Abstract
Dendritic cells (DCs) are important regulators in graft-versus-host disease (GVHD). To gain insight into cord blood (CB) DC immunology, we compared chemotactic responses of mature monocyte-derived DCs and maturation agent lipopolysaccharide (LPS)–induced signaling between CB and adult blood (AB). Mature CB DCs expressed reduced CCR7, but increased CXCR4. This was associated with reduced migratory efficiency toward both CCR7 ligand CCL19 and CXCR4 ligand CXCL12. LPS induced higher extracellular signal-regulated kinase (ERK) phosphorylation in CB than in AB DCs. Specific inhibition of ERK during CB DC maturation enhanced LPS-induced up-regulation of CCR7 and CXCR4 on CB DCs and their chemotaxis toward CCL19 and CXCL12, to a level similar to that of mature AB DCs. Overall, monocyte-derived CB DCs responded to LPS with stronger and sustained ERK activation, which negatively correlated with LPS-induced up-regulation of CCR7 and CXCR4 on CB DCs and their migratory responses. These findings may have potential relevance to better understanding DC function in CB transplantation.
Introduction
Mature dendritic cell (DC) function is linked to their capacity to migrate.1 Inflammatory stimuli, such as lipopolysaccharide (LPS), promote DC maturation and DC chemotactic response toward CCR7 ligand CCL19 and CXCR4 ligand CXCL12.2-4 CCR7-deficient DCs do not traffic to secondary lymphoid organs where CCL19 is secreted. CCR7-deficient mice are deficient in initiating primary immune reactions.5,6
LPS-induced maturation of DCs is associated with activation of mitogen-activated protein kinases (MAPKs; extracellular signal-regulated kinase [ERK], c-Jun N-terminal kinase [JNK], p38 kinase).7-9 LPS-induced MAPK activation is involved in the regulation of DCs.7-9 However, little is known of MAPK's role in mature DC migration.
Cord blood (CB) is a transplantable source of hematopoietic stem cells with relatively low risk of graft-versus-host disease (GVHD).10-12 Donor DCs have been implicated in GVHD and graft rejection.13 Monocytes are DC precursors giving rise to DCs under the influence of granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4). LPS induces functional maturation of CB and AB DCs.14,15 Here, we compared migration and LPS-induced MAPK activation of mature CB and AB DCs.
Materials and methods
Monocytes and cell culture
Monocytes (purity > 96%) were isolated from fresh CB and adult buffy coats using MACS CD14+ magnetic beads, and were cultured15 in RPMI 1640 culture medium (BioWhittaker, Walkersville, MD) with 10 ng/mL human (h) IL-4 (Peprotech, Rocky Hill, NJ) and 40 ng/mL hGM-CSF (Immunex/Amgen, Seattle, WA) for 5 days. To induce DC maturation, 1 μg/mL LPS (Sigma-Aldrich, St Louis, MO) was added on day 5 for 24 hours. Some cells were incubated for 1 hour with PD98059 (40 μM; Calbiochem, San Diego, CA) or DMSO (0.2%) before LPS addition.
Chemotaxis assay
Chemotaxis assay was done in duplicate at 37°C for 3 hours16 with modification. Cells (1 × 105-2 × 105) were added to the upper chambers of Transwell (5 μm pore; Costar, Cambridge, MA). Chemotaxis buffer (0.6 mL RPMI 1640; 0.5% bovine serum albumin [BSA]) with 100 ng/mL CCL19 or CXCL12 (predetermined for optimal DC migration) was added to the lower chambers. Percent migration was determined as migrated/total events using FACScan (Becton Dickinson, San Jose, CA).
Calcium influx
Calcium influx was assayed by flow cytometry17 with modification. Cells were washed and resuspended in Iscove modified Dulbecco medium (IMDM) plus 2% BSA. Premixed Fluo-3AM/pluronic acid (Molecular Probes, Eugene, OR) was added at final concentration of 4 μM. After 45 minutes at room temperature, cells were washed in Ca2+ flux assay buffer (Hanks balanced salt solution containing 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] and 0.2% BSA, pH 7.4) to remove extracellular dye, incubated 10 minutes at room temperature, and analyzed by FACScan. Background fluorescence was measured, CCL19 added to samples, and Ca2+ influx recorded.
Immunostaining
Cells were stained on ice with PE-conjugated CXCR4 Ab (clone 12G5; R&D Systems, Minneapolis, MN), FITC-conjugated CD83 (BD Bioscience, San Jose, CA) and CD86 Abs (eBioscience, San Diego, CA). For CCR7 detection, cells were incubated with anti-CCR7 Ab (clone 2H4), PE-conjugated anti–murine IgM, or biotin-conjugated anti–murine IgM, followed by streptavidin-PE (BD Pharmingen, San Diego, CA). Percent positive cells was calculated by histogram subtraction using FCS Express 2 (De Novo Software, Thornhill, ON, Canada).
Cell lysates and Western blotting
Immature DCs were growth factor–starved in signaling buffer (RPMI medium with 0.5% BSA and 25 μM HEPES) for 3 hours, cells stimulated with 1 μg/mL LPS in silicon-treated tubes, cell pellets solubilized in lysis buffer, and equal amounts of protein electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes for Western blot.18 PVDF membranes were probed with Abs against p-ERK (Cell Signaling Technology, Beverly, MA), stripped, and reprobed with Abs against total ERK.
Statistics
Two-tailed paired Student t test and nonparametric Wilcoxon sum rank test were used. An asterisk indicates significance (P < .05).
Results and discussion
LPS induces maturation of DCs derived from monocytes
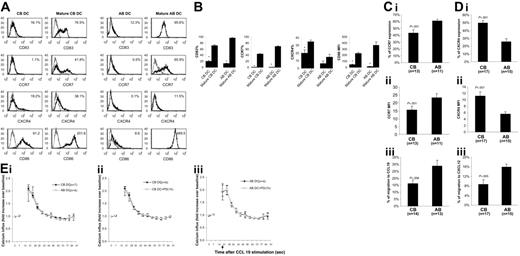
Monocyte-derived CB and AB DCs were activated with LPS. Upon LPS addition, CB and AB DCs acquired enhanced surface expression of CD83, CD86, and CCR7 (Figure 1A-B), indicating that LPS induced maturation of CB and AB DCs. Compared with mature AB DCs, fewer mature CB DCs expressed CCR7 and the expression level was lower (Figure 1A-B). In contrast, CB DCs expressed higher levels of CXCR4 than AB DCs before and after maturation (Figure 1A-B).
Comparison of phenotype, migration, and chemokine receptor expression by mature CB and AB DCs. (A) LPS induced phenotypic maturation of both CB and AB DCs. One representative histogram of CB and AB DCs before and after LPS-induced maturation is shown. Cells were stained with indicated molecules (black histogram) and isotype controls (gray histogram). Expression levels of indicated molecules (percentage of positive cells for CD83, CCR7, and CXCR4, and median fluorescence intensity for CD86) are shown in the upper right corner. (B) Average expression level of indicated molecules plus or minus the standard error of the mean (SEM) from 3 independent experiments is shown in panel A. (C) Fewer mature CB DCs express CCR7 (i) and mature CB DCs express a lower CCR7 median fluorescence intensity (MFI) (ii) on the surface, and mature CB DCs migrate with significantly lower efficiency to CCL19 (iii). (D) Mature CB DCs have a higher percentage of CXCR4+-expressing cells (i) and higher CXCR4 MFI (ii) on the surface than mature AB DCs, but migrate at significantly lower efficiency to CXCL12 (iii). In panels C and D, mature CB and AB DCs were tested in Transwell chemotaxis assays for the ability to migrate toward medium alone or toward medium supplemented with 100 ng/mL CCL19 or CXCL12 as indicated. (E) CCL19 induces calcium influx in CB DCs as well as in AB DCs (i), a process independent of ERK activation in both CB DCs (ii) and AB DCs (iii). In panels Eii and Eiii, cells were pretreated with PD98059 for 1 hour before adding CCL19.
Comparison of phenotype, migration, and chemokine receptor expression by mature CB and AB DCs. (A) LPS induced phenotypic maturation of both CB and AB DCs. One representative histogram of CB and AB DCs before and after LPS-induced maturation is shown. Cells were stained with indicated molecules (black histogram) and isotype controls (gray histogram). Expression levels of indicated molecules (percentage of positive cells for CD83, CCR7, and CXCR4, and median fluorescence intensity for CD86) are shown in the upper right corner. (B) Average expression level of indicated molecules plus or minus the standard error of the mean (SEM) from 3 independent experiments is shown in panel A. (C) Fewer mature CB DCs express CCR7 (i) and mature CB DCs express a lower CCR7 median fluorescence intensity (MFI) (ii) on the surface, and mature CB DCs migrate with significantly lower efficiency to CCL19 (iii). (D) Mature CB DCs have a higher percentage of CXCR4+-expressing cells (i) and higher CXCR4 MFI (ii) on the surface than mature AB DCs, but migrate at significantly lower efficiency to CXCL12 (iii). In panels C and D, mature CB and AB DCs were tested in Transwell chemotaxis assays for the ability to migrate toward medium alone or toward medium supplemented with 100 ng/mL CCL19 or CXCL12 as indicated. (E) CCL19 induces calcium influx in CB DCs as well as in AB DCs (i), a process independent of ERK activation in both CB DCs (ii) and AB DCs (iii). In panels Eii and Eiii, cells were pretreated with PD98059 for 1 hour before adding CCL19.
Migratory response of mature CB and AB DCs to CCL19 and CXCL12
Side-by-side comparison between CB and AB (n = 14-17) showed that fewer mature CB DCs expressed CCR7 than mature AB DCs, and the level of expression was lower (Figure 1Ci-ii) and fewer mature CB DCs migrated to CCL19, a CCR7 ligand (Figure 1Ciii). Although expressing increased surface CXCR4 (Figure 1Di-ii), mature CB DCs migrated significantly less well to CXCL12 than did mature AB DCs (P = .005; Figure 1Diii). Although CB DCs expressed reduced levels of CCR7 and migrated less well to CCL19, CCL19 induced a rapid rise in intracellular calcium in CB DCs and AB DCs (Figure 1Ei). Even though expressed at a reduced level, CCR7 was detected (43% ± 5% CCR7-positive cells) on CB DCs. This suggests that a rapid rise of calcium influx upon CCL19 activation might not be closely dependent on the expression level of CCR7. This phenomenon has been observed in T lymphocytes.19 Reduced chemotaxis of CB DCs to CCL19 might be due to calcium-dependent or -independent downstream events. Stimulation of CCR7 on DCs by CCL19 induced ERK phosphorylation, and ERK inhibitor strongly suppressed CCL19-induced DC chemotaxis.20 We performed calcium influx assays in the presence of PD98059, a specific inhibitor of MEK1, the kinase that phosphorylates and activates ERK.21 PD98059 failed to inhibit intracellular calcium influx of both CB and AB DCs (Figure 1Eii-iii), suggesting that activation of ERK is not required for CCL19-induced calcium influx, but is required for CCL19-induced DC migration. These data, plus those from others,20 imply that CCL19-induced migration and calcium influx in DCs are uncoupled or are not coupled proportionally.
It is known that neonates are highly susceptible to various infectious factors, such as bacteria, viruses, and parasites.22 Our results indicate a possible mechanism for this susceptibility that might involve decreased migratory capacity of CB DCs and subsequent inadequate priming of neonatal T cells. Expressing reduced levels of CCR7, CB DCs, after ingestion of pathogens, might migrate less efficiently to T-cell–rich areas of lymphoid organs to present Ag to T cells for T cells to initiate Ag-specific immunity. Although the function of CXCR4 in DCs is less well documented, expression of CXCL12 is detected in various tissues including normal and inflamed skin,23 liver,24 and intestines,25 where GVHD pathogenesis usually occurs. Reduced migratory response of CB DCs to CXCL12 might reduce local accumulation of activated DCs in pathologic tissues where CXCL12 is present, thereby alleviating local inflammation initiated by DCs. It is possible, although not proven, that these migratory features of CB DCs could have implications in the known reduced GVHD associated with CB transplantation.11,12
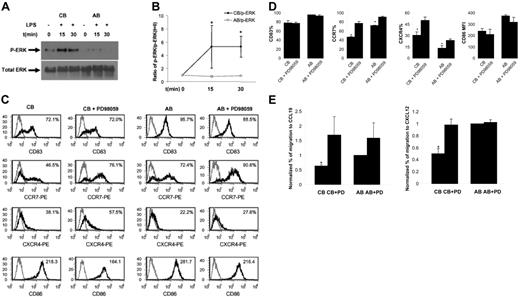
CB DCs show enhanced phosphorylation of ERK in response to LPS. As LPS up-regulates CCR7 expression on AB DCs,4 whereas CB DCs expresse reduced levels of CCR7 upon LPS stimulation, we hypothesized that CB DCs may not respond to LPS as well as AB DCs at an intracellular MAPK level (p38, JNK, and ERK). We did not detect significant p-ERK in response to LPS in AB DCs (Figure 2A-B), which is in agreement with a report that LPS failed to induce ERK activity in AB DCs.8 In contrast, we did detect increased and sustained p-ERK in CB DCs (Figure 2A-B). Therefore, the ERK signaling pathway is more responsive in CB than in AB DCs after LPS-induced maturation of CB DCs. No consistent differences in p-P38 and p-JNK were observed between CB and AB samples (not shown).
Comparison of LPS-induced ERK activation and effects of ERK inhibitor on LPS-induced changes in phenotype, chemokine receptor expression, and chemotaxis between CB and AB DCs. (A) LPS induced p-ERK in CB but not AB DCs. One representative of Western blotting data was shown. CB and AB DCs were either not treated (as a control) or treated side by side for 15 minutes and 30 minutes with LPS. Cell lysate was subjected to Western blotting using Abs specific for p-ERK. PVDF membranes were stripped and reprobed with Abs for total ERK as a loading control. (B) Average density of p-ERK band of CB and AB DCs plus or minus SEM from 3 independent experiments as shown in panel A was presented. Density of individual p-ERK bands was calculated relative to that of unstimulated cells (defined as 1.0). (C) PD98059 significantly enhanced LPS-induced up-regulation of CCR7 and CXCR4, but not CD83 and CD86, on mature CB DCs. Cells were pretreated with or without PD98059 for 1 hour. LPS was then added directly into cell culture for 1 day. Cells were harvested and stained with indicated molecules (black histogram) or isotype controls (gray histogram). Expression levels of indicated molecules (percentage of positive cells for CD83, CCR7, and CXCR4, and MFI for CD86) are shown in the upper right corner. (D) Average expression levels plus or minus SEM of 3 to 4 experiments as shown in panel C were presented. (E) PD98059-pretreated mature CB DCs, but not mature AB DCs, functionally migrated at significantly enhanced levels to CCL19 and CXCL12, compared with nontreated cells. Mature CB and AB DCs pretreated with or without inhibitor were assayed side by side in Transwells for chemotaxis toward both CCL19 and CXCL12. The data represent the average level of chemotaxis plus or minus SEM from 3 independent experiments. To minimize sample variation, percentage of migration was calculated relative to percentage of migration of mature AB DCs (defined as 1.0).
Comparison of LPS-induced ERK activation and effects of ERK inhibitor on LPS-induced changes in phenotype, chemokine receptor expression, and chemotaxis between CB and AB DCs. (A) LPS induced p-ERK in CB but not AB DCs. One representative of Western blotting data was shown. CB and AB DCs were either not treated (as a control) or treated side by side for 15 minutes and 30 minutes with LPS. Cell lysate was subjected to Western blotting using Abs specific for p-ERK. PVDF membranes were stripped and reprobed with Abs for total ERK as a loading control. (B) Average density of p-ERK band of CB and AB DCs plus or minus SEM from 3 independent experiments as shown in panel A was presented. Density of individual p-ERK bands was calculated relative to that of unstimulated cells (defined as 1.0). (C) PD98059 significantly enhanced LPS-induced up-regulation of CCR7 and CXCR4, but not CD83 and CD86, on mature CB DCs. Cells were pretreated with or without PD98059 for 1 hour. LPS was then added directly into cell culture for 1 day. Cells were harvested and stained with indicated molecules (black histogram) or isotype controls (gray histogram). Expression levels of indicated molecules (percentage of positive cells for CD83, CCR7, and CXCR4, and MFI for CD86) are shown in the upper right corner. (D) Average expression levels plus or minus SEM of 3 to 4 experiments as shown in panel C were presented. (E) PD98059-pretreated mature CB DCs, but not mature AB DCs, functionally migrated at significantly enhanced levels to CCL19 and CXCL12, compared with nontreated cells. Mature CB and AB DCs pretreated with or without inhibitor were assayed side by side in Transwells for chemotaxis toward both CCL19 and CXCL12. The data represent the average level of chemotaxis plus or minus SEM from 3 independent experiments. To minimize sample variation, percentage of migration was calculated relative to percentage of migration of mature AB DCs (defined as 1.0).
Inhibition of ERK signaling in CB DCs enhances their chemotactic response toward CCL19 and CXCL12
To explore the functional relevance of elevated p-ERK in response to LPS in CB DCs, we pretreated both CB and AB DCs for 1 hour with a highly selective ERK inhibitor, PD98059, before adding LPS. Pretreatment of CB DCs with PD98059 dramatically enhanced LPS-induced up-regulation of CCR7 (from 46% ± 2% without inhibitor to 78% ± 4% CCR7+ cells with inhibitor; n = 4) and CXCR4 (from 30% ± 5% to 50% ± 5% CXCR4+ cells with inhibitor; n = 4) (Figure 2C-D). Functionally, inhibitor-treated CB DCs migrated at an increased level to CCL19 and CXCL12 (Figure 2E). Although PD98059 also slightly increased the percentage of CCR7+ DCs (from 72% ± 1% to 91% ± 1% with inhibitor) and the percentage of CXCR4+ DCs (from 13% ± 3% to 24% ± 2% with inhibitor) on AB (Figure 2C-D), such effect did not lead to significantly enhanced chemotaxis of AB DCs to CCL19 and CXCL12 (Figure 2E). PD98059 failed to alter the surface level of CD83 and CD86 (Figure 2C-D). Therefore, ERK effects appear to be specific to CCR7 and CXCR4. Although ERK was not directly involved in CCL19-induced calcium influx of DCs (Figure 1Eii-iii), ERK was highly activated in response to LPS in CB DCs and played an indirect role in regulation of CB DC migratory responses toward CCL19 and CXCL12 by inhibiting LPS-induced up-regulation of their receptors, CCR7 and CXCR4.
In summary, we demonstrated a reduced ability of CB monocytes to differentiate into CCR7+CXCR4high mature DCs with potent migratory capability. Such reduced migration of CB DCs could be enhanced to a level comparable to that of mature AB DCs by inhibiting maturation-induced ERK signaling in CB DCs.
Authorship
Contribution: G.L. conceived, designed, and performed experiments, analyzed data, and drafted the paper; S.B. designed and performed experiments, and analyzed data; M.-K.H. designed and performed experiments; Y.-J.K. performed experiments; H.E.B. participated in design, coordination, performance of experiments, in writing the manuscript, and in funding the studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hal E. Broxmeyer, Walther Oncology Center, Indiana University School of Medicine, 950 West Walnut St, R2-302, Indianapolis, IN 46202-5181; e-mail: hbroxmey@iupui.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by US Public Health Service Grants R01 HL56 416 and R01 HL67 384, and a project in P01 HL053 586 from the National Institutes of Health (H.E.B.).
The current addresses for G.L. and M.-K.H. are as follows: Geling Li, Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, WA; and Myung-Kwan Han, Department of Microbiology and Immunology, Chonbuk National University Medical Center, San 2-20, Kenam-Dong, Dukjin-Gu, Jeonju 561-182, South Korea.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal