Every therapy comes with a cost, both fiscal and physical. Hirbe and colleagues report in this issue of Blood that granulocyte colony-stimulating factor (filgrastim) can promote tumor metastases by enhancing osteoclast function.
Since its approval in 1992 for use of chemotherapy-induced myelosuppression, filgrastim has become widely used, with a worldwide market of $3.5 billion. Filgrastim is effective in shortening the length of profound neutropenia in a diverse population of patients being treated with increasingly more myelosuppressive regimens, mobilizing peripheral blood stem cells for autotransplantation or allotransplantation, and protecting children with severe chronic neutropenia. Concerns about the long-term safety of filgrastim have been raised regarding the last group. Children with severe chronic neutropenia are treated to boost their neutrophil counts to a level protective against opportunistic infections. Whereas once these children died from life-threatening infection, they now lead a normal life, albeit one punctuated with near daily subcutaneous injections of filgrastim. Reports of an increasing incidence of myelodysplasia or acute myeloid leukemia in children and adolescents with severe chronic neutropenia have raised suspicions of a putative role for filgrastim in enhancing or accelerating leukemogenesis.1,2 In addition, osteoporosis/osteopenia, as defined by abnormal bone densities, was found in half of studied patients.3 Some of the children have benefited from bisphosphonate therapy.4
For patients with myeloid malignancies, the use of filgrastim has been a bone of contention. First identified as an inducer of myeloid leukemia differentiation,5 granulocyte colony-stimulating factor has been used to ameliorate patients with acute myeloid leukemia undergoing time-intensive induction chemotherapy. Spirited debates arose in myeloid leukemia protocol committees over filgrastim's growth stimulation of acute myeloid leukemia blasts, many of which express the granulocyte colony-stimulating factor receptor. Fortunately, those who were treated with filgrastim to reduce adverse effects of chemotherapy had remission and overall survival rates that were superior to those without treatment.6 Filgrastim has been used in an adult cohort to drive blasts into cell cycle for enhanced cytarabine cytotoxicity. Overall survival was not different between the groups.7 Lack of adverse outcome was also observed in patients with myelodysplastic syndromes.8
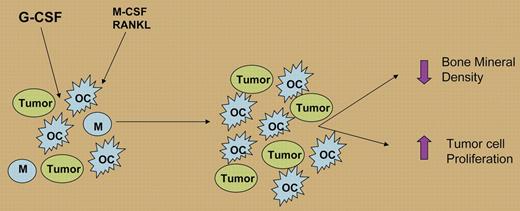
Many more patients with nonmyeloid cancers today receive filgrastim, but is this practice safe? Breast, colorectal, prostate, and lung cancers frequently metastasize to the bone, causing severe morbidity. Injecting a melanoma and a breast cancer line directly into the bone, Hirbe and colleagues observed greater tumor growth through increased osteoclast and decreased osteoblast activities. Several molecules, including PU.1, RANKL, and M-CSF, directly regulate osteoclastogenesis.9 Since the authors found G-CSF receptors on both osteoclasts and precursor cells, suggesting a direct role for filgrastim (see figure).
G-CSF (filgrastim) stimulation in the bone promotes, in concert with M-CSF and RANKL, the expansion and differentiation of macrophages (Ms) to osteoclasts (OCs), increasing osteoclast activity and leading to decreased bone mineral density and increased tumor cell production.
G-CSF (filgrastim) stimulation in the bone promotes, in concert with M-CSF and RANKL, the expansion and differentiation of macrophages (Ms) to osteoclasts (OCs), increasing osteoclast activity and leading to decreased bone mineral density and increased tumor cell production.
Mouse models that have been developed to study metastases have been useful, but in the end, highly passaged cell lines of a particular type in a genetically defined mouse strain cannot mimic the complexity of human cancer. Nonetheless, this study underscores the dynamic relationship between tumor graft and microenvironment, which can be modified by cytokine therapy. Better therapeutic results may arise from greater understanding of the “seed and soil” of cancer metastases. Many factors likely contribute to the declining deaths due to cancer. Filgrastim treatment has become an important part of supportive care that allows patients to tolerate intensive chemotherapy. Investigation into possible secondary effects of this treatment has important clinical implications. To date, increased metastases have not been observed in the clinic.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal