Abstract
Polycomb group (PcG) proteins are chromatin modifiers that are necessary for the maintenance and renewal of embryonic and adult stem cells. However, overexpression of the PcG protein, Bmi-1, causes lymphoma in transgenic mice. We show that Bmi-1 is up-regulated in Hodgkin lymphoma (HL) cells by the Epstein-Barr virus (EBV) oncogene latent membrane protein-1 (LMP1) and that this up-regulation is mediated by NF-κB signaling. We also show that Bmi-1 is up-regulated by NF-κB in EBV-negative HL cells. Down-regulation of LMP1 and Bmi-1 decreased the survival of HL cells, suggesting that Bmi-1 may mediate the prosurvival effects of LMP1-induced NF-κB signaling in HL cells. Transcriptional targets of Bmi-1 were identified after its knockdown in an HL cell line. We show here that Bmi-1 and LMP1 down-regulate the ataxia telangiectasia–mutated (ATM) tumor suppressor and conclude that Bmi-1 contributes to LMP1-induced oncogenesis in HL.
Introduction
Classical Hodgkin lymphoma (HL) is derived from germinal center (GC) B cells and is characterized by malignant Hodgkin/Reed-Sternberg (HRS) cells in a background of nonmalignant “reactive” cells.1 The Epstein-Barr virus (EBV) is present in HRS cells in approximately half of all patients with HL, in whom it expresses a restricted set of virus-latent genes; these include the major EBV oncogene latent membrane protein-1 (LMP1).2 By mimicking a constitutively active CD40 receptor, LMP1 activates signaling pathways, such as NF-κB, which enhance B-cell survival and are essential for EBV-induced transformation.3,4
Polycomb group (PcG) genes are necessary for the maintenance and renewal of embryonic and adult stem cells, embryogenesis, and cell cycle regulation.5,6 Two polycomb repressive complexes, PRC1 and PRC2, are required for the initiation and maintenance of gene silencing, respectively.7–9 Bmi-1/PCGF4 (B lymphoma Mo-MLVinsertion region/polycomb group ring finger 4) is a component of PRC1.10,11 Bmi-1 induces lymphoid proliferation and the development of lymphomas in transgenic mice.12–15 Bmi-1 is highly expressed in high-grade large B-cell lymphomas, mantle cell lymphoma, and nonlymphoid malignancies, such as colorectal cancer and non–small cell lung cancer.16–18 Although Bmi-1 is highly expressed in HRS cells,19–21 its regulation and contribution to the pathogenesis of HL are unknown. We show here that Bmi-1 is a transcriptional target of LMP1, that the expression of Bmi-1 promotes the survival of HL cells, and that Bmi-1 induces transcriptional changes in HL cells that include the down-regulation of the ataxia telangiectasia mutated (ATM) gene.
Materials and methods
The work undertaken in the study received ethical approval from the South Birmingham Research Ethics Committee (LREC no.0844).
Cell lines and tissue samples
EBV-negative cell lines from mixed cellularity (MC) HL (KM-H2) and nodular sclerosis (NS) HL (L428)22 were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, and 1% penicillin-streptomycin solution (Sigma-Aldrich, Poole, United Kingdom). An EBV-positive cell line from a patient with NS HL (L591) and an EBV-negative clone of this line (L591-SD3) were grown in the same way.23 Paraffin-embedded HL biopsies were obtained from Queen Elizabeth Hospital, (Birmingham, United Kingdom), and their EBV status was determined by immunohistochemical staining for LMP1.24
Transient transfection of HL lines
Transfection of HL-derived cell lines was performed using the nucleofector unit supplied by Amaxa GmbH and described by Schakowski et al.25 In brief, 2 × 106 KM-H2 cells and 4 × 106 other HL cell lines were pelleted at 1500g for 9 minutes. After resuspension in 100 μL freshly prepared nucleofector solution kit T (catalog no. VCA-1002; Amaxa, Cologne, Germany), 2 μg plasmid DNA was added to KM-H2 and 4 μg was added to the other HL cells. Subsequently, KM-H2 cells were pulsed using program T-01, and the other HL cells were pulsed using program U-09; cells were then incubated in culture media and analyzed after 24 and 48 hours.
Reverse transcription–polymerase chain reaction
Total RNA was extracted from cell lines using the StrataPrep Total RNA Microprep Kit (catalog no. 400805; Stratagene, La Jolla, CA), according to the protocol of the manufacturer. cDNA was synthesized using gene-specific primers in a reverse transcription reaction using AMV reverse transcriptase (Roche, Welwyn Garden City, United Kingdom). Gene transcripts were amplified with the following primers: Bmi-1 forward, 5′-GCCTTCTCTGCTATGTCTGAA-3′, Bmi-1 reverse, 5′-CTGATGAACACACACCAACTT-3′; LMP1 forward, 5′-ACAATGCCTGTCCGTGCAAA-3′, LMP1 reverse, 5′-CTTCAGAAGAGACCTTCTCT-3′; GAPDH forward, 5′-GGTGAAGGTCGGAGTCAACGGA-3′, GAPDH reverse, 5′-GAGGGATCTCGCTCCTGGAAGA-3′; HK2 forward, 5′-GGTGGACAGGATACGAGAAAA-3′, HK2 reverse, 5′-GGGTCCTCTCTGCCAGCAA-3′, ATM forward, 5′-GTGGGTATTCCGACTTTGTT-3′, ATM reverse, 5′-GTGGGTATTCCGACTTTGTT-3′.
PCR was performed using “hot start” (Red Hot Taq DNA Polymerase; Abgene, Epsom, United Kingdom), which consisted of an initial 2-minute denaturation at 94°C, followed by 25 cycles, consisting of a denaturing step for 30 seconds at 94°C, an annealing step for 1 minute at 45°C, and an extension for 1 minute at 72°C. For semiquantitative analysis, 15, 20, and 25 PCR cycles were performed. PCR products were visualized on 2% agarose gels.
Western blot analysis
HL cell lines were washed in cold PBS and lysed in 80 μL lysis buffer (20 mM Tris HCl buffer [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100). Protein concentrations were determined using the Dc protein assay (Bio-Rad, Hercules, CA), and proteins were transferred to nitrocellulose membranes after their separation by 10% SDS-PAGE. After a 1-hour incubation in blocking solution (5% milk in PBS, 0.01% Tween 20), blots were probed overnight with primary antibody for Bmi-1 (clone 229F6; mouse monoclonal diluted 1/1000; Upstate Laboratories, Syracuse, NY), LMP1 (CS1-4, prepared in house; mouse monoclonal diluted 1/50), HK2 (clone-14, goat polyclonal diluted 1/200; Santa Cruz Biotechnology, Santa Cruz, CA) or actin (clone-2, mouse monoclonal 1/500; Santa Cruz Biotechnology). Fifty micrograms of whole cell lysates separated by 6% SDS-PAGE were immunoblotted with ATM antibody (11G12 mouse monoclonal antibody, 1:500).26 After washing in PBS, the secondary peroxidase-labeled antibody (Dako, Bucks, United Kingdom) was added at 1:1000. Proteins were visualized with the enhanced chemiluminescence (ECL) technique (Amersham, Little Chalfont, Buckinghamshire, United Kingdom).
Inhibition of NF-κB activity
HL cells were incubated with the NF-κB inhibitor tosyl-l-lysine chloromethyl ketone hydrochloride (TLCK; Sigma-Aldrich) for 6 hours at a 1:500 dilution (100-mM stock) in growth medium; this proteasome inhibitor prevents the degradation of IκB-alpha and inhibits the processing of the p105 subunit of NF-κB. After treatment, cells were centrifuged, washed twice in cold PBS (pH 7.6), and resuspended in RNA extraction buffer or protein lysis buffer. HL cells were also transiently transfected with an expression vector encoding a mutant form of IκBκ. Because the phosphorylation of this mutant IκBκ is prevented by the substitution of alanine for serine residues at positions 32 and 36, it has a dominant-negative phenotype that prevents the activation of NF-κB by sequestering it in the cytoplasm.27 Finally, HL cells were transfected with pSG5 vectors expressing either wild-type LMP1 or a mutant LMP1 (AxAxA 386 stop) that lacks the CTAR1 and CTAR2 domains, each of which is responsible for NF-κB activation.4
RNA interference
LMP1 expression was knocked down using exogenously supplied oligonucleotides (5′-UUUGCACGGACAGGCAUUG-3′ and 3′-AAACGUGCCUGUCCGUAAC-5′), designed and manufactured by Eurogentec (Seraing, Belgium). Before transfection, 30 μL each of the RNA oligonucleotide solutions (50 μM) was combined with 15 μL annealing buffer (final buffer concentration: 100 mM potassium acetate, 30 mM HEPES-KOH [pH 7.4], 2 mM magnesium acetate). These solutions were incubated for 1 minute at 90°C, briefly centrifuged, and incubated for another hour at 37°C. siRNAs (20 μM) were stored at −20°C until transfection. HL cells were diluted with media to a density of 2.5 × 105 cells per 250 μL in 24-well plates. A mixture containing 3 μL RiboJuice (Novagen, Freiburg, Germany) transfection reagent, 47 μL serum-free medium, and siRNA (final concentration, 2 μM) was added to each well. Plates were left overnight at 37°C in 5% CO2; samples were then centrifuged and resuspended in 1 mL culture medium. Bmi-1 expression was knocked down either by nucleofection of HL cell lines with a pSUPER-retro vector expressing a Bmi-1–specific short hairpin (sh) RNA (target sequence gta ttg tcc tat ttg tga t [gift of Maarten van Lohuizen, The Netherlands Cancer Institute, Amsterdam]) or with an exogenous oligonucleotide (5′-CCAGACCACUACUGAAUAU-3′), as described.
Trypan blue cell viability assay
Cell viability after siRNA transfection was assessed in triplicate using the trypan blue reagent (Sigma-Aldrich). Cell suspension (100 μL) was removed from plates and mixed with 100 μL trypan blue reagent for 2 minutes. Viability was determined by direct counting of unstained cells in a hemocytometer.
Immunohistochemistry
Paraffin-embedded tissues were cut at 5 μm onto adhesive-coated slides (Vectabond reagent; Novocastra Laboratories, Newcastle, United Kingdom). After dewaxing of paraffin sections, endogenous peroxidase activity was blocked by incubation of all slides for 10 minutes in 3% hydrogen peroxide in methanol. Antigens were retrieved by incubation overnight in EDTA 1 mM (pH 8.0)/Tween 20 (0.1%) buffer on a hot-plate stirrer at 65°C. Primary antibodies used were Bmi-1 (dilution 1:50; Upstate Laboratories), LMP1 (dilution 1:50; CS1-4),24 and HK2 (dilution 1:50; Santa Cruz Biotechnology). Detection of bound primary antibody was performed using the Envision IHC Select kit (Dako).
Gene expression analysis
For analysis of gene expression after Bmi-1 knockdown, L428 cells were transfected with a pSUPER retro vector expressing Bmi-1–specific shRNA or control vector, as described. Successful knockdown of Bmi-1 was confirmed using RT-PCR and immunoblotting. Knockdown was performed in triplicate, and pooled RNA from each transfection was used to prepare biotinylated RNA hybridized to HG-U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA). Differentially expressed probe sets were identified using the change algorithm of Affymetrix GCOS with the default settings; only those with “increase” or “decrease” calls were included.
Results
LMP1 induces Bmi-1 expression in HL cells
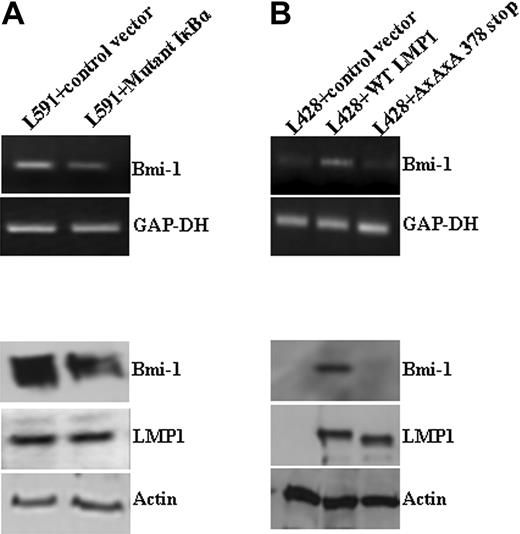
First, we investigated the expression of Bmi-1 in HL cell lines. Figure 1A shows that Bmi-1 was expressed in EBV-positive (L591) and EBV-negative (KMH2, L428) cell lines. We examined the influence of LMP1 on Bmi-1 expression by comparing its expression in EBV-positive and EBV-negative L591 HL cells; loss of EBV was associated with the down-regulation of Bmi-1 expression (Figure 1A). Next we investigated whether LMP1 regulated Bmi-1 expression. The knockdown of LMP1 in L591 cells by specific siRNA led to the down-regulation of Bmi-1 expression (Figure 1B), though the ectopic expression of LMP1 in EBV-negative L428 cells led to its up-regulation (Figure 1C). We concluded that LMP1 can induce the expression of Bmi-1 in HL cells. Ectopic expression of LMP1 in EBV-negative Burkitt lymphoma cell lines did not result in the up-regulation of Bmi-1 expression. However, LMP1 was able to up-regulate the expression of TRAF1, an established LMP1 target in BL cells (Figure S1). These data suggest that among B cells the up-regulation of Bmi-1 by LMP1 might be restricted to certain B-cell lineages.
LMP1 induces Bmi-1 expression in HL cells. (A) Expression of Bmi-1 in EBV-negative HL cell lines L428 and KMH2 and in the EBV-positive (LMP1-positive) L591 HL cell line compared with the EBV-negative variant, L591-SD3. Although Bmi-1 is highly expressed in all parental lines, irrespective of EBV status, loss of the EBV episome from L591 cells resulted in its down-regulation (for all figures, mRNA and protein are shown in upper and lower panels, respectively). (B) Knockdown of LMP1 expression (L591+LMP1 siRNA) down-regulates Bmi-1 expression in EBV-positive L591 cells compared with cells treated with transfection reagent (RiboJuice; Novagen) alone. (C) Ectopic expression of LMP1 in EBV-negative L428 cells up-regulates Bmi-1 expression.
LMP1 induces Bmi-1 expression in HL cells. (A) Expression of Bmi-1 in EBV-negative HL cell lines L428 and KMH2 and in the EBV-positive (LMP1-positive) L591 HL cell line compared with the EBV-negative variant, L591-SD3. Although Bmi-1 is highly expressed in all parental lines, irrespective of EBV status, loss of the EBV episome from L591 cells resulted in its down-regulation (for all figures, mRNA and protein are shown in upper and lower panels, respectively). (B) Knockdown of LMP1 expression (L591+LMP1 siRNA) down-regulates Bmi-1 expression in EBV-positive L591 cells compared with cells treated with transfection reagent (RiboJuice; Novagen) alone. (C) Ectopic expression of LMP1 in EBV-negative L428 cells up-regulates Bmi-1 expression.
LMP1 induction of Bmi-1 is NF-κB dependent
A number of approaches were used to determine whether NF-κB mediates the up-regulation of Bmi-1 by LMP1. First, EBV-positive L591 cells were treated with the broad-spectrum NF-κB inhibitor TLCK; this led to the down-regulation of Bmi-1 (data not shown). Next, L591 cells were transfected with a mutant form of IκBκ, which inhibits NF-κB activity.27 This also down-regulated Bmi-1 expression (Figure 2A). Finally, we showed that whereas the ectopic expression of wtLMP1 in EBV-negative L428 cells resulted in Bmi-1 up-regulation, the expression of a mutant LMP1 (AxAxA386stop LMP1), which is incapable of activating NF-κB, did not (Figure 2B). In keeping with these observations, we were able to demonstrate that loss of the EBV genome, and therefore also LMP1 expression, from L591 cells results in significantly decreased NF-κB activity (data not shown). We concluded that LMP1 induces Bmi-1 expression by activating the NF-κB pathway.
LMP1 induction of Bmi-1 is NF-κB dependent. (A) L591 cells transfected with a plasmid expressing an IκBκ mutant that inhibits NF-κB activation. Expression of the IκBκ mutant resulted in the down-regulation of Bmi-1 expression compared with cells transfected with control vector. (B) L428 cells transfected with control plasmid (empty vector), wild-type (wt) LMP1, or a mutant LMP1 incapable of inducing NFκB (AxAxA378stop). WtLMP1, but not AxAxA378stopLMP1, induced Bmi-1 expression.
LMP1 induction of Bmi-1 is NF-κB dependent. (A) L591 cells transfected with a plasmid expressing an IκBκ mutant that inhibits NF-κB activation. Expression of the IκBκ mutant resulted in the down-regulation of Bmi-1 expression compared with cells transfected with control vector. (B) L428 cells transfected with control plasmid (empty vector), wild-type (wt) LMP1, or a mutant LMP1 incapable of inducing NFκB (AxAxA378stop). WtLMP1, but not AxAxA378stopLMP1, induced Bmi-1 expression.
Bmi-1 expression in EBV-negative HL cells is also NF-κB dependent
We found that Bmi-1 expression in primary HRS cells did not vary with EBV status (Figure 3A; Table 1). Therefore, we examined the influence of NF-κB activity on Bmi-1 expression in EBV-negative HL cells. Bmi-1 was down-regulated in EBV-negative L428 and KMH2 cells after treatment with the NF-κB inhibitor TLCK (Figure 3B) and after expression of the mutant IκBκ (Figure 3C). We concluded that Bmi-1 expression in HL cells is regulated by NF-κB in EBV-positive and EBV-negative HL cells.
Bmi-1 expression in EBV-negative HL cells is also NF-κB dependent. (A) Immunohistochemistry for Bmi-1 in primary HL. (Top) Typical nuclear staining of HRS cells (arrow) observed in most patients. Only 3 of 60 patients lacked Bmi-1 expression. (Middle) Typical image showing lack of HRS cell expression of Bmi-1 (arrow). (Bottom) Mantle zone (MZ) B cells and centrocytes (CC) were Bmi-1 positive in control tonsil, whereas centroblasts (CBs) were negative. Images were acquired using a Zeiss Photomicroscope II equipped with a Nikon Coolpix990 camera (Nikon, Kingston upon Thames, United Kingdom) and using Corel Paint Shop Pro v.10 software (Maidenhead, United Kingdom). Top two images were acquired with a 60 ×/1.40 NA oil-immersion objective lens (Nikon, Kingston upon Thames, United Kingdom); the bottom image, with a 40 ×/0.65 NA dry objective lens. (B) Treatment of L428 cells with the NF-κB inhibitor TLCK resulted in the down-regulation of Bmi-1 expression. Changes in Bmi-1 mRNA (top) and protein (bottom). Similar results were obtained with EBV-negative KMH2 cells (data not shown). (C) KM-H2 cells transfected with a plasmid expressing an IκBκ mutant that inhibits NF-κB activation. Expression of the IκBκ mutant resulted in the down-regulation of Bmi-1 expression compared with cells transfected with control vector.
Bmi-1 expression in EBV-negative HL cells is also NF-κB dependent. (A) Immunohistochemistry for Bmi-1 in primary HL. (Top) Typical nuclear staining of HRS cells (arrow) observed in most patients. Only 3 of 60 patients lacked Bmi-1 expression. (Middle) Typical image showing lack of HRS cell expression of Bmi-1 (arrow). (Bottom) Mantle zone (MZ) B cells and centrocytes (CC) were Bmi-1 positive in control tonsil, whereas centroblasts (CBs) were negative. Images were acquired using a Zeiss Photomicroscope II equipped with a Nikon Coolpix990 camera (Nikon, Kingston upon Thames, United Kingdom) and using Corel Paint Shop Pro v.10 software (Maidenhead, United Kingdom). Top two images were acquired with a 60 ×/1.40 NA oil-immersion objective lens (Nikon, Kingston upon Thames, United Kingdom); the bottom image, with a 40 ×/0.65 NA dry objective lens. (B) Treatment of L428 cells with the NF-κB inhibitor TLCK resulted in the down-regulation of Bmi-1 expression. Changes in Bmi-1 mRNA (top) and protein (bottom). Similar results were obtained with EBV-negative KMH2 cells (data not shown). (C) KM-H2 cells transfected with a plasmid expressing an IκBκ mutant that inhibits NF-κB activation. Expression of the IκBκ mutant resulted in the down-regulation of Bmi-1 expression compared with cells transfected with control vector.
Bmi-1 expression in primary HL
| . | Bmi-1 expression, no./no. total (%) . | ||
|---|---|---|---|
| Negative . | Positive . | Strong positive . | |
| EBV positive | 1/14 (7) | 5/14 (36) | 8/14 (57) |
| EBV negative | 2/46 (4) | 21/46 (46) | 23/46 (50) |
| Total | 3/60 (5) | 26/60 (43) | 31/60 (52) |
| . | Bmi-1 expression, no./no. total (%) . | ||
|---|---|---|---|
| Negative . | Positive . | Strong positive . | |
| EBV positive | 1/14 (7) | 5/14 (36) | 8/14 (57) |
| EBV negative | 2/46 (4) | 21/46 (46) | 23/46 (50) |
| Total | 3/60 (5) | 26/60 (43) | 31/60 (52) |
Immunohistochemistry was used to study Bmi-1 expression in HRS cells, which was recorded as negative, positive (Bmi-1 expression weaker than or as strong as that observed in surrounding lymphocytes), or strong positive (Bmi-1 expression stronger than surrounding lymphocytes).
Bmi-1 promotes the survival of HL cells
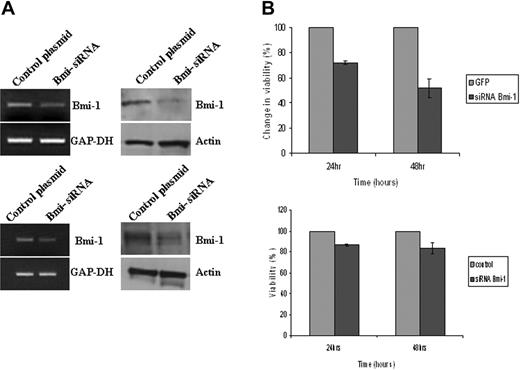
Given that Bmi-1 is an NF-κB target and that NF-κB activation is critical for the survival of HL cells,3 we investigated the contribution of Bmi-1 to HL cell survival. Knockdown of Bmi-1 led to a decrease in the viability of L591 cells and of L428 cells (Figure 4). To confirm the specificity of these effects, we also knocked down Bmi-1 in L591 and L428 cells using an exogenous siRNA directed to a different region of Bmi-1. Compared with cells transfected with a scrambled siRNA, Bmi-1–specific exogenous siRNA reduced the viability of these HL cells (Figure S2). We concluded that in HL cells, Bmi-1 may mediate the prosurvival effects of NF-κB activation.
Bmi-1 promotes the survival of HL cells. (A) Knockdown of Bmi-1 expression in L591 cells (top) and in L428 cells (bottom). mRNA and protein are shown in the left and right panels, respectively. (B) Knockdown of Bmi-1 led to a marked reduction in the viability of L591 cells (upper) at 24 hours and at 48 hours after transfection compared with control cells (GFP-only vector). Knockdown of Bmi-1 in L428 cells had a significant but less marked effect on cell viability (bottom). Error bars represent the standard error of the mean.
Bmi-1 promotes the survival of HL cells. (A) Knockdown of Bmi-1 expression in L591 cells (top) and in L428 cells (bottom). mRNA and protein are shown in the left and right panels, respectively. (B) Knockdown of Bmi-1 led to a marked reduction in the viability of L591 cells (upper) at 24 hours and at 48 hours after transfection compared with control cells (GFP-only vector). Knockdown of Bmi-1 in L428 cells had a significant but less marked effect on cell viability (bottom). Error bars represent the standard error of the mean.
Gene expression profiling reveals Bmi-1–regulated genes in HL cells
Gene expression profiling of L428 cells after Bmi-1 knockdown revealed the up-regulation of 771 probe sets and the down-regulation of 383 (including Bmi-1). The complete list of differentially expressed genes is available in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Bmi-1 down-regulated a large number of genes associated with differentiation (Figure S2), including a number of B-cell lineage markers (eg, CD20/MS4A1, BLK, LY9).
A number of genes induced by Bmi-1 are known to be overexpressed in HL.28–30 These include STAT1 and c-MET; both are known transcriptional targets of LMP1.31–33 We confirmed the changes in the expression of several Bmi-1–induced genes, including hexokinase 2 (HK2), Bcl-2/adenovirus E1B 19-kDa interacting protein-3 (BNIP3), and prolyl 4-hydroxylase alpha subunit (P4HA1) (Figure 5); these genes are induced by LMP1. We were also able to show that ectopic expression of LMP1 in L428 cells led to the up-regulation of HK2 (Figure 6A). Although HK2 was overexpressed in primary HRS cells (Figure 6B), this was unrelated to EBV status.
Validation of Bmi-1 target genes identified by microarray analysis. Knockdown of Bmi-1 expression in L428 cells resulted in the down-regulation of HK2, c-MET, BNIP3, and P4HA1 mRNA (left). Changes in the protein levels of HK2, c-MET, and BNIP3 were also demonstrated (right). P4HA1 could not be confirmed at the protein level because of the lack of a suitable antibody.
Validation of Bmi-1 target genes identified by microarray analysis. Knockdown of Bmi-1 expression in L428 cells resulted in the down-regulation of HK2, c-MET, BNIP3, and P4HA1 mRNA (left). Changes in the protein levels of HK2, c-MET, and BNIP3 were also demonstrated (right). P4HA1 could not be confirmed at the protein level because of the lack of a suitable antibody.
HK2 is a transcriptional target of LMP1 and is overexpressed in HRS cells. (A) Ectopic expression of LMP1 in L428 cells up-regulated the expression of HK2 (mRNA [left] and protein [right]). (B) Immunohistochemistry was used to study the expression of HK2 in primary HL. (Top) Low-level HK2 expression in germinal center (GC) and mantle zone (MZ) B cells of normal tonsil. (Middle, bottom) Strong staining of HK2 in HRS cells (arrow). Images were acquired using a Zeiss Photomicroscope II (Zeiss UK) equipped with a Nikon Coolpix990 camera and using Corel Paint Shop Pro v.10 software. All images were acquired using a 60 ×/1.4 NA oil-immersion objective lens.
HK2 is a transcriptional target of LMP1 and is overexpressed in HRS cells. (A) Ectopic expression of LMP1 in L428 cells up-regulated the expression of HK2 (mRNA [left] and protein [right]). (B) Immunohistochemistry was used to study the expression of HK2 in primary HL. (Top) Low-level HK2 expression in germinal center (GC) and mantle zone (MZ) B cells of normal tonsil. (Middle, bottom) Strong staining of HK2 in HRS cells (arrow). Images were acquired using a Zeiss Photomicroscope II (Zeiss UK) equipped with a Nikon Coolpix990 camera and using Corel Paint Shop Pro v.10 software. All images were acquired using a 60 ×/1.4 NA oil-immersion objective lens.
Bmi-1 and LMP1 down-regulate the ATM tumor suppressor in HL cells
Bmi-1–repressed genes included several tumor-suppressor genes epigenetically inactivated in other cancers (Table 2). Given that we have previously shown that one of these genes, ATM, is not expressed at the protein level in HRS cells,26 this gene was selected for further study. We confirmed that ATM gene expression is induced after Bmi-1 knockdown in the L428 and KM-H2 cell lines using either endogenous knockdown (Figure 7A) or exogenously supplied Bmi-1–specific siRNA, which targets a different region of Bmi-1 (Figure S2). This exogenous siRNA also down-regulates the expression of HK2 (data not shown). Next we showed that ATM expression is lower in EBV-positive L591 cells than in EBV-negative L591 cells and that the knockdown of LMP1 in EBV-positive L591 cells up-regulated ATM expression (Figure 7B). Finally, we showed that ATM was also up-regulated after the inhibition of NF-κB in L428 cells (Figure 7C). We concluded that the down-regulation of ATM by NF-κB may be mediated by Bmi-1.
Tumor-suppressor genes epigenetically inactivated in other cancers that were up-regulated by Bmi-1 knockdown in L428 cells
| Gene symbol . | Gene ID . | Fold change after Bmi-1 knockdown . | Gene title/description . |
|---|---|---|---|
| DLC1 | NM024767 | 2.0 | Deleted in liver cancer 1 |
| ATM | BG623786 | 2.3 | Ataxia telangiectasia mutated gene |
| CASP8 | A1830471 | 2.5 | Caspase 8, apoptosis-related cysteine peptidase |
| ID4 | U16153 | 2.4 | Inhibitor of DNA-binding 4, dominant-negative helix-loop-helix protein |
| IGSF4 | AU146373 | 4.6 | Immunoglobulin superfamily, member 4/TSLC1 |
| TUSC3 | A1760262 | 4.6 | Tumor-suppressor candidate 3 |
| GLIPR1 | AK024153 | 5.2 | GLI pathogenesis related-1 |
| FAM45A | AK025354 | 6.06 | Family with sequence similarity 45, member A |
| SERPINB5 | NM002639 | 7.5 | Serpin peptidase inhibitor, clade B (ovalbumin), member 5 |
| LOX | BE503425 | 21.1 | Lysyl oxidase |
| ST18 | BC025662 | 29.8 | Suppression of tumorigenicity 18 |
| MEG3 | BC036602 | 36.7 | Maternally expressed 3 |
| Gene symbol . | Gene ID . | Fold change after Bmi-1 knockdown . | Gene title/description . |
|---|---|---|---|
| DLC1 | NM024767 | 2.0 | Deleted in liver cancer 1 |
| ATM | BG623786 | 2.3 | Ataxia telangiectasia mutated gene |
| CASP8 | A1830471 | 2.5 | Caspase 8, apoptosis-related cysteine peptidase |
| ID4 | U16153 | 2.4 | Inhibitor of DNA-binding 4, dominant-negative helix-loop-helix protein |
| IGSF4 | AU146373 | 4.6 | Immunoglobulin superfamily, member 4/TSLC1 |
| TUSC3 | A1760262 | 4.6 | Tumor-suppressor candidate 3 |
| GLIPR1 | AK024153 | 5.2 | GLI pathogenesis related-1 |
| FAM45A | AK025354 | 6.06 | Family with sequence similarity 45, member A |
| SERPINB5 | NM002639 | 7.5 | Serpin peptidase inhibitor, clade B (ovalbumin), member 5 |
| LOX | BE503425 | 21.1 | Lysyl oxidase |
| ST18 | BC025662 | 29.8 | Suppression of tumorigenicity 18 |
| MEG3 | BC036602 | 36.7 | Maternally expressed 3 |
Bmi-1 and LMP1 down-regulate expression of the ATM tumor suppressor in HL cells. (A) Knockdown of Bmi-1 expression in L428 cells resulted in the up-regulation of ATM expression. (left) Changes in mRNA. (right) Changes in protein. Knockdown of Bmi-1 in the KM-H2 cell line produced similar effects on ATM expression (data not shown). (B) ATM expression was higher in EBV-negative L591 cells than in EBV-positive L591 cells (left). Knockdown of LMP1 in EBV-positive L591 cells led to the up-regulation of ATM transcription (right). These data show that ATM expression is suppressed by LMP1 in EBV-positive HL cells. (C) Inhibition of NF-κB in L428 and cells up-regulated ATM expression. Similar effects were also observed in KM-H2 cells (data not shown).
Bmi-1 and LMP1 down-regulate expression of the ATM tumor suppressor in HL cells. (A) Knockdown of Bmi-1 expression in L428 cells resulted in the up-regulation of ATM expression. (left) Changes in mRNA. (right) Changes in protein. Knockdown of Bmi-1 in the KM-H2 cell line produced similar effects on ATM expression (data not shown). (B) ATM expression was higher in EBV-negative L591 cells than in EBV-positive L591 cells (left). Knockdown of LMP1 in EBV-positive L591 cells led to the up-regulation of ATM transcription (right). These data show that ATM expression is suppressed by LMP1 in EBV-positive HL cells. (C) Inhibition of NF-κB in L428 and cells up-regulated ATM expression. Similar effects were also observed in KM-H2 cells (data not shown).
Discussion
Here we demonstrate that LMP1, the major transforming protein of EBV, can up-regulate the Bmi-1 oncogene and that the up-regulation of Bmi-1 in EBV-positive and EBV-negative HL cells is mediated by NF-κB. Our observations directly link the overexpression of Bmi-1 in HRS cells to the aberrant NF-κB signaling characteristic of this tumor. LMP1 may be the major regulator of Bmi-1 expression in EBV-positive HL, whereas other activators of NF-κB, such as IκBα mutations, may be responsible for Bmi-1 up-regulation in EBV-negative disease.34,35 Given that Bmi-1 is overexpressed in a wide range of cancers and that NF-κB deregulation is strongly associated with oncogenesis,36 a similar relationship between NF-κB and Bmi-1 should be explored in other cancers.
We found that the viability of HL cells was decreased after Bmi-1 knockdown. Constitutive NF-κB activation is important for the survival of HL cells3,37,38 ; therefore, the influence of NF-κB on HL cell survival may in part be mediated by the ability of NF-κB to induce Bmi-1 expression. Our findings are consistent with those of a recent report that showed Bmi-1 is required for the short-term survival of cancer cells.39
Microarray analysis after the knockdown of Bmi-1 in L428 cells revealed for the first time the impact of Bmi-1 on the cellular transcriptional program in a transformed cell. Consistent with a recent study in which human embryonic fibroblasts were depleted of PRC1 and PRC2 proteins,40 we observed that Bmi-1 down-regulated a large number of differentiation-related genes; several of these were B-cell lineage markers (CD20/MS4A1, BLK, LY9) previously shown to be down-regulated in HRS cells.41 Thus, Bmi-1 may contribute to the loss of B-cell identity, which is characteristic of HL.
Bmi-1 up-regulated a number of genes, including STAT1 and c-MET, which are overexpressed in HL28–30 and which are also known transcriptional targets of LMP1.31–33 MET is the receptor tyrosine kinase for hepatocyte growth factor that induces ERK and PI3K activation and contributes to oncogenesis in other lymphomas.42 Furthermore, we have shown that other Bmi-1–induced genes were also up-regulated by LMP1. One of these, HK2, is overexpressed in HRS cells and in other cancers, where it is essential for the maintenance of high glycolytic activity.43
Bmi-1 also down-regulated a number of tumor-suppressor genes that are epigenetically silenced in cancer. These include IGSF4, which directly binds the PRC1 and PRC2 complexes40 and which we have recently shown to be methylated in most patients with primary HL (P.G.M., G. Davies, H. Li, Y. Tsang, G. Kapatai, J.R.F., W.W., G. Reynolds, A. Ito, C.B.W., R. F. Ambinder, L.S.Y., and Q. Tao, manuscript submitted). The tumor-suppressor gene ATM, whose biallelic inactivation increases susceptibility to lymphoma, was also down-regulated by Bmi-1 and LMP1. We have shown previously that ATM protein expression is lost in most patients with primary HL.26 We show here that NF-κB down-regulates ATM. Although mutation and promoter hypermethylation of ATM are responsible for the inactivation of this gene in hematopoietic and solid malignancies, we have been unable to detect any evidence for either of these mechanisms in HL (S. Bose, J. Starczynski, M.B.C., K.R.N.B., W.W., S. Morgan, P. Byrol, R. Grundy, J. R. Mann, Q. Tao, A.M.R.T., P.G.M., and T. Stankovic, manuscript in preparation). Although our data suggest that the up-regulation of Bmi-1 may be responsible for the loss of ATM expression in HRS cells, the mechanism for the transcriptional down-regulation of ATM has yet to be identified; the observation that Bmi-1 has H2A-K119 ubiquitin E3 ligase activity merits further investigation.44
In conclusion, our data suggest that Bmi-1 contributes to LMP1-induced oncogenesis in HL and provide additional insight into how this PcG protein might contribute to oncogenesis at other sites of cancer.
Authorship
Contribution: A.D. performed the research and wrote the paper. C.B.W. analyzed the data and wrote the paper. M.B.C. performed the immunohistochemistry experiments. J.I.K.L. performed ATM immunoblotting. W.W. analyzed the data. M.V. analyzed the data. K.R.N.B. assisted with microarray work. J.F. generated L591/SD3 cells. M.R. supervised the work with BL cell lines. A.M.R.T. supervised the ATM studies. L.S.Y. designed the research and wrote the paper. P.G.M. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul G. Murray, CRUK Institute for Cancer Studies, The Medical School, University of Birmingham, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: p.g.murray@bham.ac.uk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank the Leukaemia Research Fund, United Kingdom for supporting this project.




![Figure 6. HK2 is a transcriptional target of LMP1 and is overexpressed in HRS cells. (A) Ectopic expression of LMP1 in L428 cells up-regulated the expression of HK2 (mRNA [left] and protein [right]). (B) Immunohistochemistry was used to study the expression of HK2 in primary HL. (Top) Low-level HK2 expression in germinal center (GC) and mantle zone (MZ) B cells of normal tonsil. (Middle, bottom) Strong staining of HK2 in HRS cells (arrow). Images were acquired using a Zeiss Photomicroscope II (Zeiss UK) equipped with a Nikon Coolpix990 camera and using Corel Paint Shop Pro v.10 software. All images were acquired using a 60 ×/1.4 NA oil-immersion objective lens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-05-020545/4/m_zh80060709540006.jpeg?Expires=1769112326&Signature=A46VQ-XapXNi8PrRPKvUV~MUgdhlKQMPMKbHxi4vRKQvMU3inLVJpvqy~KDz3IGLt26QyfASDanMt3ASdbKbsY0L0ja2YCIvuiG72xdmfZnkK9tZbKwHtVPQ2mP0R-30kx6h5UP-zkPxUR2bQPv9zyIYSLXwzMxS8sf5Qj3cbM5jFd0vgqDvdJ8vYbneqXaUEFecXnm5KZMmqHsPp~V2401tNeE~vwVQxH1Krc3D3G7al0E1kf4tpq0eVIPpXYs-3xplDHhQdWvPn4cx8d~PjfIgHz8PlbOpohjbgQ3pQxqp9FAeRnJeZUe3AdfQS-ltrBYoq6d7pemYlO5q0LEfDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal