Abstract
Red blood cells from patients with sickle cell disease (SCD) exhibit increased electrogenic cation permeability, particularly following deoxygenation and hemoglobin (Hb) polymerisation. This cation permeability, termed Psickle, contributes to cellular dehydration and sickling, and its inhibition remains a major goal for SCD treatment. Nevertheless, its characteristics remain poorly defined, its molecular identity is unknown, and effective inhibitors have not been established. Here, patch-clamp methodology was used to record whole-cell currents in single red blood cells from healthy individuals and patients with SCD. Oxygenated normal red blood cells had a low membrane conductance, unaffected by deoxygenation. Oxygenated HbS cells had significantly increased conductance and, on deoxygenation, showed a further rise in membrane conductance. The deoxygenation-induced pathway was variable in magnitude. It had equal permeability to Na+ and K+, but was less permeable to NMDG+ and Cl−. Conductance to Ca2+ was also of a similar magnitude to that of monovalent cations. It was inhibited by DIDS (100 μM), Zn2+ (100 μM), and by Gd3+ (IC50 of approximately 2 μM). It therefore shares some properties with Psickle. These findings represent the first electrical recordings of single HbS cells and will facilitate progress in understanding altered red blood cell cation transport characteristics of SCD.
Introduction
Sickle cell disease (SCD) is caused by the presence in red blood cells of mutant hemoglobin, HbS (HbS-containing red blood cells are here called HbS cells, whereas normal HbA-containing red blood cells are called HbA cells). The reduced lifespan of HbS cells contributes to the prevailing anemia which characterizes the disease.1 Furthermore, deoxygenated HbS polymerizes, distorting the red blood cell shape into a variety of elaborate patterns, including the eponymous sickle. Sickled cells participate in vascular occlusion and associated sequelae, including ischemia, organ dysfunction, pain, and, ultimately, death.1 Although the molecular nature of the Hb defect underlying SCD is well established,2,3 details of the pathophysiology are uncertain, and treatment remains largely supportive.4
Dehydration of HbS cells, and particularly of certain HbS cell subpopulations,5 markedly promotes polymerization by reducing the lag time to polymer formation.6 Several membrane transport pathways promote solute loss, but a deoxygenation-induced cation permeability, called Psickle, is pre-eminent (reviewed by Joiner,7 Gibson,8 and Lew9 ). Psickle increases cell membrane permeability to Ca2+ (as well as to monovalent cations), thereby elevating cytosolic [Ca2+]. Subsequent activation of the Ca2+-activated K+ channel (also known as the Gardos channel) mediates particularly rapid K+ loss, with Cl− following via separate pathways.10 Inhibition of Psickle, which will reduce the propensity of cells to shrink, represents an immediate goal for SCD therapy.7,9,11
Permeability studies on Psickle date from the seminal work of Tosteson and colleagues.12 Subsequent radioactive tracer studies indicate that Psickle behaves like a conductive cation channel, lacking selectivity between alkali cations (including Na+ and K+), with moderate permeability to Ca2+ and Mg2+,13–16 whose activation upon deoxygenation is probably stochastic.17 Notwithstanding, its permeability characteristics remain poorly defined, and its molecular identity remains unknown. There is marked variation in the magnitude of Psickle activity between different HbS cells, but conventional techniques have the disadvantage in that Psickle activity in a cell sample is the average from many thousands of cells.17,18 Although DIDS and dipyrimadole inhibit Psickle partially, effective inhibitors have not been identified.14,19
The mechanisms accounting for Psickle activation remain unclear. However, given the clear association with HbS polymerization, and the accompanying increased membrane stress and tension, mechanosensitive cation conductances are a potential candidate. Membrane tension in sickle erythrocytes has been predicted to be greater than 400 dyne/cm2, which is the maximum stress experienced by HbA cells.20 Shear stresses of this magnitude can increase cation permeability of HbA cells, and it is entirely conceivable that this could account for increased cation conductance in deoxygenated HbS erythrocytes. In support of this, Psickle-like stress-induced erythrocyte cation fluxes can be inhibited by DIDS,20 and a variety of other mechanosensitive fluxes and currents have been reported.21,22
In recent years, the application of patch-clamp methodology to erythrocytes has become relatively commonplace.23–27 When examining a heterogeneous cell population like that provided by sickle cells, it has the distinct advantage in that single cells can be studied. Results can be compared with conductances obtained using alternative methodologies.13,14,16,28 In the work reported here, we used the technique to measure the effects of O2 tension on whole-cell conductance of normal and sickle human red blood cells. While our findings constitute the first electrical characterization of sickle cells, we also report a deoxygenation-induced increase in conductance that shares certain properties with Psickle. In addition, we show significant Ca2+ conductance, together with partial inhibition of whole-cell conductance by DIDS and also by Zn2+, which has been used in clinical trials to treat SCD but whose mechanism has not been fully established,29 together with even greater inhibition by Gd3+. Radioactive tracer studies were used to confirm some of our electrical findings.
Materials and methods
Human volunteers
HbA and HbS (HbSS and HbSC) blood samples were obtained by venepuncture from volunteers with full ethical consent (NHS REC reference no. 04/Q1604/20), collected into tubes containing heparin or EDTA, and handled as previously described.30 O2 tension (PO2) was controlled by incubating cells at 37°C in Eschweiler tonometers.31 PO2 was varied (0 or 150 mmHg) by replacement of air with N2 (all at 1 atmosphere). Samples in MOPS buffered saline (MBS; “Solutions,” below) were incubated at approximately 0.1 proportion of 1 hematocrit for at least 15 minutes at each PO2. For electrophysiologic recordings, aliquots were then removed and diluted approximately 10 000-fold into the appropriate bath solution pre-equilibrated to the same PO2. For deoxygenated preparations, the bath was gently superfused with N2 to prevent reoxygenation. Blood samples were kept refrigerated until required, and subsequently studied at room temperature for patch-clamping and at 37°C for radioactive tracer fluxes.
Electrophysiology
Erythrocyte membrane currents were recorded under conventional whole-cell patch-clamp conditions at room temperature, as previously described.32 Pipettes with tip resistances in the range of 8 to 20 MΩ, when filled with the standard pipette solution (“Solutions,” below), were prepared from borosilicate glass capillaries pulled and fire-polished on a Werner Zeitz DMZ programmable puller (Augsburg, Germany). Currents were measured using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA) under the control of pCLAMP software (version 9.2; Axon Instruments), digitized at 10 kHz and filtered at 5 kHz with a 4-pole Bessel filter.
To obtain whole-cell recordings, components of pipette capacitance were first nulled using the Axopatch controls, with the pipette adjacent to the target cell in the bath. The pipette was then pressed gently against the cell and suction was applied to form a gigaseal, which was measured electronically, followed by application of either a brief 1.3 V hyperpolarizing voltage or a short suction pulse to rupture the cell membrane and obtain the whole-cell configuration. No differences in the electrophysiologic responses were observed using these 2 techniques to rupture the membrane. Cell capacitance was then calculated using pCLAMP and compensated electronically, as was series resistance (by > 80%). During recordings, the cell was lifted up to minimize mechanical trauma and care was taken to ensure there was no negative pressure applied to the pipette interior.
Current-voltage (I-V) relationships were obtained by applying a series of 300 ms test potentials from −100 or −80 mV to +100 or +80 mV in 10-mV increments, from a holding potential of 0 or −10 mV. Data for the construction of I-V curves were obtained by averaging the final 50 ms of the current records evoked by the test potential. Data are presented without correcting for the background seal conductance.
Membrane slope-conductance (g) at positive and negative potentials were estimated by calculating the gradient of I-V curves at +85 mV (for g+) and −85 mV (for g−), and also at −10 mV (which approximates erythrocyte resting membrane potential). None of the currents measured in this study demonstrated time-dependent activation or inactivation. This was confirmed by using ramp protocols, in which clamp potential was increased from +100 to −100 mV over 2000 ms from a holding potential of −10 mV, and comparing the resulting I-V relations with those obtained using step protocols.
Solutions
MBS comprised NaCl (145 mM), MOPS (10 mM), and glucose (5 mM) pH-adjusted to 7.4 at 21°C using NaOH. Bath solution contained NaCl (115 mM), MgCl2 (10 mM), CaCl2 (5 mM), and HEPES (10 mM) pH-adjusted to 7.4 at 21°C using NaOH. High levels of divalent cations were used because they facilitate the formation of tight seals.24,25 The standard pipette solution contained NaCl (120 mM), HEPES (5 mM), EGTA (1 mM), and Mg-ATP (1 mM) pH-adjusted to 7.4 at 21°C using NaOH. The presence of EGTA in the pipette solution prevents activation of the Gardos channel. Where required, NaCl in the bath solution was replaced with NMDG-Cl (N-methyl D-glucamine chloride) or KCl. Low Cl− pipette solution contained NaCl (10 mM), sodium gluconate (110 mM), HEPES (5 mM), and EGTA (1 mM) pH-adjusted to 7.4 at 21°C using NaOH. For measurement of Ca2+ conductance, a high-Ca2+ bath solution was used, comprising CaCl2 (82 mM), MgCl2 (10 mM), and HEPES (10 mM) pH-adjusted to 7.4 at 21°C using NaOH, together with a high-Ca2+ pipette solution containing CaCl2 (80 mM), HEPES (5 mM), and Mg-ATP (1 mM) pH-adjusted to 7.4 at 21°C using NaOH. Stock solutions of ZnCl2 (10 mM) and GdCl3 (10 mM) were prepared in distilled water. Stock solutions of ouabain (100 mM), bumetanide (10 mM), clotrimazole (10 mM), and DIDS (10 mM) were prepared in dimethyl sulfoxide. All chemicals were obtained from Sigma-Aldrich, (Poole, United Kingdom).
Radioactive tracer fluxes
K+ influx measurements were made using standard protocols.33 Red blood cell samples were removed from the tonometers and diluted 10-fold into saline (final hematocrit about 0.04 proportion of 1) at 37°C pre-equilibrated at the same O2 tension. Ouabain (100 μM), bumetanide (10 μM), and clotrimazole (10 μM) were always present. 86Rb+ was used as a tracer for K+ and added in 150 mM KNO3 solution to give a final concentration of K+ of 7.5 mM. K+ influx was measured over a 10-minute period at the appropriate O2 tension, after which unincorporated radioisotope was removed by washing in ice-cold isotonic MOPS-buffered MgCl2 saline (pH 7.4). Hematocrit was measured by the cyanomethemoglobin method.
Statistics
Averaged data are shown as the means ± SEM. In all cases, “n” denotes the number of cells tested. Statistical significance was assessed using Student unpaired t test.
Results
Effect of O2 tension on the electrical parameters of normal (HbA) red blood cells
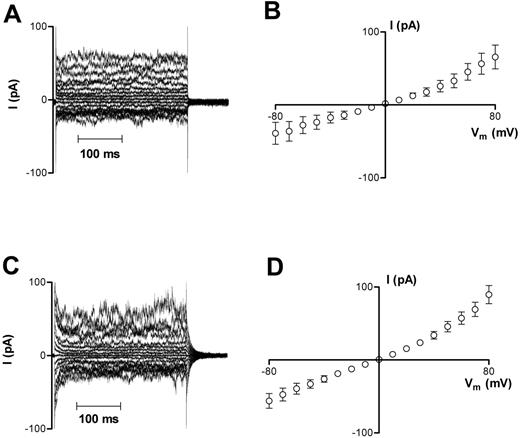
Whole-cell currents were recorded from oxygenated HbA erythrocytes using the protocols outlined in “Materials and methods.” These cells had initially a normal biconcave morphology, which reverted to a sphere upon patching; whole-cell capacitance was 1.20 ± 0.03 pF (n = 48). Measured whole-cell currents were small (< 100 pA; Figure 1A), with a mean membrane slope conductance of 540 ± 189 pS at −10 mV (n = 13) and I-V curves demonstrating outward rectification (g+ = 1292 ± 306 pS, g− = 485 ± 203 pS; Figure 1B) with a reversal potential near 0 mV. Deoxygenated HbA cells had similar characteristics, with membrane currents (Figure 1C) and conductances (Figure 1D) not significantly different from those recorded in oxygenated HbA cells (648 ± 106 pS at −10 mV, n = 34; P > .05).
Representative recordings of whole-cell current measurements in HbA cells. (A-B) Oxygenated HbA cells. (C-D) Deoxygenated HbA cells. Measurements were made using Na+-containing bath and pipette solutions. Current measurements were only included for analysis in this study after they remained stable for 3 successive recordings over a 6 minute period. Averaged results for panels A and C are presented in panels B and D as means ± SEM, n = 13 (oxygenated cells) and n = 34 (deoxygenated cells).
Representative recordings of whole-cell current measurements in HbA cells. (A-B) Oxygenated HbA cells. (C-D) Deoxygenated HbA cells. Measurements were made using Na+-containing bath and pipette solutions. Current measurements were only included for analysis in this study after they remained stable for 3 successive recordings over a 6 minute period. Averaged results for panels A and C are presented in panels B and D as means ± SEM, n = 13 (oxygenated cells) and n = 34 (deoxygenated cells).
Effect of O2 tension on the electrical parameters of red blood cells of patients with sickle cell disease
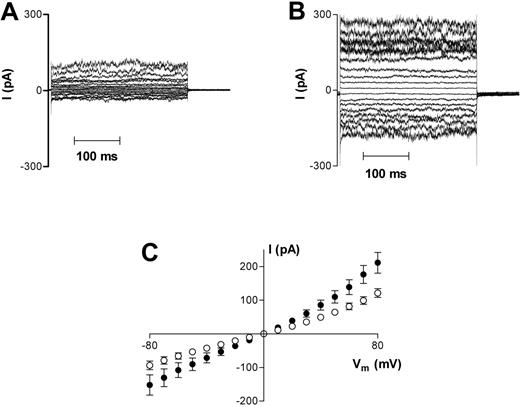
Oxygenated HbS cells also had normal biconcave morphology before patching, reverting to spherical morphology on adhesion to the patch pipette. However, mean whole-cell capacitance of 1.35 ± 0.03 pF in 82 cells was significantly larger than that measured in HbA cells (P < .05). Oxygenated HbS cells (n = 56) also demonstrated significantly (P < .05) larger whole-cell conductances than in HbA (1041 ± 142 pS vs 540 ± 189 pS at −10 mV), but with a smaller degree of rectification (g+ = 2473 ± 258 pS, g− = 1613 ± 208 pS; Figure 2A,C). Reversal potential was again approximately 0 mV.
Representative recordings of whole-cell current measurements in HbS cells. (A) Oxygenated HbS cells. (B) Deoxygenated HbS cells. Measurements were made using Na+-containing bath and pipette solutions. Current measurements were only included for analysis in this study after they remained stable for 3 successive recordings over a 6-minute period. Averaged results are presented in panel C as means ± SEM, n = 56 (oxygenated cells; □) and n = 26 (deoxygenated cells; ▪).
Representative recordings of whole-cell current measurements in HbS cells. (A) Oxygenated HbS cells. (B) Deoxygenated HbS cells. Measurements were made using Na+-containing bath and pipette solutions. Current measurements were only included for analysis in this study after they remained stable for 3 successive recordings over a 6-minute period. Averaged results are presented in panel C as means ± SEM, n = 56 (oxygenated cells; □) and n = 26 (deoxygenated cells; ▪).
When HbS cells were deoxygenated, approximately 15% of the cells adopted the classical “sickle” morphology, and a further 15% to 20% were of irregular shape, as expected. HbS cells with sickle or irregular morphology were selected for whole-cell voltage clamp measurements. These cells also adopted a spherical appearance shortly after adhesion to the pipette. On deoxygenation, HbS cell capacitance was not significantly different from HbA cells (1.24 ± 0.04 pF; n = 34). Whole-cell conductance increased significantly (P < .05) at all membrane potentials tested (g+ = 4772 ± 685 pS, n = 26; g− = 2140 ± 391 pS; at −10 mV, 1798 ± 359 pS; Figure 2B-C).
Effect of ion substitution on membrane currents in HbA and HbS cells
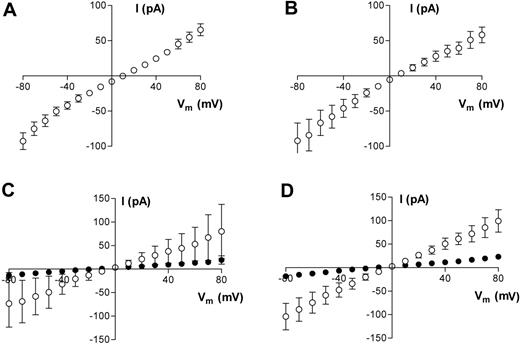
To determine the main charge carriers for the membrane currents that we have measured, we replaced Na+ in the pipette with NMDG+. Under these conditions, outward membrane currents were decreased markedly in both HbA (data not shown) and HbS cells, and I-V reversal potentials were shifted to more positive potentials (Figure 3A-B). Since, by definition, membrane currents are small near the reversal potential, there is significant contamination in the current recordings due to flow through the seal conductance. We adopted a very simplistic method to assess qualitatively the shift in reversal potential upon replacing Na+ in the pipette with NMDG+. Since gigaseal values were measured in each cell, we subtracted from each current record the ohmic component of current arising from the calculated seal leakage. With this simple procedure, we predicted that the reversal potential in both oxygenated and deoxygenated HbS cells shifts by approximately +20 to +25 mV when Na+ in the pipette is replaced by NMDG+. In contrast, when Na+ was replaced with K+, neither conductance nor reversal potentials were significantly affected (P > .05; data not shown). The question then arises of whether Ca2+ is also a permeant cation under our experimental conditions. This is important because of the central role of Ca2+ permeability in the dehydration of HbS cells. We conducted pilot experiments to address this point, using both bath and pipette solutions containing Ca2+ as the main charge carrier. Under these conditions, robust inward and outward membrane currents were recorded in both oxygenated and deoxygenated HbS cells (Figure 3C-D). In oxygenated HbS cells, membrane slope conductance (−10mV) was 830 ± 556 pS (n = 3) and 1129 ± 304 (n = 3) in deoxygenated HbS cells. Since there was also Mg2+ at much lower concentrations in both bath and pipette solutions, we cannot rule out a small component of Mg2+ conductance. These results imply that the measured permeation pathway is equipermeable to Na+ and K, has a reduced permeability to NMDG+, and is also permeable to Ca2+.
Averaged results of whole-cell current measurements in HbS cells. (A,C) Oxygenated HbS cells. (B,D) Deoxygenated HbS cells. All measurements were made using either Na+-containing bath solution and Na+-free pipette solution (NMDG+ substituted for Na+; A-B) or Ca2+-containing bath and pipette solution (C-D). For panels C and D, data are presented before (□) and at least 3 minutes after (▪) Gd3+ (50 μM) addition. Data are presented as means ± SEM, n = 10 (A), n = 8 (B), and n = 3 (C-D).
Averaged results of whole-cell current measurements in HbS cells. (A,C) Oxygenated HbS cells. (B,D) Deoxygenated HbS cells. All measurements were made using either Na+-containing bath solution and Na+-free pipette solution (NMDG+ substituted for Na+; A-B) or Ca2+-containing bath and pipette solution (C-D). For panels C and D, data are presented before (□) and at least 3 minutes after (▪) Gd3+ (50 μM) addition. Data are presented as means ± SEM, n = 10 (A), n = 8 (B), and n = 3 (C-D).
Inhibition of membrane currents in HbA and HbS cells
It is well known that the formation of gigaseals, followed by membrane rupture to obtain the whole-cell configuration of the patch-clamp technique, can result in the activation of endogenous mechanosensitive membrane channels in a variety of cell types.34 To examine whether this response occurs under our experimental conditions, we examined the effects of the classical mechanosensitive channel blocker Gd3+.34
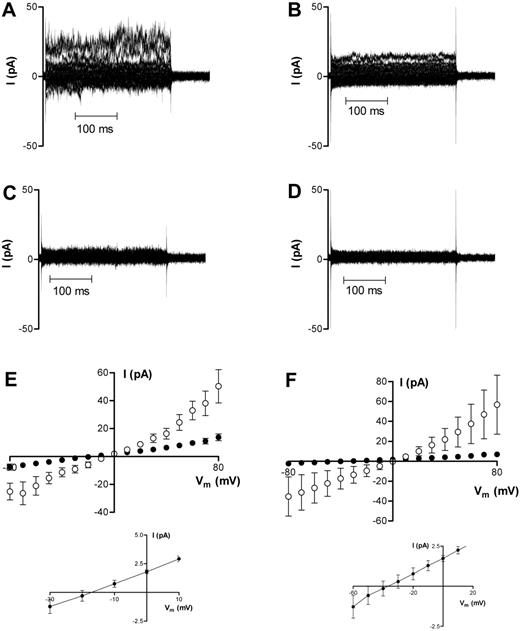
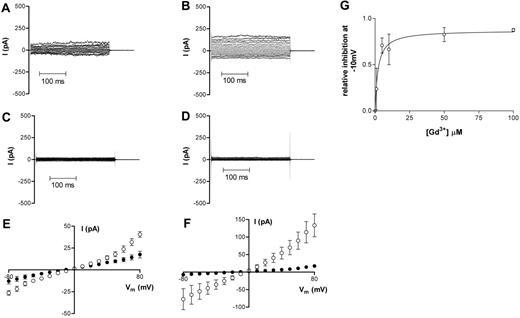
In HbA cells, application of 50 μM Gd3+ to the bath blocked approximately 80% of membrane current at all potentials tested (Figure 4A,C,E), with g+ reduced from 992 ± 291 pS to 273 ± 51 pS in 8 cells and g− reduced to 194 ± 48 pS from 303 ± 73 pS. At −10 mV, 50 μM Gd3+ decreased membrane conductance from 327 ± 90 pS to 105 ± 20.6 pS. Given that the mean seal resistance for these experiments was 11.6 GΩ, this implies that HbA cells have a Gd3+-insensitive membrane conductance of the order 19 pS at −10 mV. With a mean capacitance of 1.2 pF, the specific membrane conductance is then about 16 μS cm−2, assuming that most cell types have a specific membrane capacitance of 1 μF cm−2. When similar experiments were performed with low Cl− solution in the pipette, outward currents were strongly suppressed and the reversal potential of the I-V curve after Gd3+ shifted to more negative potentials, suggesting that the Gd3+-insensitive component of membrane conductance is a Cl− conductance (Figure 4B,D,F).
Representative recordings of whole-cell current measurements in oxygenated HbA cells before and after Gd3+ addition (50 μM). (A-B) Before Gd3+ addition. (C-D) At least 3 minutes after Gd3+ addition. Corresponding averaged data are presented in panels E and F as means ± SEM before (○) and after (▪) Gd3+ addition; n = 6 (E) and n = 5 (F). Measurements presented in panels A, C, and E were made using NaCl-containing bath and pipette solution; measurements presented in panels B, D, and F were made using NaCl-containing bath and Na-gluconate–containing pipette solution. The insets to panels E and F highlight indicate more clearly reversal potential following Gd3+ addition.
Representative recordings of whole-cell current measurements in oxygenated HbA cells before and after Gd3+ addition (50 μM). (A-B) Before Gd3+ addition. (C-D) At least 3 minutes after Gd3+ addition. Corresponding averaged data are presented in panels E and F as means ± SEM before (○) and after (▪) Gd3+ addition; n = 6 (E) and n = 5 (F). Measurements presented in panels A, C, and E were made using NaCl-containing bath and pipette solution; measurements presented in panels B, D, and F were made using NaCl-containing bath and Na-gluconate–containing pipette solution. The insets to panels E and F highlight indicate more clearly reversal potential following Gd3+ addition.
Application of 50 μM Gd3+ to the bath also markedly attenuated membrane currents in HbS cells (Figure 5). For oxygenated cells, g+ was reduced from 1598 ± 871 pS to 348 ± 71 pS in 6 cells and g− was reduced to 257 ± 73 pS from 1034 ± 719 pS (Figure 5A,C,E). After deoxygenation, g+ decreased from 2740 ± 365 pS to 350 ± 103 pS and g− was reduced from 1127 ± 322 pS to 149 ± 37.6 pS (n = 5; Figure 5B,D,F). The IC50 value for the inhibition of whole-cell current in deoxygenated HbS cells by Gd3+ was 2.1 ± 0.85 μM (Figure 5G). Gd3+ had similar inhibitory effects on the Ca2+ conductance in both oxygenated and deoxygenated HbS cells (Figure 3C-D).
Representative recordings of whole-cell current measurements in HbS cells before and at least 3 minutes after Gd3+ addition (50 μM). (A,C,E) Oxygenated HbS cells. (B,D,F) Deoxygenated HbS cells. Data in panels A and B are from before Gd3+ addition; data in panels C and D are from at least 3 minutes after Gd3+ addition. All measurements were made using Na+-containing bath and pipette solutions. Corresponding averaged data are presented in panels E and F as means ± SEM, n = 5 (HbS oxygenated) and n = 5 (HbS deoxygenated). (G) The effect of Gd3+ on whole-cell conductance of deoxygenated HbS cells; dose-response curve. Relative conductance was determined at −10mV in the absence of Gd3+. Serial additions of Gd3+ were then made to the bath solution and, after each addition, a new stable value for whole-cell conductance was obtained. Data are presented as means ± SEM, n ≥ 2-5 for each concentration.
Representative recordings of whole-cell current measurements in HbS cells before and at least 3 minutes after Gd3+ addition (50 μM). (A,C,E) Oxygenated HbS cells. (B,D,F) Deoxygenated HbS cells. Data in panels A and B are from before Gd3+ addition; data in panels C and D are from at least 3 minutes after Gd3+ addition. All measurements were made using Na+-containing bath and pipette solutions. Corresponding averaged data are presented in panels E and F as means ± SEM, n = 5 (HbS oxygenated) and n = 5 (HbS deoxygenated). (G) The effect of Gd3+ on whole-cell conductance of deoxygenated HbS cells; dose-response curve. Relative conductance was determined at −10mV in the absence of Gd3+. Serial additions of Gd3+ were then made to the bath solution and, after each addition, a new stable value for whole-cell conductance was obtained. Data are presented as means ± SEM, n ≥ 2-5 for each concentration.
Other potential inhibitors were also studied on whole-cell currents in deoxygenated HbS cells using Na+-containing bath and pipette solutions. Application of either Co2+ (100 μM) or Cd2+ (100 μM) was without effect (data not shown). Zn2+ (100 μM), however, when added to the bath solution, partially inhibited both inward and outward currents while leaving reversal potential unchanged (Zn2+ reduced conductance at −10 mV to 58% ± 8% of its original value).
In 9 deoxygenated HbS cells, application of 100 μM DIDS decreased membrane slope conductance at +85 mV from 4067 ± 873 pS to 3224 ± 710 pS, representing a significant 25.7% ± 7.0% decrease in conductance (P < .02; each cell used as its own control). The corresponding values at −10 mV were 1408 ± 321 pS versus 1174 ± 304 pS after DIDS addition. This corresponds to a significant 20.0% ± 8.4% decrease (P < .05). The mean membrane slope conductance of this group of cells after DIDS addition was not significantly different from those measured in oxygenated HbS cells (g+ = 2473 ± 258 pS; −10 mV, 1041 ± 142 pS; P > .05). DIDS had no effect on membrane slope conductance in oxygenated HbS cells (g+ 2246 ± 741 pS vs 2371 ± 802 pS after DIDS; n = 7).
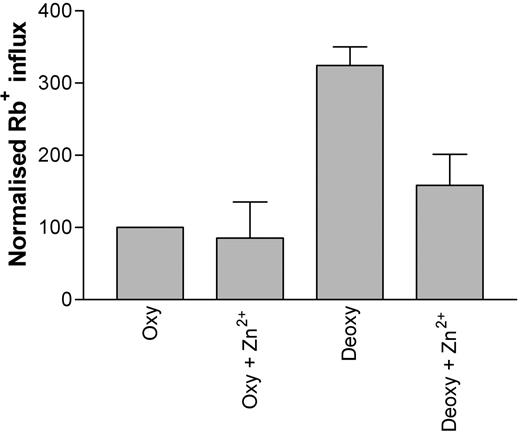
The inhibitor Zn2+ (100 μM) was tested for its ability to inhibit deoxygenation-induced Rb+ influx into HbS erythrocytes. In the presence of Zn2+, a significant attenuation (approximately 50%) of deoxygenation-induced Rb+ influx was noted (Figure 6; P < .05, n = 3). The effect of Gd3+ on deoxygenation-induced Rb+ influx was also tested. However, while Gd3+ (at concentrations greater than 5 μM) was also able to reduce the deoxygenation-induced increase in Rb+ flux, it was noted that the cation caused agglutination of red blood cells, thus preventing a reliable influx measurement.
The effect of Zn2+ (100 μM) on deoxygenation-induced Rb+ influx into HbS erythrocytes. Influx was measured for 10 minutes using 86Rb+ under deoxygenated conditions. Data were normalized due to variations between datasets obtained on different days (*P < .05; n = 3).
The effect of Zn2+ (100 μM) on deoxygenation-induced Rb+ influx into HbS erythrocytes. Influx was measured for 10 minutes using 86Rb+ under deoxygenated conditions. Data were normalized due to variations between datasets obtained on different days (*P < .05; n = 3).
Discussion
In this paper, the whole-cell patch-clamp technique has been used to measure the conductance of individual human red blood cells under oxygenated and deoxygenated conditions. We provide the first report of altered whole-cell currents in HbS cells, from which it is apparent that HbS erythrocytes are different from HbA cells in that they exhibit an oxygen-sensitive conductance. This pathway was permeable to both monovalent and divalent cations. The action of a number of inhibitors has also been described, namely DIDS, Gd3+, and Zn2+, the latter of which has been used clinically in the amelioration of SCD.
Conductance of normal red blood cells
In the experiments reported here, whole-cell conductance of normal red blood cells had a magnitude of approximately 600 pS per cell at −10 mV, which was not significantly affected by O2 tension. Other estimates have been reported using a range of different techniques. Thus, early studies using ionophores gave an anion conductance of approximately 1 μS·cm−2 (using valinomycin35 ). This equates to approximately 1.5 pS per cell, given the surface area of the human red blood cell of 163 × 10−8 cm2. A similar value was calculated for the K+ conductance by estimating the electrogenicity of the Na+/K+ pump in Cl−-free conditions.36 In contrast, human red blood cell membranes extracted from ghosts were found to have a total conductance some 2 orders of magnitude higher, greater than 100 μS·cm−2 or 200 pS per cell.34,37
Although red blood cells have proved difficult to patch because of their small size, high deformability, and atypical membrane composition, in the last few years there have been several reports of both whole-cell and cell-attached recordings of red blood cells. Despite this, there are relatively few reports of whole-cell conductance in normal red blood cells, as opposed to those infected with intraerythrocytic parasites or treated with oxidants or anisotonic media. A low conductance (100 pS per cell) was obtained by Desai and coworkers,24 which is the first report of whole-cell recordings from intact red blood cells. The highest published values, some 1 to 2 orders of magnitude greater, have been reported by Lang and coworkers26 and Rodighiero and colleagues.38 However, more recent reports from Lang have been toward the lower end of this spectrum,39 around 250 pS per cell.
Notwithstanding, even the lowest measurements of erythrocyte whole-cell conductance by patch-clamp methodology are significantly higher than those inferred from the alternate methodologies described, even taking into account the differences to be expected from measurements on single cells and those obtained from multiple cells in suspension. It is well known that application of the patch-clamp technique can activate endogenous mechanosensitive channels, and, in particular, several recent accounts have described cation currents induced in HbA cells by osmotic shock.26,40 Shear-activated cation fluxes permeable to Ca2+, as well as to monovalent ions, and of sufficient magnitude to activate the Gardos channel, have also been reported in HbA cells,20 as well as Gd3+-sensitive Ca2+ fluxes.21 In this context, it is interesting to note that the whole-cell conductance of HbA cells measured here in the presence of Gd3+ (a classical blocker of such channels) more closely approximates that predicted by the alternative methodologies described, on the order of 16 μS cm−2 at −10 mV. Furthermore, in the presence of an inwardly directed Cl− gradient, the reversal potential in the presence of Gd3+ shifts to negative values, indicating that this “background” conductance is mediated by Cl− movement (which is in agreement with previous measurements of Cl− permeability).
Conductance of sickle cells
A major goal of this work was to record whole-cell currents from sickle cells. We found that conductance values of oxygenated HbS cells were increased compared with those obtained from HbA cells. They were also more variable, particularly after deoxygenation, reflecting the marked heterogeneity associated with red blood cells from patients with SCD.41 These findings agree with previous reports of cation permeability of HbS cells, for example, those obtained with radioactive tracer fluxes, which are generally several fold higher than those observed in HbA cells (eg, Joiner and Gibson et al30 ).
Membrane capacitance values were in the range previously reported for erythrocytes.38 Capacitance was slightly, although significantly, increased in oxygenated HbS cells, but there was no statistically significant difference between capacitance values from HbA and deoxygenated HbS cells. This may reflect alterations in membrane morphology resulting from altered interactions of HbA and HbS with the erythrocyte membrane. Alternatively, HbS cells may be simply larger as a consequence of the regenerative anemia characteristic of SCD,1 which leads to a young cell population with greater mean corpuscular volumes (MCVs).
Cell morphology and red blood cell conductance
Activation of Psickle is thought to occur following HbS polymerization.7,42–44 Certainly, the deoxygenation-induced flux in HbS cells correlates with morphologic sickling,42 while substituted benzaldehydes have parallel effects on O2 affinity of HbS, sickling, and Psickle activation.44 Our recordings show a deoxygenation-induced increase in conductance in HbS cells that is not observed in HbA cells, which is reminiscent of the properties of Psickle. We speculate that many intracellular events that mediate activation of Psickle are patent under our experimental conditions, for key intracellular constituents that activate Psickle (HbS or kinase/phosphatase enzymes) may remain membrane associated after adoption of the whole-cell configuration. In support of this postulate, we have preliminary evidence that a dexoygenation-induced flux pathway remains in pink ghosts made from HbS cells, in which bulk hemoglobin is reduced to about 20% of normal concentrations. Notwithstanding, the detailed cellular events responsible for activating Psickle remain to be elucidated.
Inhibition of currents in sickle cells
Finally, we addressed potential inhibitors of the deoxygenation-induced conductance described in HbS cells. We show that DIDS inhibits membrane conductance only in deoxygenated HbS cells. Total membrane slope conductance at −10 mV was inhibited by about 20%, so, since the deoxygenation-induced conductance is of approximately the same magnitude as that of membrane slope-conductance in oxygenated cells (Figure 2C), DIDS blocks approximately 40% of the deoxygenation sensitive component. This is about half the value for DIDS inhibition reported by Joiner and others using detailed flux studies of Rb+ influx.14 Although subtle methodologic differences might account for some of this discrepancy, we must emphasize that the precise quantitative level of DIDS inhibition of membrane conductance in single deoxygenated HbS cells remains open for future determination, for in our experiments the significant between-cell variability in both oxygenated and deoxygenated HbS cells results in the finding that the mean membrane slope conductance in DIDS-treated deoxygenated cells is not significantly different from that in oxygenated cells. An ideal experimental protocol would be to record from an oxygenated HbS cell deoxygenate under conditions identical to those used in the tonometer, and then apply DIDS. This is not technically possible at the present time.
Our demonstration that a significant component of membrane conductance in HbS cells is Gd3+ sensitive also has important implications. Since this is also true, under our experimental conditions, for HbA cells, one conclusion would be that red blood cells have endogenous mechanosensitive cation conductances, which are more active in HbS cells and further potentiated by deoxygenation. While one important caveat is the assumption that Gd3+ sensitivity is a conclusive marker of mechanosensitive conductances, a distinct possibility is that polymerization of HbS upon deoxygenation induces further activation of mechanosensitive channels in HbS cells. However, the situation must be more complex than this, since Gd3+ blocks most membrane conductance in both oxygenated and deoxygenated HbS cells, whereas DIDS (and Zn2+) targets only the deoxygenation-induced component. This raises the possibilities that there are different members of the mechanosensitive channel family (or their subtypes) involved in these responses, and that DIDS (and Zn2+) may interfere with the mechanisms by which the additional conductance is activated. Detailed and comprehensive single-channel recordings and a careful dissection of the signaling cascades involved will clearly be required to address these questions.
Following ion substitutions, the changes in conductance and shifts in reversal potential showed that the permeation pathway could not distinguish between K+ and Na+, was less permeable to NMDG+, and was also permeable to Ca2+. Our data are consistent with the induced current being wholly or mostly carried by both monovalent and divalent cations, and being blocked by DIDS. These features are similar to those ascribed for Psickle, which is DIDS sensitive, shows approximately a 5-fold increase in K+ influx and a nonselectivity of Na+ over K+, and is relatively impermeable to NMDG+.7,9 We speculate tentatively that the deoxygenation-induced current in HbS cells described here is an electrical manifestion of Psickle. If this is the case, permeability via Psickle might be modulated by red blood cell volume changes. There is little information on this aspect in the literature, and concomitant changes in HbS polymerization complicate interpretation, but there are some suggestions that deoxygenation-induced cation fluxes are stimulated by shrinkage.45
We also studied inhibition by a number of divalent cations. Neither Co2+ nor Cd2+ affected the conductances measured in HbS cells, suggesting that it was not mediated by classic Ca2+ channels, several of which have been described in red blood cells.21,46 By contrast, Zn2+ inhibits deoxygenation-induced ion movements in HbS cells, both electrically and in radioisotopic experiments. At a cellular level, the physiology and pathology of Zn2+ remains uncertain, although it is associated with dermal and neuronal function as well as apoptosis.47–49 Notwithstanding, Zn2+ supplementation (given as zinc sulfate at 220 mg 3 times daily) has been shown to ameliorate SCD,29,50 but its mechanism of action has not been investigated. Normal plasma Zn2+ levels are some 10 to 15 μM, and these can be doubled on moderate Zn2+ supplementation (50 mg/day−1),51 making an inhibitory effect on Psickle a distinct possibility.
Summary
In conclusion, the application of patch-clamp methodology to the investigation of HbS cells, allowing examination of single cells, represents a novel and important adjunct to complement more classical studies. The deoxygenation-induced increase in membrane conductance we have observed shares many features of Psickle and may be the electrical manifestation of this entity. Its inhibition by Gd3+, DIDS, and Zn2+ is particularly exciting and warrants further study.
Authorship
Author contributions: J.A.B. executed electrophysiologic recordings. H.M.S. and T.P. advised on electrophysiologic experiments. H.C.R. performed radioisotopic flux measurements. J.C.E. and J.S.G. designed the study and are the grant holders.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Joseph Browning, Department of Physiology, Anatomy and Genetics, Sherrington Building, University of Oxford, Parks Road, Oxford OX1 3PT, United Kingdom; e-mail: joseph.browning@physiol.ox.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Timothy Wallis and Amanda Tattersall for technical assistance.
Supported by Action Medical Research (SP3968) and The Wellcome Trust (071662 and 076441). H.M.S. is a Wellcome Trust Research Career Development Fellow. J.A.B. is a Foulkes Foundation Fellow.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal