In this issue of Blood, Subramanian and colleagues provide new insight into the function of PTEN and demonstrate that deletion of this 3′ inositol phosphate phosphatase renders neutrophils hyperresponsive to low concentrations of chemoattractants.
Accumulation of neutrophils at sites of infection is essential for host defense, and tight regulation of cell activation is essential for control of the inflammatory response. It is well established that neutrophil chemoattractants, such as fMLP, act via G protein–coupled receptors and that the lipid products of PI3 kinase, PI(3,4,5)P3 and PI(3,4)P2, are essential signaling intermediates that accumulate at the leading edge of motile cells. During migration, local enrichment of 3′-phosphoinositides is enhanced by reciprocal accumulation of phosphatase and tensin homologue (PTEN) at the rear, where it catalyzes the conversion of PI(3,4,5)P3 into PI(4,5)P2.
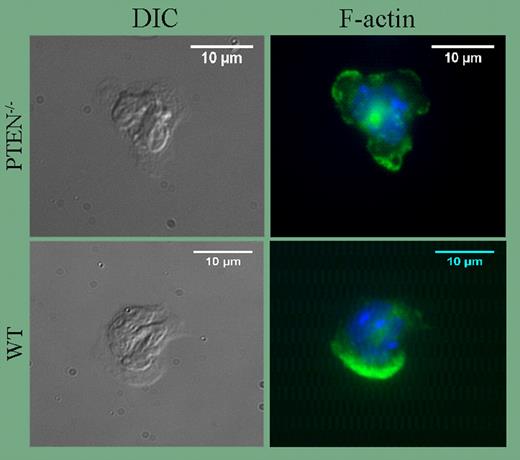
In their current study, Subramanian and colleagues used the Cre/lox system to generate PTEN-null neutrophils. The results of this study are of considerable interest since they demonstrate for the first time that when PTEN is absent, neutrophils are exquisitely sensitive to activation by fMLP and other chemoattractants. Thus, concentrations of fMLP, C5a, and IL-8 that have little or no effect on wild-type cells trigger robust ruffling and polarization of PTEN-null neutrophils (see figure), and cell migration in vivo and in vitro is significantly enhanced. Basal and stimulated rates of superoxide synthesis are also elevated. In this regard, it is noteworthy that the altered responsiveness of mutant neutrophils is diminished by priming, and is nearly ablated as agonist concentrations reach saturation. Thus, the data suggest PTEN, via its ability to prevent accumulation of PI(3,4,5)P3 and PI(3,4)P2, may be an essential regulator of polymorphonuclear leukocyte cell (PMN) quiescence that suppresses basal responsiveness as a means to prevent untoward inflammatory tissue damage.
Formation of multiple pseudopodia in PTEN−/− neutrophils. See the complete figure in the article beginning on page4028.
Formation of multiple pseudopodia in PTEN−/− neutrophils. See the complete figure in the article beginning on page4028.
In this regard, it is noteworthy that neutrophils that lack the SH2-domain–containing 5′-inositol phosphatase (SHIP) have a distinctly different phenotype that in many respects is the opposite of cells lacking PTEN.1 Unstimulated SHIP-null neutrophils are spread and resemble fried eggs, whereas wild-type and PTEN-null cells are round. Moreover, cell polarization is nearly ablated by deletion of SHIP. Indeed, overall neutrophil morphology is largely unaffected by chemoattractants such as fMLP, and PI(3,4,5)P3 and PI(4,5)P2 accumulate throughout the plasma membrane. As polarity is an essential prerequisite for chemotaxis, it is perhaps not surprising that migration of SHIP-null neutrophils is profoundly impaired. Conversely, PI(3,4,5)P3 and PI(4,5)P2 accumulate transiently throughout the plasma membrane of wild-type cells and neutrophils and then become enriched at the leading edge as cells polarize.1
Of note, Nishio et al1 also generated PTEN-null neutrophils, and in their hands these cells had no apparent phenotype. However, concentrations of fMLP used in their study were relatively high, and as such the hyperresponsiveness described by Subramanian and colleagues may have been masked. Nevertheless, the results of both studies show that PTEN is dispensable for directional sensing by neutrophils, which contrasts sharply with the phenotype of PTEN-null Dictyostelium.1–2
The data discussed here demonstrate definitively that PTEN and SHIP serve nonredundant functions in neutrophils. At the same time, many important questions remain unanswered. For example, the extent to which downstream effectors are altered by deletion of PTEN or SHIP is unclear and whether transient formation of 2 pseudopodia in PTEN-null neutrophils (see figure) indicates decreased stability of the leading edge remains to be determined.
Conflict-of-interest disclosure: The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal