcription is involved in this process. A model for how RA might stimulate proliferation and differentiation of human memory B cells is summarized in Figure 7.
Model for RA-mediated activation of memory B cells. The question mark indicates that a formal proof for a direct connection is lacking.
Model for RA-mediated activation of memory B cells. The question mark indicates that a formal proof for a direct connection is lacking.
An important finding was that RA both stimulated a rapid burst of proliferation and induced memory B cells to differentiate into Ig-secreting plasma cells. This is consistent with the generally accepted view that cell division is required for the generation of plasma cells.65,66 However, we have not so far been able to determine whether the proliferation induced by RA is required for driving the CpG-stimulated memory B cells into Ig-secreting cells. The RA-mediated early activation of p38MAPK phosphorylation was necessary for IL-10 and IgG secretion, and cyclin D3 expression and proliferation, and IL-10 was in turn essential for both cyclin D3 expression and IgG secretion. Cyclin D3 plays an important role in B-cell proliferation,67 but although some reports point to an important function of cyclin D3 in the differentiation of certain cell types,68–70 its contribution to differentiation of B cells is still unresolved. One way to elucidate whether cyclin D3 is required for the enhanced differentiation of B cells is to knock down cyclin D3 by using siRNA. Yet, so far we have not been able to successfully use siRNA in our cultures of primary human B cells.
Two recent reports demonstrate B-cell differentiation in the presence of RA, and in these reports the induced differentiation was associated with inhibition of proliferation.56,71 In these studies, tonsillar B cells were stimulated via CD40/L cells and IL-10,71 or murine splenic B cells were stimulated via BcR and CD38.56 In our previous report on BcR-stimulated peripheral-blood B cells, the RA-mediated inhibition of proliferation was not associated with differentiation of the cells.19 Taken together, this strongly indicates that the effects of RA depend not only on the source of B cells used, but also on the way the cells are stimulated. This emphasizes the importance of using defined cell populations when performing in vitro studies on human B cells, but even more important, it reveals the distinct effects of RA on functionally different subpopulations of B cells.
The results of this study may have important physiologic implications. In a current model of humoral memory, it is proposed that periodic activation of memory B cells by polyclonal stimulation produces antibodies specific for immunogenes of past immune responses.32,33 Our demonstration of RA's ability to enhance both proliferation and differentiation of polyclonal activated memory B cells into Ig-secreting effector cells suggests that RA may have an important role in maintaining such long lived humoral memory.
Authorship
Contribution: A.E. and H.K.B. designed research; A.E., H.-C.A., and S.N. performed research; A.E., H.-C.A., S.N., and H.K.B. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heidi Kiil Blomhoff, Department of Biochemistry, Institute of Basic Medical Sciences, University of Oslo, PO Box 1112 Blindern, N-0317 Oslo, Norway; e-mail h.k.blomhoff@basalmed.uio.no.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Norwegian Cancer Society, The Norwegian Research Council, The Freia Research Foundation, The Jahre Research Foundation, The Blix Family Legacy, and The Rakel and Otto Christian Bruuns Legacy.
We thank Camilla Solberg, Hilde R. Haug, and Hanne Sagsveen Hjorthaug for excellent technical assistance.

![Figure 1. RA potentiates CpG-mediated DNA synthesis and proliferation of human memory B cells. (A) Peripheral-blood B cells (0.5 × 106/mL) were cultured in triplicates with medium alone or with CpG (1 μg/mL) or SAC (0.005%) in the presence or absence of RA (100 nM). [3H]thymidine was added for the last 20 hours of a 72-hour period, and thymidine incorporation was determined. Data represent mean cpm ± SEM of the median of triplicates (**P < .01, paired sample t test; n = 7). (B) B cells were cultured with CpG (1 μg/mL) in the presence or absence of increasing concentrations of RA, and thymidine incorporation was determined. The results are presented as mean cpm ± SD of triplicates from 1 representative experiment of 3. (C) Peripheral-blood B cells were fractionated into CD27+ or CD27− cells as described in “Materials and methods,” and cells of the fractionated and unfractionated populations were analyzed for the expression of CD27, IgD, and IgM as indicated. Isotype-matched control antibodies were included as negative controls (dotted lines), and the flow cytometry histograms represent 1 of 3 reproducible experiments. (D) CD27+ and CD27− B cells were cultured in medium alone or with CpG or SAC in the presence or absence of RA as described in panel A, and thymidine incorporation was determined. The results are presented as mean cpm ± SEM of the median of triplicates (*P < .05, Wilcoxon signed-rank test; **P = .01, paired sample t test; n = 7). (E) CD27+ B cells (0.5 × 106/mL) were labeled with CFSE, and cultured in medium alone or with CpG (1 μg/mL) with or without RA (100 nM). Cell division was analyzed by flow cytometry on successive days as indicated. During the acquisition, events were obtained for a fixed time so that the amplitude of the peak is proportional to the cell yield. The data were gated for living cells based on forward- and side-scatter distribution, and the results are from 1 representative experiment of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-09-046748/4/m_zh80090700010001.jpeg?Expires=1763470860&Signature=EEJ3AKSK8d7h8dQD4XBnWivxWTJxI5Xdc6Q0MJrMRkbf0zYmMHmSQhV2qPJmurXwLKcrG7gX4kVaUUTBwME0b1NJyWEWx0ef0wqnDVHkjD11BserSgqMGzlYkyzv1ySST3~uLTTkGfLxVEDb9MmLkkVI0C10XFCqE5wU0AsIEYdMdS-qDHaeiu74sirFXskMyCEDdPSAb-pQtJs2rTyHDAt3TM6OS2gGjkAWfd7vdqQTR-a9peDPVn4OCeWUvBoB5~VtYFogalB6rVa1X3d5S0KHHU8BPdVvgt~Lb1TShXUU1TODu1T-Ev0xU07tvpvWhsFgAP9FjZpHfrTau5c9bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal