Abstract
Granulocyte colony-stimulating factor (G-CSF) regulates the production, maturation, and function of neutrophils. Its expression is often induced during infection, resulting in high concentrations of G-CSF in inflammatory exudates and in the blood, suggesting that it may regulate both local and systemic neutrophil responses. Herein, we characterize the neutrophil response in G-CSFR−/− mice following intratracheal injection with Pseudomonas aeruginosa–laden agarose beads, modeling the pulmonary infection observed in many patients with cystic fibrosis. G-CSFR−/− mice are markedly susceptible to bronchopulmonary P aeruginosa infection, exhibiting decreased survival and bacterial clearance as well as extensive damage to lung tissue. The systemic neutrophil response was mediated primarily by enhanced neutrophil release from the bone marrow rather than increased neutrophil production and was attenuated in G-CSFR−/− mice. Despite normal to increased local production of inflammatory chemokines, neutrophil accumulation into the infected lung of G-CSFR−/− mice was markedly reduced. Moreover, the percentage of apoptotic neutrophils in the lung was elevated, suggesting that G-CSF signals may play an important role in regulating neutrophil survival at the inflammatory site. Collectively, these data provide new evidence that G-CSF signals play important but specific roles in the regulation of the systemic and local neutrophil response following infection.
Introduction
Granulocyte colony-stimulating factor (G-CSF) is a hematopoietic cytokine widely used to treat neutropenic patients in a wide range of clinical settings.1,2 G-CSF stimulates the proliferation of granulocytic precursors, enhances their terminal differentiation, and stimulates their release from the bone marrow.2 In addition, G-CSF modulates the survival and function of mature neutrophils.3 The importance of G-CSF in the regulation of granulopoiesis was confirmed by the characterization of G-CSF– and G-CSF receptor (G-CSFR)–deficient mice.4-6 These mice display chronic neutropenia with a uniform decrease in granulocytic precursors in the bone marrow. Recent studies suggest that both decreased neutrophil production and impaired release from the bone marrow contribute to the peripheral blood neutropenia.7 These studies establish G-CSF as the principal cytokine regulating basal granulopoiesis.
G-CSF expression is often induced during infections, resulting in high levels both systemically (ie, in the plasma) and locally in inflammatory fluids in both mice8,9 and in humans.10-12 However, the importance of G-CSF in regulating the stress granulopoiesis response is controversial. G-CSF−/− mice infected intravenously with Candida albicans or intraperitoneally with Listeria monocytogenes demonstrated a neutrophilia that matched that of wild-type littermates, suggesting a nonessential role for G-CSF in mediating stress granulopoiesis.13,14 In contrast, G-CSF−/− mice infected intravenously with L monocytogenes demonstrated reduced neutrophil recruitment into the blood compared with wild-type littermates.4 Of importance, each of these models used large doses of infectious agents administered parenterally. Thus, their relevance to the more common local-regional infections seen in the clinical setting is unclear.
Pseudomonas aeruginosa is a Gram-negative organism responsible for a high percentage of nosocomial lower respiratory tract infections and is also a leading cause of mortality in patients with cystic fibrosis. In patients with cystic fibrosis, P aeruginosa often produces a chronic pulmonary infection, leading to excessive inflammation and lung injury. The chronic recruitment of neutrophils into the lung parenchyma, while important in the clearance of bacteria, may also contribute to the excessive inflammation. The role of G-CSF in maintaining the delicate balance between bacterial clearance and inflammation is unknown.
To address these issues, we studied the immune response in G-CSFR−/− mice following endobronchial infection with P aeruginosa–laden agarose beads. We previously showed that this procedure produces a subacute bronchopulmonary P aeruginosa infection that reproduces many of the features found in patients with cystic fibrosis.15 Herein, we show that G-CSFR−/− mice are highly susceptible to this infection and develop extensive tissue damage in the lung. Evidence is provided suggesting that G-CSF regulates specific aspects of the immune response that contribute to both bacterial clearance and local inflammation.
Materials and methods
Animals
G-CSFR−/− mice inbred onto a C57BL/6 background were produced as previously described.5 All mice used in these studies were male and ranged from 8 to 18 weeks in age. All mice were housed in a specific pathogen-free environment and examined twice weekly by veterinary staff for signs of illness. Mice were given food and water ad libitum. All studies were approved by the Animal Studies Committee at Washington University.
Murine model of endobronchial inflammation
An adaptation of the agarose bead method developed by Starke et al was used to create a subacute pulmonary infection in mice.16 Briefly, P aeruginosa cystic fibrosis patient isolate PAM57-15 (or laboratory strain PAO1) was grown to log phase and then mixed with 2% agarose, low electroendosmosis (EEO) (Fisher, Fairlawn, NJ). This mixture was added to heavy mineral oil (Fisher), equilibrated at 55°C, then rapidly stirred at room temperature and cooled, generating P aeruginosa–laden agarose beads. Beads were washed once with 0.5% deoxycholic acid sodium salt in phosphate-buffered saline (PBS), once with 0.25% deoxycholic acid sodium salt in PBS, and 4 times in PBS alone (all solutions: pH 7.4). Quantitative bacteriology was performed on serial dilutions of homogenized bead slurries to determine bacterial concentration, measured as colony-forming units of P aeruginosa per milliliter (CFUs/mL) of bead slurry.
Mice underwent intratracheal inoculation with P aeruginosa–embedded beads. The animals were anesthetized with 2.5% Tribromoethanol, Sigma-Aldrich, St Louis, MO) and placed in a dorsal recumbent position, and the ventral cervical area was surgically prepared. A transverse skin incision was made, and the trachea was visualized by blunt dissection. Transtracheal insertion of a 22-G 1-inch catheter was used to instill 50 μL bead slurry into the right mainstem bronchus. Consequently, the right lung is the primary site of infection in this model.
Bronchoalveolar lavage (BAL)
BAL was performed by cannulating the trachea in situ with a 22-G 1-inch catheter, instilling 3, 1-mL aliquots of sterile PBS, and collecting the fluid by gentle aspiration. The total lavage fluid recovered from all animals was centrifuged at 4°C for 10 minutes at 500g. The lavage supernatant was then supplemented with protease inhibitors (100 μM phenylmethanesulfonyl fluoride [PMSF] and 5 mM ethylenediaminetetraacetic acid [EDTA]) and stored at −80°C until time of analysis. The cell pellet obtained from lavage was resuspended in PBS, and cell counts with leukocyte differential were performed as described in “Peripheral blood and bone marrow analysis.”
Peripheral blood and bone marrow analysis
Blood was collected in EDTA-coated polypropylene tubes at defined time points during the infection by retro-orbital venous plexus sampling or by cardiac puncture. Bone marrow was harvested by flushing femoral bones with Opti-MEM (Gibco, Carlsbad, CA) containing 1% fetal calf serum. A total leukocyte cell count was performed on BAL fluid (BALF), blood, and bone marrow samples using a Hemavet automated cell counter (CDC Technologies, Oxford, CT). Manual leukocyte differentials were performed on Wright-stained blood smears (minimum 200 cells) or cytospin preparations of bone marrow and BALF cells (minimum 500 cells). As described previously, the percentage of total body neutrophils in the blood was estimated using the neutrophil distribution index.7 This index is computed by dividing the absolute number of neutrophils in the blood by the absolute number of neutrophils in the blood plus bone marrow. Blood and bone marrow neutrophils were calculated assuming a blood volume of 1.8 mL and a whole femur equivalent to 6% of the total bone marrow.17
Enzyme-linked immunosorbent assay (ELISA) for secreted murine cytokines
Murine cytokines G-CSF, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), macrophage inflammatory protein-2 (MIP-2), and keratinocyte-derived chemokine (KC) were measured using commercially available sandwich enzyme immunoassays (EIAs) according to the manufacturer's recommended protocols (R&D Systems, Minneapolis, MN). The limit of detection for each cytokine is 5.0 pg/mL. Since respiratory epithelial lining fluid (ELF) is diluted by BAL, the concentration of cytokines and chemokines in ELF was calculated using a dilution factor observed for urea (plasma urea/BALF urea).18
In vitro TNF-production assay
Blood was collected by cardiac puncture, red blood cells were lysed in Tris (tris(hydroxymethyl)aminomethane)–buffered ammonium chloride (pH 7.2) buffer, and the remaining cells were incubated with Alexa 488–conjugated anti–mouse F4/80 (Caltag Laboratories, Burlingame, CA) and phycoerythrin (PE)–conjugated anti–mouse Gr-1 (eBiosciences, San Diego, CA). Monocytes, identified as F4/80+Gr-1low/− cells, were sorted using a MoFlo high-speed flow cytometer (Dako Cytomation, Fort Collins, CO) into wells containing RPMI 1640 at a concentration of 1 × 105 cells per well. Cells were stimulated with 10 μg/mL lipopolysaccharides (LPS) from Salmonella abortus equi (Sigma-Aldrich) ± 100 ng/mL G-CSF (Amgen, Thousand Oaks, CA) for 24 hours at 37°C and 5% CO2. TNF-α levels in culture supernatants were measured by ELISA.
Intracapillary accumulation assay
Mice were infected with P aeruginosa and intracapillary accumulation was measured as described by Reutershan et al.19 In detail, 5 minutes prior to being killed, mice were intravenously injected with 0.6 μg PE-conjugated anti–mouse Gr-1. Nonadherent blood neutrophils were removed from the pulmonary vasculature by severing the vena cava to exsanguinate, followed by injection of 10 mL PBS into the right ventricle to flush. Lungs were then excised from the animal and ground into a single cell suspension using a Wheaton Dounce tissue grinder (Wheaton Scientific, Millville, NJ). Cells were ground in the presence of excess (5 μg) unlabeled Gr-1 (eBiosciences) to prevent binding of any remaining PE-Gr-1, liberated from the vessels during homogenization, to extravascular neutrophils. Blood and lung cell suspensions were then stained with fluorescein isothiocyanate (FITC)–conjugated anti–mouse neutrophil-7/4 (Serotec, Raleigh, NC). Intravascular (7/4+Gr-1+) and extravascular (7/4+Gr-1−) neutrophil populations were assessed using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Mansfield, MA).
Quantitative bacteriology
Cell-free supernatants obtained from BALF or whole lung homogenates were cultured quantitatively by serial dilution on tryptic soy agar plates. Splenic homogenates and blood were streaked on tryptic soy agar plates. The plates were incubated at 37°C and inspected for P aeruginosa colonies after 24 hours.
Histopathologic grading of lung sections
Paraffin-embedded tissue sections (5 μm) from 4 different lungs of each experimental group were stained with hematoxylin and eosin (H&E). Light microscopic images were visualized using a Nikon Microphot-SA microscope (Nikon, Melville, NY) equipped with a Nikon Plan Apo 40×/1.0 numerical aperture (NA) or a Nikon Plan Apo 100× (oil)/1.4 NA objective. Images were obtained and analyzed using a ColorView camera and analySIS software version 3.1 (Soft Imaging Systems, Münster, Germany). Analyses of lung specimens were performed by blinded observation, focusing on 5 independent fields containing an inflammatory lesion. As previously described,20 4 pathologic features were scored on a scale of 0 to 4: (a) alveolar congestion, (b) hemorrhage, (c) leukocyte infiltration in airspace or the vessel wall, and (d) thickness of the alveolar wall. A score of 0 represented normal lungs; 1, mild, less than 25% involvement in the visualized field; 2, moderate, 25% to 50% involvement; 3, severe, 50% to 75% involvement; and 4, very severe, more than 75% involvement.
Apoptosis assay
Cells recovered from BALF were washed once in binding buffer (20 mM HEPES [pH 7.4], 132 mM NaCl, 6 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 1.2 mM KH2PO4, 5.5 mM glucose, and 0.5% bovine serum albumin), incubated with FITC-conjugated annexin V (NeXins Research BV, Roermond, The Netherlands) for 30 minutes at 4°C, washed twice in binding buffer, stained with actinomycin D, 7-amino (7-AAD; Calbiochem, La Jolla, CA), and analyzed using flow cytometry. To obtain viable and apoptotic neutrophil populations with which to set flow cytometry gates, bone marrow neutrophils from control C57BL/6 mice were isolated over a Percoll density gradient as previously described,21 and then cultured in Opti-MEM/1% fetal calf serum (FCS) with or without 100 ng/mL G-CSF. Viable neutrophils were collected at one hour after G-CSF culture. Early apoptotic and late apoptotic neutrophil populations were collected after 24 and 48 hours of culture, respectively, in the absence of G-CSF.
Statistics
Statistical significance was determined using the Student t test assuming equal variance. Time course experiments were analyzed using 2-way ANOVA with Bonferroni posttesting at individual time points. All values reported are means ± SEM.
Results
Loss of the G-CSFR increases susceptibility to pulmonary P aeruginosa infection
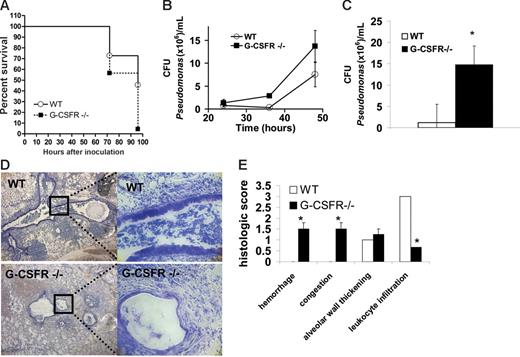
Wild-type and G-CSFR−/− mice inbred onto a C57BL/6 background underwent pulmonary challenge with P aeruginosa–laden agarose beads, a well-characterized model of endobronchitis and peribronchitis.16,22-24 In wild-type mice, inoculation of 4 × 104 CFUs/mouse of P aeruginosa strain PAM57-15 induced a severe pulmonary infection with 45% of mice surviving at 96 hours after infection (Figure 1A). In contrast, only 4% of G-CSFR−/− mice survived at 96 hours following infection with an identical dose of P aeruginosa. To assess P aeruginosa clearance in the lung, the number of bacterial CFUs in BALF was measured at 24 to 48 hours after infection; later time points were not examined to avoid confounding data from preterminal mice (Figure 1B). A trend toward increased bacterial CFUs was observed in G-CSFR−/− mice at 36 and 48 hours after infection. Moreover, G-CSFR−/− lung homogenates harbored significantly higher P aeruginosa CFUs than wild type at 48 hours (Figure 1C). Of interest, no bacterial CFUs were detected in the blood or spleen of either wild-type or G-CSFR−/− mice at any time point. This lack of bacteremia suggests that this mode of inoculation produces a local infection. Histopathologic analysis of wild-type animals showed extensive infiltration of inflammatory cells (predominantly neutrophils) into airways, with some extension into adjacent alveoli (Figure 1D-E), however tissue destruction was generally limited. In contrast, extensive destruction of normal tissue architecture was observed in the lungs of G-CSFR−/− mice (Figure 1D-E). Of note, despite this extensive tissue damage, a striking paucity of neutrophils in the lungs of G-CSFR−/− mice was observed (Figure 1D-E). G-CSFR−/− mice also displayed increased susceptibility to infection with PAO1, a well-characterized laboratory strain of P aeruginosa. Infection with 2 to 4 × 104 CFUs PAO1/mouse resulted in the death of 93.3% of G-CSFR−/− animals by 48 hours compared with only 12.5% death in wild-type littermates. Collectively, these data show that G-CSFR−/− mice are highly susceptible to pulmonary P aeruginosa infection.
G-CSFR−/− mice display increased susceptibility to bronchopulmonary infection with P aeruginosa. (A) Wild-type (n = 22) and G-CSFR−/− mice (n = 23) were challenged with a single intratracheal injection of P aeruginosa–laden beads. Kaplan-Meier estimates demonstrate significantly decreased survival in G-CSFR−/− mice compared with wild-type mice (log rank, P = .006). Bacterial load of P aeruginosa in the lung was estimated by measuring the number of bacterial CFUs in the BALF (B) at the indicated times. The number of animals at each time point ranges from n = 4 at 24 hours to n = 21 at 48 hours. Bacterial load was also measured in whole lung homogenates (C) at 48 hours (n = 4). (D) Representative Giemsa-stained sections of lung tissue 72 hours after infection with Pseudomonas-laden beads are shown. Original magnification, × 40 (left panel) or × 200 (right panel). (E) Lungs harvested at 72 hours were sectioned and assigned a histologic score as described in “Materials and methods” (n = 4). Data represent the mean ± SEM; *P ≤ .05.
G-CSFR−/− mice display increased susceptibility to bronchopulmonary infection with P aeruginosa. (A) Wild-type (n = 22) and G-CSFR−/− mice (n = 23) were challenged with a single intratracheal injection of P aeruginosa–laden beads. Kaplan-Meier estimates demonstrate significantly decreased survival in G-CSFR−/− mice compared with wild-type mice (log rank, P = .006). Bacterial load of P aeruginosa in the lung was estimated by measuring the number of bacterial CFUs in the BALF (B) at the indicated times. The number of animals at each time point ranges from n = 4 at 24 hours to n = 21 at 48 hours. Bacterial load was also measured in whole lung homogenates (C) at 48 hours (n = 4). (D) Representative Giemsa-stained sections of lung tissue 72 hours after infection with Pseudomonas-laden beads are shown. Original magnification, × 40 (left panel) or × 200 (right panel). (E) Lungs harvested at 72 hours were sectioned and assigned a histologic score as described in “Materials and methods” (n = 4). Data represent the mean ± SEM; *P ≤ .05.
Neutrophil mobilization from the bone marrow in response to pulmonary P aeruginosa infection is impaired in G-CSFR−/− mice
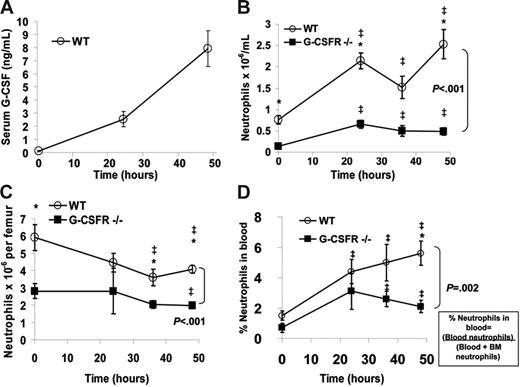
The paucity of neutrophils in the lungs of G-CSFR−/− mice suggested that the stress granulopoiesis response to bronchopulmonary P aeruginosa infection was impaired. To investigate this further, we first determined whether systemic expression of G-CSF was induced. Indeed, the serum concentration of G-CSF in wild-type mice increased 59-fold during the first 48 hours after infection, reaching a concentration of 7.9 ± 1.4 ng/mL (Figure 2A); this concentration is within the range of that observed in patients treated with G-CSF.2 Of interest, in G-CSFR−/− mice, the serum concentration of G-CSF was markedly elevated at baseline (6.4 ± 0.6 ng/mL) and further increased during the infection, reaching 258 ± 13.4 ng/mL at 48 hours.
The systemic neutrophil response following P aeruginosa infection is impaired in G-CSFR−/− mice. (A) The serum concentration of G-CSF in wild-type mice at the indicated times following P aeruginosa infection was measured by ELISA. (B) The absolute neutrophil count in the blood was measured in wild-type and G-CSFR−/− mice (n = 8-26, each time point). (C) The number of mature neutrophils per femur was measured in wild-type and G-CSFR−/− mice (n = 4-17, each time point). (D) The percentage of the total body pool of mature neutrophils present in the blood was estimated as described in “Materials and methods” (n = 4-17, each time point). All data represent the mean ± SEM. ‡P < .05 compared with untreated mice of the same genotype. *P < .05 compared with G-CSFR−/− mice.
The systemic neutrophil response following P aeruginosa infection is impaired in G-CSFR−/− mice. (A) The serum concentration of G-CSF in wild-type mice at the indicated times following P aeruginosa infection was measured by ELISA. (B) The absolute neutrophil count in the blood was measured in wild-type and G-CSFR−/− mice (n = 8-26, each time point). (C) The number of mature neutrophils per femur was measured in wild-type and G-CSFR−/− mice (n = 4-17, each time point). (D) The percentage of the total body pool of mature neutrophils present in the blood was estimated as described in “Materials and methods” (n = 4-17, each time point). All data represent the mean ± SEM. ‡P < .05 compared with untreated mice of the same genotype. *P < .05 compared with G-CSFR−/− mice.
In wild-type mice, the number of neutrophils in the blood increased from 0.8 ± 0.1 × 106/mL at baseline to 2.5 ± 0.3 × 106/mL at 48 hours after infection (Figure 2B). G-CSFR−/− mice were neutropenic at baseline and, while a significant increase in blood neutrophils was observed throughout the course of infection, it did not match the robust peripheral neutrophilia observed in wild-type animals (Figure 2B). To further characterize the systemic neutrophil response, the number of neutrophils in the bone marrow was quantified (Figure 2C). As reported previously, G-CSFR−/− mice have a decreased number of mature neutrophils in the bone marrow at baseline.5 Following P aeruginosa infection, the number of neutrophils in the bone marrow decreased to a similar degree in both wild-type and G-CSFR−/− mice. As described previously, neutrophil release from the bone marrow was estimated by determining the percentage of neutrophils in the blood versus the total number of neutrophils in the bone marrow and blood.7 Following P aeruginosa infection, the percentage of neutrophils in the blood of wild-type mice increased, reaching a peak at 48 hours after infection (Figure 2D). In G-CSFR−/− mice, though the percentage of neutrophils in the blood increased, the magnitude of this increase was reduced compared with wild-type mice, particularly at 48 hours after infection when serum G-CSF levels are maximal (% of neutrophils in the blood at 48 hours after infection ± SEM: 5.60% ± 0.81% [wild-type mice] and 2.07% ± 0.37% [G-CSFR−/− mice]; P < .05). Collectively, these data suggest that the systemic neutrophil response in this infectious model is dependent upon G-CSF signals and is primarily mediated by increased neutrophil release from the bone marrow rather than increased neutrophil production.
Expression of key inflammatory chemokines and cytokines in the lung is normal to increased in G-CSFR−/− mice following P aeruginosa infection
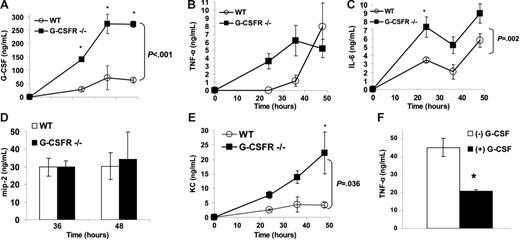
The recruitment of neutrophils from the blood to inflammatory sites and their subsequent activation and clearance are regulated by a host of chemokines and cytokines. We first determined that G-CSF expression is strongly induced in BALF during P aeruginosa infection. The concentration of G-CSF in BALF of wild-type mice increased from undetectable levels at baseline to 63 ± 7 ng/mL at 48 hours after infection (Figure 3A). Similar to serum, G-CSF levels in BALF from G-CSFR−/− mice were markedly elevated.
Local production of key inflammatory chemokines and cytokines in the lung is normal to increased in G-CSFR−/− mice following P aeruginosa infection. The concentration of (A) G-CSF, (B) TNF-α, (C) IL-6, (D) MIP-2, and (E) KC in BALF was measured by ELISA (n = 4-7 for each time point). *P ≤ .05 compared with wild-type mice. (F) Blood monocytes isolated from wild-type mice were stimulated with LPS (10 μg/mL) for 24 hours in the presence (▪) or absence (□) of 100 ng/mL G-CSF (n = 6). TNF-α in the supernatant of these cultures was measured by ELISA. Graph shown is representative of 3 independent experiments. *P ≤ .05. All data represent the mean ± SEM.
Local production of key inflammatory chemokines and cytokines in the lung is normal to increased in G-CSFR−/− mice following P aeruginosa infection. The concentration of (A) G-CSF, (B) TNF-α, (C) IL-6, (D) MIP-2, and (E) KC in BALF was measured by ELISA (n = 4-7 for each time point). *P ≤ .05 compared with wild-type mice. (F) Blood monocytes isolated from wild-type mice were stimulated with LPS (10 μg/mL) for 24 hours in the presence (▪) or absence (□) of 100 ng/mL G-CSF (n = 6). TNF-α in the supernatant of these cultures was measured by ELISA. Graph shown is representative of 3 independent experiments. *P ≤ .05. All data represent the mean ± SEM.
TNF-α is a key regulator of the immune response to infection25 ; consequently, we measured TNF-α levels in BALF. Of interest, though a similar maximum level was achieved, the kinetics of the increase in TNF-α in BALF was more rapid in G-CSFR−/− mice (Figure 3B). In particular, TNF-α expression in BALF was near maximum levels in G-CSFR−/− mice by 24 hours after infection, whereas it was undetectable in wild-type mice. Although decreased bacterial clearance may contribute to increased TNF-α production, at 24 hours after infection, the number of bacteria is similar in wild-type and G-CSFR−/− mice (Figure 1B). It is also possible that G-CSF signals directly regulate TNF-α production by inflammatory cells in the lung. Since cells of the monocytic lineage are a major source of TNF-α,26 we next asked whether G-CSF could directly regulate TNF-α production in murine monocytes in vitro. Sorted blood monocytes were stimulated with LPS in the presence or absence of G-CSF, and TNF-α production was measured. As expected, blood monocytes treated with LPS released significant amounts of TNF-α (Figure 3F). Treatment with G-CSF suppressed TNF-α production by 2.2-fold (P < .05). In contrast, G-CSF did not affect TNF-α production in LPS-stimulated cultures of G-CSFR−/− blood monocytes (data not shown). These data suggest a direct role for G-CSF signaling in downmodulating TNF-α production.
Interleukin-6 (IL-6) is likewise a key regulator of the recruitment of leukocytes to inflammatory sites.27 Indeed, local expression of IL-6 in the lung was strongly induced in wild-type mice during P aeruginosa infection, as evidenced by the high concentration of IL-6 in BALF (Figure 3C). Although the kinetics of IL-6 accumulation in BALF was similar in wild-type and G-CSFR−/− mice, higher levels of IL-6 were achieved in G-CSFR−/− mice at all analyzed time points.
In mice, neutrophil emigration into inflammatory sites is thought to be regulated primarily by the CXC chemokines MIP-2 and KC. MIP-2, which has been shown to be undetectable in the BALF of uninfected C57BL/6 mice,15 was produced in similar amounts by infected wild-type and G-CSFR−/− mice (Figure 3D). Likewise, local expression of KC in the lungs was strongly induced during P aeruginosa infection in both wild-type and G-CSFR−/− mice (Figure 3E). However, in contrast to MIP-2, higher levels of KC were detected in the BALF of G-CSFR−/− mice at all time points.
Together, these data demonstrate that G-CSF signals are not required for the induction of key inflammatory mediators in the lung in response to P aeruginosa infection. Rather, in vitro evidence (Figure 3F) suggests that G-CSF signals may down-regulate expression of certain inflammatory mediators via a direct effect on pulmonary macrophages.
Neutrophil accumulation in the lung is reduced in G-CSFR−/− mice following P aeruginosa infection
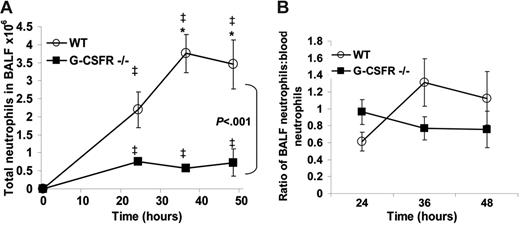
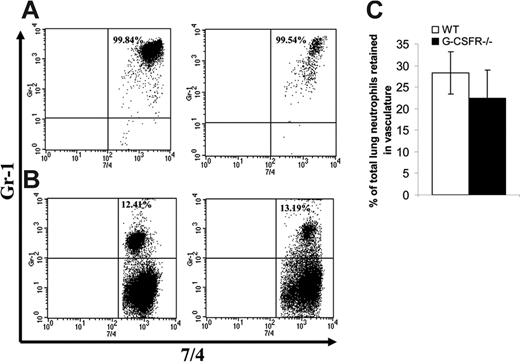
Having established that key inflammatory mediators are present in the lung of G-CSFR−/− mice following P aeruginosa infection, we next characterized the local neutrophil response. In wild-type mice, robust neutrophil infiltration into the bronchoalveolar space was observed, with a maximum of 3.8 ± 0.5 × 106 neutrophils recovered in the BALF at 36 hours. As predicted by the histopathology, neutrophil number in the BALF of G-CSFR−/− mice was significantly reduced compared with wild-type mice (Figure 4A); at 36 hours after infection, only 0.6 ± 0.06 × 106 neutrophils were recovered. However, G-CSFR−/− mice are neutropenic at baseline and remained neutropenic throughout the study (Figure 2B). In order to address this issue, BALF neutrophil counts were corrected for the degree of neutropenia observed in the peripheral blood (Figure 4B). Indeed, after correcting for the blood neutropenia, neutrophil accumulation in the BALF of G-CSFR−/− mice was comparable with wild-type mice. However, the peripheral blood count that provides the basis for this correction may not accurately reflect the population of neutrophils that is sequestered in pulmonary capillaries. This population has been described in several models of G-CSF–induced28,29 as well as infection-driven30,31 neutrophil mobilization. If G-CSF–mobilized neutrophils were, in fact, selectively retained in the pulmonary capillaries, the linear relationship assumed for migration of circulating neutrophils to the inflammatory site may not hold true. To address this possibility, we used a technique recently described by Reutershan et al19 in which adherent neutrophils in the pulmonary vasculature can be measured. Briefly, intravenous neutrophils were labeled in vivo with PE-conjugated anti–Gr-1; after depletion of nonadherent neutrophils in the vasculature by exsanguination and flushing, the percentage of remaining neutrophils in lung homogenates that stained with Gr-1 was measured (Figure 5). If neutrophils preferentially sequester in the pulmonary capillaries of either wild-type or G-CSFR−/− animals, the number of 7/4+Gr-1+ neutrophils in lung homogenates should be increased. In fact, the percentage of adherent intravascular neutrophils in lung homogenates does not differ significantly between wild-type (28.2% ± 6.4%) and G-CSFR−/− (22.4% ± 4.9%) mice (Figure 5D). We therefore conclude that peripheral blood values can be used to accurately correct for neutropenia in this model. Applying this correction for peripheral blood values, neutrophil accumulation into the BALF of G-CSFR−/− mice was similar to wild-type mice (Figure 4B). These data indicate that the decrease in circulating neutrophils likely accounts for most of the neutrophil accumulation defect in the lungs of G-CSFR−/− mice, suggesting that G-CSFR−/− neutrophils retain the ability to efficiently emigrate into the bronchoalveolar space.
Neutrophil accumulation into the BALF of G-CSFR−/− mice is reduced following P aeruginosa infection. (A) Kinetics of neutrophil accumulation into the BALF of wild-type and G-CSFR−/− mice following P aeruginosa infection (n = 3-13, each time point). (B) To correct for the effect of circulating neutrophil number on neutrophil recruitment to the lungs, the number of neutrophils in the BALF of each mouse was divided by the total number of neutrophils present in the blood. All data represent the mean ± SEM. ‡P < .05 compared with untreated mice of the same genotype. *P < .05 compared with G-CSFR−/− mice.
Neutrophil accumulation into the BALF of G-CSFR−/− mice is reduced following P aeruginosa infection. (A) Kinetics of neutrophil accumulation into the BALF of wild-type and G-CSFR−/− mice following P aeruginosa infection (n = 3-13, each time point). (B) To correct for the effect of circulating neutrophil number on neutrophil recruitment to the lungs, the number of neutrophils in the BALF of each mouse was divided by the total number of neutrophils present in the blood. All data represent the mean ± SEM. ‡P < .05 compared with untreated mice of the same genotype. *P < .05 compared with G-CSFR−/− mice.
Accumulation of neutrophils in pulmonary vessels. Forty-eight hours after P aeruginosa inoculation, wild-type and G-CSFR−/− animals were injected with PE-Gr-1 antibody. Five minutes later, neutrophils in blood (A) and lung homogenate (B) were harvested as described in “Materials and methods.” Shown are representative fluorescence-activated cell sorted (FACS) plots of wild-type (left) and G-CSFR−/− mice (right) stained ex vivo with FITC-7/4 (antineutrophil) antibody. (C) Quantitation of the percentage of 7/4+Gr-1+ cells in lung homogenate of wild-type (□) and G-CSF−/− (▪) animals (n = 5). Data represent the mean ± SEM.
Accumulation of neutrophils in pulmonary vessels. Forty-eight hours after P aeruginosa inoculation, wild-type and G-CSFR−/− animals were injected with PE-Gr-1 antibody. Five minutes later, neutrophils in blood (A) and lung homogenate (B) were harvested as described in “Materials and methods.” Shown are representative fluorescence-activated cell sorted (FACS) plots of wild-type (left) and G-CSFR−/− mice (right) stained ex vivo with FITC-7/4 (antineutrophil) antibody. (C) Quantitation of the percentage of 7/4+Gr-1+ cells in lung homogenate of wild-type (□) and G-CSF−/− (▪) animals (n = 5). Data represent the mean ± SEM.
Neutrophil survival in the lungs of P aeruginosa–infected G-CSFR−/− mice is reduced
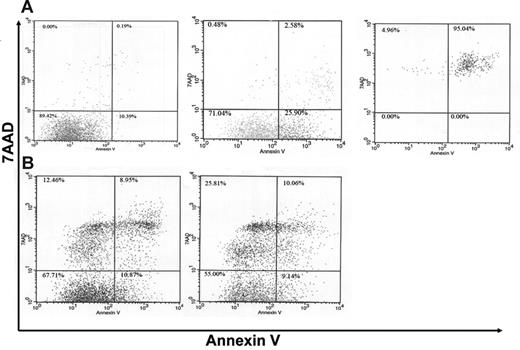
Neutrophil accumulation in an inflammatory site is dependent upon both neutrophil emigration into the site and their subsequent clearance. G-CSF is a potent survival signal for neutrophils,32 thus the high local concentration of G-CSF induced during P aeruginosa infection may contribute to the regulation of neutrophil survival in the lungs. To examine this possibility, we determined the percentage of apoptotic neutrophils in BALF using a well-characterized flow cytometric assay to measure cell surface annexin V expression.33-35 Control experiments were performed with wild-type bone marrow neutrophils to define populations of viable (annexin V−, 7AAD−), early apoptotic (annexin V+, 7AAD−), and late apoptotic (annexin V+, 7AAD+) neutrophils (Figure 6A). We next examined neutrophils in BALF at 48 hours after infection. The percentage of viable, early apoptotic, and late apoptotic neutrophils in the BALF of wild-type mice was 59.0% ± 4.0%, 10.6% ± 1.7%, and 10.7% ± 2.5%, respectively (Figure 6B). In G-CSFR−/− mice, the percentage of viable, early apoptotic, and late apoptotic neutrophils was 39.5% ± 3.8% (P < .05), 17.0% ± 5.1% (P = .25), and 19.7% ± 3.1% (P < .05), respectively. These data show that the percentage of viable neutrophils in the bronchoalveolar space of G-CSFR−/− mice is significantly reduced compared with wild-type mice, suggesting that neutrophil survival may be decreased. Of interest, an unusual population of 7AAD+, annexin V− cells was observed in the BALF from both wild-type and G-CSFR−/− mice. It has been shown that neutrophils exposed to a clinical isolate of P aeruginosa can die from a process of bacterial intoxication that results in cell swelling and the disintegration of the cell membrane.36 This process is morphologically and mechanistically distinct from apoptosis and thus could lead to 7AAD permeability in the absence of phosphatidylserine exposure.
A higher percentage of apoptotic neutrophils is present in the BALF of G-CSFR−/− mice following P aeruginosa infection. (A) Neutrophils isolated from the bone marrow of wild-type mice were cultured for 48 hours under conditions that induced apoptosis (“Materials and methods”). Cells were harvested at the indicated times and stained for Gr-1, annexin V, and 7AAD. Whereas the majority of neutrophils is viable (annexin V−, 7AAD−) at time 0 (left panel), an increasing percentage of early apoptotic (annexin V+, 7AAD−) and then late apoptotic (annexin V+, 7AAD+) cells was seen with prolonged culture (middle panel, right panel). Histograms are gated for Gr-1+ (granulocytic) cells. (B) Representative dot plots of neutrophils recovered from the BALF of wild-type (left panel) and G-CSFR−/− (right panel) mice. Data are gated for Gr-1+ (granulocytic) cells.
A higher percentage of apoptotic neutrophils is present in the BALF of G-CSFR−/− mice following P aeruginosa infection. (A) Neutrophils isolated from the bone marrow of wild-type mice were cultured for 48 hours under conditions that induced apoptosis (“Materials and methods”). Cells were harvested at the indicated times and stained for Gr-1, annexin V, and 7AAD. Whereas the majority of neutrophils is viable (annexin V−, 7AAD−) at time 0 (left panel), an increasing percentage of early apoptotic (annexin V+, 7AAD−) and then late apoptotic (annexin V+, 7AAD+) cells was seen with prolonged culture (middle panel, right panel). Histograms are gated for Gr-1+ (granulocytic) cells. (B) Representative dot plots of neutrophils recovered from the BALF of wild-type (left panel) and G-CSFR−/− (right panel) mice. Data are gated for Gr-1+ (granulocytic) cells.
Discussion
G-CSF plays a major role in maintaining basal neutrophil homeostasis in the blood primarily through the regulation of neutrophil release from the bone marrow.7 However, its importance in mediating the increase in neutrophil number in the blood following infection is less clear. In response to infection, a host of cytokines (GM-CSF, TNF-α, IL-1, IL-8, and IL-6) as well as chemotactic factors (C5a, leukotriene B4 [LTB4], platelet-activating factor [PAF], and N-formyl-methionyl-leucyl-phenylalanine [fMLP]) are generated in addition to G-CSF. These inflammatory mediators likewise have the ability to stimulate neutrophil production and release from the bone marrow.37,38 Thus, it is unclear whether there is functional redundancy in the signals regulating the systemic neutrophil response to infection. Indeed, 2 prior studies suggested that G-CSF signals were dispensable in the systemic neutrophil response following C albicans or L monocytogenes infection. In response to intravenous injection of C albicans, the increase in blood and bone marrow neutrophils in G-CSF−/− mice was comparable with wild-type mice.13 Likewise, the systemic neutrophil response in G-CSF−/− mice following intraperitoneal injection of L monocytogenes was similar to wild-type mice, despite a marked reduction in the number of myeloid progenitors in the bone marrow.14 In contrast, G-CSF−/− mice were found to have a reduced peripheral blood neutrophil response to intravenous administration of L monocytogenes compared with wild-type controls.4 It is therefore unclear based on these studies what role G-CSF plays in regulating the neutrophil response to infection and whether its effects differ in response to pathogen type or route of administration. Moreover, in each of these studies, relatively large inoculums of microbes were injected parenterally, producing severe systemic infections. In the present study, intratracheal injection of P aeruginosa coupled to agarose beads was used to produce a localized bronchopulmonary infection. In this infectious model, a significant increase in G-CSF concentration was observed at local (BALF) and systemic (blood) sites. In wild-type mice, a significant increase in circulating neutrophils was observed despite a decrease in bone marrow neutrophils. Accordingly, there was a significant redistribution of neutrophils from the bone marrow to the blood. These data suggest that the systemic neutrophil response to bronchopulmonary P aeruginosa infection is mediated primarily by enhanced neutrophil release rather than increased neutrophil production. In G-CSFR−/− mice, neutrophil release early during infection (24 hours) was comparable with wild-type mice, suggesting that non–G-CSF–dependent pathways mediate early neutrophil release. However, at later stages of infection (36-48 hours) when blood G-CSF levels peaked, neutrophil release was impaired. Thus, despite evidence for a more severe infection, neutrophil release from the bone marrow and corresponding increase in blood neutrophils were attenuated in G-CSFR−/− mice, suggesting that G-CSF signals are a key mediator of the late systemic neutrophil response in this model.
Neutrophil emigration from the blood to inflammatory sites is mediated by a well-defined sequence of neutrophil rolling, firm adhesion on endothelium, and diapedesis. The role of G-CSF in the regulation of this process remains unclear. G-CSF treatment modulates neutrophil adhesion39 and chemokinesis40 in vitro. Consistent with these observations, G-CSFR−/− neutrophils display defective chemotaxis in vitro, and neutrophil emigration into the skin in response to local interleukin-8 injection is impaired.41 In contrast, neutrophil emigration into the peritoneum following thioglycollate or casein injection is nearly normal in G-CSFR−/−5 or G-CSF−/−42 mice, respectively. In the present study, we show that neutrophil accumulation in the bronchoalveolar space in response to P aeruginosa was significantly reduced compared with wild-type mice, and histologic analysis likewise revealed decreased neutrophil infiltration into the pulmonary interstitial space. However, neutrophil emigration into inflammatory sites is likely to be dependent upon the number of neutrophils in the circulation, and in particular the number marginated in the pulmonary vasculature. We show that the number of circulating neutrophils correlates well to the adherent neutrophil fraction in the pulmonary vasculature in both wild-type and G-CSFR−/− mice, and thus we assumed a direct, linear relationship between neutrophil number in the blood and neutrophil emigration into the lung. After applying this correction, the number of neutrophils in the BALF of G-CSFR−/− mice was comparable with wild-type mice, suggesting that G-CSF signals are not required for the efficient emigration of neutrophils into the lung. As neutrophil apoptosis was found to be increased in G-CSFR−/− mice, the possibility exists that neutrophil emigration into the BAL may, in fact, be increased in these animals. Together with the studies already described, these data suggest that G-CSF signals play an important role in the emigration of neutrophils into some sites (skin) but not other sites (lung or peritoneum). Of note, similar observations were made with CD18−/− mice, which lack all β2-integrins. Neutrophil emigration into inflamed peritoneum and lung was normal, while emigration into inflamed skin of CD18−/− mice was markedly impaired.43 Of interest, we previously showed that chemoattractant-induced β2-integrin activation is impaired in G-CSFR−/− neutrophils,41 providing a potential explanation for the concordant neutrophil emigration defect in G-CSFR−/− and CD18−/− mice.
Neutrophil apoptosis and subsequent clearance are key steps in the resolution of acute inflammation. A large number of inflammatory cytokines has been shown to regulate neutrophil survival in vitro, including G-CSF, GM-CSF, IL-6, IL-15, TNF-α, and IFN-γ.44-46 However, with few exceptions,47 the importance of individual cytokines in the regulation of neutrophil survival at inflammatory sites is less clear. G-CSF treatment significantly prolongs the in vitro survival of neutrophils, likely through reductions in active caspase-348 and Bax49 and up-regulation of survivin.50 However, the half-life of circulating neutrophils is normal in G-CSF−/− mice.51 In the present study, we show that during P aeruginosa infection, local G-CSF expression in the lung is increased to a level (approximately 63 ng/mL at 48 hours after infection) exceeding that required to regulate neutrophil survival in vitro.46 Indeed, the percentage of apoptotic neutrophils in BALF was significantly increased in G-CSFR−/− mice compared with wild-type mice. It remains a possibility that altered expression of other inflammatory cytokines in the lung may contribute to the decreased neutrophil survival in this model. For example, McLoughlin et al recently showed that IL-6 signaling negatively regulated neutrophil survival in a model of acute inflammation in the peritoneum.47 In addition, the ability of TNF-α to increase neutrophil apoptosis has been extensively described.52,53 Since levels of IL-6 and TNF-α are significantly increased in the BALF of G-CSFR−/− mice, these cytokines may, in fact, contribute to the observed increase in neutrophil apoptosis. Despite this caveat, our data provide evidence that G-CSF signals may play an important role in vivo in regulating neutrophil survival at inflammatory sites.
In addition to its ability to stimulate neutrophil trafficking and function, there is accumulating evidence that G-CSF may contribute to the anti-inflammatory response following resolution of infection. Mice pretreated with G-CSF are protected from LPS-induced organ toxicity and death.54 In addition, endotoxin-induced increases in serum TNF-α are attenuated by pretreatment with G-CSF in humans.55 In the present study, we show that the accumulation of certain proinflammatory cytokines in the lung was increased in G-CSFR−/− mice, even at early time points (24 hours after infection) when the bacterial burden in the lungs was similar to wild-type mice. Specifically, the concentration of TNF-α, KC, and IL-6 in BALF was significantly increased in G-CSFR−/− mice. Consistent with our findings, a previous study showed that pretreatment with anti–G-CSF increased the local production of TNF-α, IL-1β, and KC in murine model of pneumococcal pneumonia.56 A direct effect of G-CSF inflammatory cytokine production by monocytes may be responsible for these biologic responses, as G-CSF treatment of elutriation-purified monocytes has been shown to inhibit their release of TNF-α, IL-12, and IL-1β in response to LPS.57 To refine this observation further, we isolated a pure population of F4/80+Gr-1low monocytes and showed a reduction of TNF-α production in the presence of G-CSF in wild-type but not G-CSFR−/− monocytes. Consistent with these data, Gorgen et al showed that pretreatment of alveolar macrophages with G-CSF in vivo reduced TNF-α production.54 While the mechanism governing G-CSF–mediated cytokine inhibition remains unclear, recent studies have suggested a role for STAT3.58,59 Collectively, these data suggest an important role for G-CSF in the down-regulation of the local inflammatory response.
In summary, this study provides evidence that G-CSF regulates the immune response in a physiological model of pulmonary P aeruginosa infection at several key steps. First, G-CSF is the primary signal inducing neutrophil release from the bone marrow during late stages of infection, providing the major mechanism by which the level of circulating neutrophils is increased. Second, within the inflammatory site, G-CSF contributes to the regulation of neutrophil clearance by suppressing their apoptosis. Finally, G-CSF may contribute to the control of local inflammation by attenuating the secretion of inflammatory cytokines by resident pulmonary macrophages.
Authorship
Contribution: A.D.G. designed research, performed research, analyzed data, and wrote the paper; L.A.H. performed research and analyzed data; T.W.F. designed research and analyzed data; D.C.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Link, Division of Oncology, Department of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: dlink@im.wustl.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL60772 [D.C.L.], R01 4L64044 [T.W.F.]), the March of Dimes (T.W.F.), and a Washington University (WU)/Howard Hughes Medical Institute (HHMI) Summer Undergraduate Research Fellowship funded by an Undergraduate Biological Sciences Education Program grant from the HHMI to WU (A.D.G.).
We thank Jill Woloszynek and Kathryn Akers for their expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal