Abstract
We have identified a population of newly formed bone marrow (BM) B cells that shares multiple characteristics with late transitional B cells in the spleen. Both late splenic transitional B cells and cells within this uncharacterized BM population expressed the cell-surface phenotype AA4+ CD23+, yet the developmental kinetics and the renewal rate of AA4+ CD23+ BM B cells mirrored recently formed BM B cells. Further, unlike the least mature B cells in the BM and spleen, AA4+ CD23+ BM B cells expressed the homing receptor CD62L, were dependent on the antiapoptotic cytokine receptor BR3 and the tec family kinase Btk, and proliferated in response to IL-4 plus CD40 stimulation. Finally, frequencies of λ light chain-positive B cells declined among AA4+ CD23+ B cells in both the BM and spleen, suggesting that V-gene selection events correlate with CD23 expression in both compartments. These observations indicate that the first step in B-cell maturation occurs in both the BM and the periphery and suggest that recently formed B cells exit the BM as a heterogeneous pool of immature and semimature B cells.
Introduction
Newly formed B cells must survive multiple selection pressures before entry into the long-lived mature B-cell pool. Selection events associated with these checkpoints shape the mature B-cell repertoire through several mechanisms that operate to either maintain self-tolerance through the elimination of autoreactive cells or select for B cells via positive selection. Tolerance mechanisms include clonal deletion,1–3 receptor editing,4–7 and exclusion of anergic cells from the long-lived recirculating B-cell pool.8 The mechanisms underlying positive selection are less well characterized but are likely associated with tonic B-cell receptor (BCR) signaling and responsiveness to the antiapoptotic cytokine BLyS/BAFF.9–12 Although the development of mature B cells is clearly influenced by both negative and positive selection, the precise point or points in development where these events occur remain unclear.
Several lines of evidence indicate that selective mechanisms that influence the mature B-cell repertoire occur in the periphery as well as the bone marrow (BM). First, the final phases of B-cell maturation occur after recently formed B cells enter peripheral lymphoid tissues as immature or transitional B cells.13–16 Second, expression of the antiapoptotic BLyS/BAFF receptor BR-3 initiates in late transitional B cells in the spleen,17 and anergic B cells accumulating in the splenic transitional B-cell pool can be induced to mature on in vivo exposure to increased concentrations of BAFF/BLyS.18,19 Third, among adult splenocytes the expressed κ light-chain repertoire is less diverse among mature peripheral B cells than their immature counterparts.20 Interestingly, the λ light-chain repertoire may also be truncated during B-cell maturation because frequencies of λ+ B cells are greater among immature BM B cells than the bulk of peripheral B cells.21 Finally, maintenance of the mature B-cell pool requires sustained surface BCR expression and signaling via Igα.9,11 Signaling via CD19 and the tec family tyrosine kinase Btk also play a role in peripheral B-cell homeostasis, because CD19-deficient and Btk-deficient B cells fail to survive when forced to compete with wild-type B cells.22,23 Collectively, these observations suggest that BCR, CD19, Btk, and BR-3 mediate peripheral B-cell maturation and homeostasis. However, whether these biochemical events act in concert or via nonoverlapping pathways, and whether dependency on each pathway initiates in the BM or the periphery, are unknown.
Immature or transitional B cells in the adult spleen can be subdivided into at least 3 distinct subsets termed T1, T2, and T3.15 Current models predict that B cells within the “T1” population either die or successively mature through the T2 and T3 subpopulations before yielding long-lived follicular or marginal zone (MZ) B cells. Although this linear model may be attractive, a previous study using mathematical modeling of steady-state population dynamics of peripheral B-cell subsets instead suggested a nonlinear model in which the peripheral T2 subset derives from immature B cells in both the BM and periphery.24 Here we describe a “T2-like” subset of BM B cells. Assessment of the steady-state cellular dynamics, expression of developmentally relevant surface molecules, dependence on Btk and the BLyS receptor BR-3, responsiveness to mitogenic stimuli, and frequencies of κ+ and λ+ cells within this and additional BM and splenic B-cell subsets together suggest that early maturation of newly formed B cells occurs in parallel in the BM and the periphery. These data provide experimental evidence in support of a nonlinear model of B-cell maturation.
Materials and methods
Mice
Eight- to 10-week-old BALB/c, C57BL/6 (Ly5B6) and congenic B6.Ly5SJL (often termed B6.Ly5.2) mice were obtained from Jackson Laboratories (Bar Harbor, ME) or the National Cancer Institute (Bethesda, MD). B6.xid congenics were kindly provided by Dr Robert Woodland from the University of Massachusetts (Worcester, MA).
Antibodies
Antibodies were purified and labeled in our laboratory using standard methods or purchased from the indicated vendor. Fluorescein (FL)–labeled antibodies included monoclonal anti-B220 (RA3-6B2) and anti-CD62L (Mel-14, eBiosciences, San Diego, CA), polyclonal Fab fragments of goat anti–mouse IgM (μ-chain specific, Jackson ImmunoResearch, West Grove, PA). Phycoerythrin (PE)–labeled antibodies included CD23 (B3B4, BD PharMingen, San Diego, CA), anti-Ly5SJL (A20, BD PharMingen), anti–mouse κ (187.1, Southern Biotechnology, Birmingham, AL), and anti–mouse λ (λ1 + λ2; JC5-1, Southern Biotechnology). Allophycocyanin (APC)–labeled antibodies included AA4 (AA4.1), B220, and CD21/35. Antibodies conjugated to tandem fluorescent molecules included PE-Cy5.5-anti-CD21/35, PE-Cy7-anti-B220, PE-Cy7 anti-Ly5SJL (A20), APC-Cy5.5 B220, APC-Cy5.5 anti-Ly5B6 (104), and APC-Cy7 anti-IgM (331.12), and anti-CD62L (eBiosciences). Biotin (BI)–conjugated antibodies included anti-Ly5SJL (BD PharMingen), anti-Ly5B6 (104, BD PharMingen), CD23 (BD PharMingen), B220, Fab fragments of goat anti–mouse IgM (μ-chain specific), and anti-IgDb (Igh-5b; 217-170, BD PharMingen). BI-conjugated antibodies were revealed with streptavidin (SA)–conjugated PE-Texas red, PE-Cy5.5, or PE-Cy7 (Caltag, Burlingame, CA).
Cell preparation and staining
BM cells were flushed from tibias and femurs and splenocytes were prepared through perfusion of spleens with FACS buffer (PBS containing 0.5% BSA, 1 mM EDTA, and 0.05% sodium azide). Following lysis of red blood cells with ACT (BioWhittaker, Walkersville, MD), 1 × 106 cells were washed then incubated with optimized dilutions of antibodies in 96-well round-bottom plates in a final volume of 50 μL. After 30 minutes on ice, plates were washed twice with FACS buffer and then, when appropriate, cells were incubated for 20 minutes on ice with an optimal dilution of SA-PE-Texas red in 25 to 40 μL, then washed twice and resuspended in FACS buffer. To determine frequencies of κ+ and λ+ cells, BM or spleen cells were first stained with PE-anti-κ or PE-anti-λ alone. Cells were subsequently washed twice, stained with the remaining antibodies, and then washed and stained with SA-PECy5.5 before analysis. Total BM cells were estimated using the approach of Opstelten and Osmond in which the average number of cells per femur is multiplied by 15.8 (conversion factor for 1 femur to whole body BM).25
Flow cytometry and cell sorting
Analyses were carried out on a dual laser flow cytometer FACScalibur, one of two 4-laser LSR II flow cytometers, or a 3-laser FACAria cell sorter (all from Becton Dickinson, San Jose, CA). All flow cytometry data were analyzed by uploading files into FlowJo 6.3 (TreeStar, San Carlos, CA). Data collected on the LSR II or Aria were subjected to the data transformation algorithm in FlowJo that allows negative cell populations to be viewed as symmetrical clusters.26 For cell sorting stained cells were applied to the FACAria at a sheath pressure of 70 psi and a drop delay frequency of approximately 98 000 drops/s. This resulted in sort trigger rates of 28 000 to 30 000 cells/s.
BrdU incorporation
Continuous in vivo BrdU labeling was performed as previously described15 with the addition of the appropriately conjugated antibodies. Briefly, adult C57BL/6 mice were inoculated intraperitoneally with 0.5 mg BrdU (Sigma, St Louis, MO) in PBS every 12 hours for up to 5 days. BM and spleen cells were stained with PE-CD23, BI anti-IgM, and APC-AA4.1 in standard fluorescence-activated cell sorting (FACS) buffer, washed twice with protein-free PBS, then permeabilized using “Fix and Perm” (Caltag, Burlingame, CA). Subsequently, cells were washed, incubated with DNaseI, washed, then stained with FL-anti-BrdU (Becton Dickinson) before analysis.
Double BM chimeras
The indicated hosts were maintained on water containing a Bactrim suspension (400 mg sulfamethoxazole and 80 mg trimethoprim/500 mL water) for 1 week prior to, and 3 weeks following, lethal (900 R) irradiation. Hosts were irradiated 1 day before retro-orbital injection of a total of 2 × 106 BM cells that were depleted of B-lineage cells with anti-B220 antibodies. Depletions were carried out on LD depletion columns (Miltenyi Biotec, Bergisch Gladbach, Germany) using BI-anti-B220 and SA microbeads (Miltenyi Biotec).
In vitro proliferation
Sorted cells were cultured in flat-bottom 96-well plates at 10 000 cells/well in RPMI 1640 with 10% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 × OPI, 55 μM β-ME, and 10 mM HEPES. The indicated cultures were supplemented with 12.5 μg/mL F(ab′)2 goat anti–mouse IgM (μ-chain specific; Jackson ImmunoResearch Laboratories), anti-CD40 (clone HM40-3, BD PharMingen), or 10 ng/mL IL-4 (R&D Systems, Minneapolis, MN). After 48 hours cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 16 hours before cells were harvested onto Filtermat A filters (PerkinElmer Life Sciences, Shelton, CT), and analyzed on a MicroBeta Trilux scintillation counter (PerkinElmer Life Sciences).
RT-PCR
BM or spleen cells were sorted directly into 400 μL RNeasy RLT buffer from the RNeasy kit (Qiagen, Valencia, CA) containing 1% BME and RNA processed according to the manufacturer's instructions. Using 2 × 105 cell equivalents, gDNA was removed with RNAse-free DNAse 1 and cDNA was prepared using the First Strand cDNA Synthesis Kit from Roche (Indianapolis, IN). Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) was carried out with oligonucleotide primers specific for CD62L using serial 1:5 dilutions of cDNA. GAPDH cDNA was coamplified to normalize RT-PCR reactions. CD62L, 5′-TGGGAAATGGAACGATGACG-3′ (forward) and 5′-AGGAATGAAGAGGGGGTTGTAGTC-3′ (reverse); GAPDH, 5′-CCAAAAGGGTCATCATCTCCG-3′ (forward) and 5′-AGACAACCTGGTCCTCAGTGTAGC-3′ (reverse). PCR products were resolved on a 1.5% agarose gel.
Results
Resolution of CD23− and CD23+ immature BM B cells
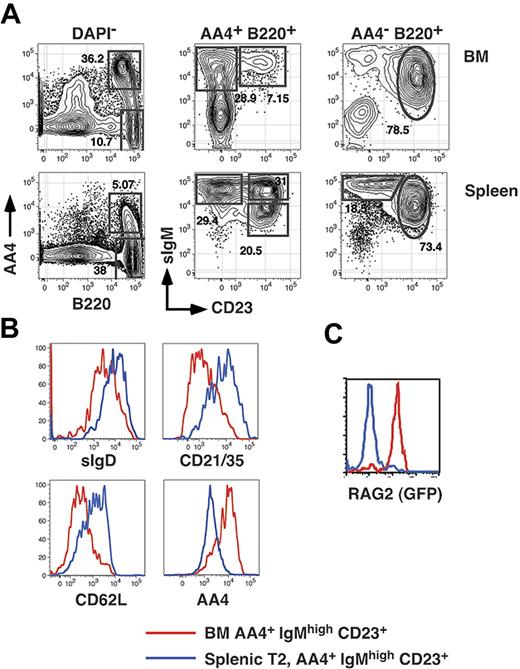
Transitional B cells in the adult spleen can be divided into 3 subpopulations termed T1 (AA4+ CD23−sIgMhigh), T2 (AA4+ CD23+ sIgMhigh), and T3 (AA4+ CD23+ sIgMlow).15 While characterizing the reconstitution kinetics of each splenic transitional B-cell subset, we noted that we were unable to define an early time point during which all splenic B cells were restricted to the least mature (T1) subset. This result was consistent regardless of whether we assessed reconstitution in sublethally irradiated adults or in adult BM chimeras established with Ly5-disparate B220-depleted BM cells (not shown). To address the basis for this result, we first examined CD23 surface expression among immature (AA4+ sIgMhigh, fraction E according to the Hardy nomenclature27 ) BM B cells in normal adult C57BL/6 mice. As shown in Figure 1A, we found that whereas most AA4+ sIgMhigh BM cells are CD23−, 15% to 20% of AA4+ sIgM+ BM cells are CD23+. To further characterize AA4+ sIgMhigh CD23+ BM cells, we compared surface expression of AA4, CD21/35, CD62L, and sIgD on CD23− and CD23+ immature B-cell subsets in the BM and spleen. Surface expression of AA4 decreases and CD21/35, CD62L, and sIgD increases as B cells mature.15,16 As shown in Figure 1B, surface expression of AA4 was higher on AA4+ CD23+ BM cells compared to splenic T2 cells, and surface expression of CD21/35, CD62L, and sIgD was lower on AA4+ CD23+ BM cells relative to cells within the splenic T2 subset.
Resolution of BM and splenic B-cell subsets. (A) BM cells and splenocytes from an 8-week-old C57BL/6 mouse were harvested and stained with FL-anti-IgM, PE-anti-CD23, APC-anti-AA4, and APC-Cy5.5-anti-B220 before collection of 200 000 events on an LSR2 flow cytometer. (B) The identical BM and spleen cells shown in panel A were also stained with PE-Cy5.5-anti-CD21/35, APC-Cy7-anti-CD62L, and BI-anti-IgDb (revealed with SA-PETR). (C) BM and spleen cells from an 8-month-old NG-BAC mouse or an age-matched control were stained with PE-anti-CD23, PE-Cy5.5-anti-B220, APC-anti-AA4, and BI-anti-IgM (revealed with SA-PETR) and RAG2/GFP expression assessed by collecting 200 000 events on an LSR2 flow cytometer. These data are representative of 2 or more separate experiments.
Resolution of BM and splenic B-cell subsets. (A) BM cells and splenocytes from an 8-week-old C57BL/6 mouse were harvested and stained with FL-anti-IgM, PE-anti-CD23, APC-anti-AA4, and APC-Cy5.5-anti-B220 before collection of 200 000 events on an LSR2 flow cytometer. (B) The identical BM and spleen cells shown in panel A were also stained with PE-Cy5.5-anti-CD21/35, APC-Cy7-anti-CD62L, and BI-anti-IgDb (revealed with SA-PETR). (C) BM and spleen cells from an 8-month-old NG-BAC mouse or an age-matched control were stained with PE-anti-CD23, PE-Cy5.5-anti-B220, APC-anti-AA4, and BI-anti-IgM (revealed with SA-PETR) and RAG2/GFP expression assessed by collecting 200 000 events on an LSR2 flow cytometer. These data are representative of 2 or more separate experiments.
In separate experiments we also assessed RAG2 expression in cells within each subpopulation in BM and spleen cells derived from middle-aged (8-month-old) RAG2/GFP reporter (NG-BAC) mice.28 Although as previously reported all splenic transitional B cells are RAG2/GFP+ in young adult NG-BAC mice28 (not shown), we found that all splenic B cells derived from middle-aged (8-month-old) NG-BAC mice were RAG2/GFP−. Significantly, in 8-month-old NG-BAC mice AA4+ sIgMhigh CD23+ BM cells were uniformly RAG2/GFP+ (Figure 1C). Together these data suggest that AA4+ sIgMhigh CD23+ BM cells are developmental intermediates between the least mature CD23− BM B cells and more mature CD23+ peripheral B cells.
Developmental kinetics of AA4+ CD23+ BM B cells
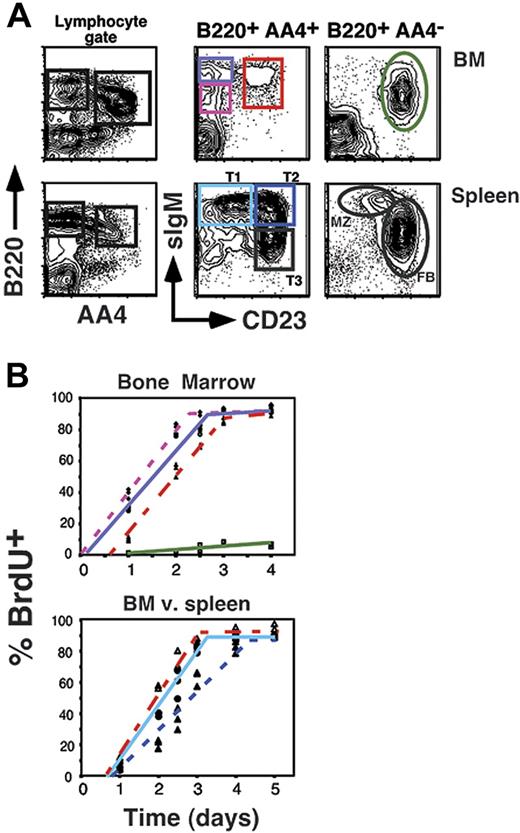
To address the steady-state developmental relationship of AA4+ sIgMhigh CD23+ BM B cells relative to other B-cell subsets in the BM and spleen, we assessed the labeling kinetics for all AA4+ B-cell subsets under conditions of continuous exposure to the thymidine analog BrdU. We previously exploited this strategy to define the developmental kinetics of B-cell subpopulations in the adult spleen.13,15,29 We further reasoned that this strategy might exclude the possibility that AA4+ sIgMhigh CD23+ BM B cells reflect the migration of splenic CD23+ T2 cells into the BM, because in this scenario AA4+ sIgMhigh CD23+ BM B cells would label after the splenic T2 population. However, as shown in Figure 2 AA4+ sIgMhigh CD23+ BM B cells labeled immediately after their CD23− counterparts in the BM, but before the AA4+ CD23+ T2 population in the spleen and congruent with the splenic T1 subset. These data show that AA4+ sIgMhigh CD23+ BM B cells exhibit a relatively rapid turnover rate with a 50% renewal rate of 2 days compared to 3 days for the splenic T2 subset. This result further suggests that AA4+ sIgMhigh CD23+ BM cells are less mature than AA4+ sIgMhigh CD23+ (T2) splenic B cells.
BrdU labeling of BM and peripheral B-cell pools. (A) Flow cytometric analysis showing gating strategy for assessing BrdU incorporation. (B) The 8-week-old C57BL/6 mice (3 mice per time point) were inoculated intraperitoneally with BrdU every 12 hours for the indicated time points. Mice were killed and BM and splenocytes were stained as shown in panel A, then stained intracellularly for BrdU incorporation as described in “Materials and methods.” Populations are as follows: AA4+ B220+ sIgMlow CD23− BM cells, ♦ with dotted pink line; AA4+ B220+ sIgMhigh CD23− BM cells, ○ with solid purple line; AA4+ B220+ sIgMhigh CD23+ BM cells, ▵ with solid red line; AA4−B220+ sIgMlow CD23+ BM cells, ▪ with solid green line; AA4+ B220+ sIgMhigh CD23− splenocytes (splenic T1), • with light blue solid line; AA4+ B220+ sIgMhigh CD23+ splenocytes (splenic T2), ▴ with dotted dark blue line. Data are representative of 2 separate experiments.
BrdU labeling of BM and peripheral B-cell pools. (A) Flow cytometric analysis showing gating strategy for assessing BrdU incorporation. (B) The 8-week-old C57BL/6 mice (3 mice per time point) were inoculated intraperitoneally with BrdU every 12 hours for the indicated time points. Mice were killed and BM and splenocytes were stained as shown in panel A, then stained intracellularly for BrdU incorporation as described in “Materials and methods.” Populations are as follows: AA4+ B220+ sIgMlow CD23− BM cells, ♦ with dotted pink line; AA4+ B220+ sIgMhigh CD23− BM cells, ○ with solid purple line; AA4+ B220+ sIgMhigh CD23+ BM cells, ▵ with solid red line; AA4−B220+ sIgMlow CD23+ BM cells, ▪ with solid green line; AA4+ B220+ sIgMhigh CD23− splenocytes (splenic T1), • with light blue solid line; AA4+ B220+ sIgMhigh CD23+ splenocytes (splenic T2), ▴ with dotted dark blue line. Data are representative of 2 separate experiments.
CD62L expression
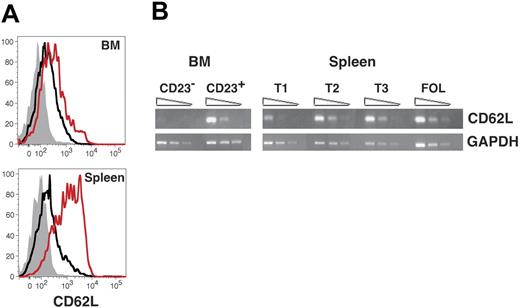
We then performed several studies to determine whether up-regulation of CD23 correlates with functional maturation of immature B cells in the BM. Previous work from Loder et al indicated that expression of the lymph node homing receptor CD62L initiates among late transitional B cells in the spleen.16 We therefore performed both flow cytometric and semiquantitative RT-PCR analyses to determine whether AA4+ sIgMhigh CD23+ BM cells express greater levels of CD62L than CD23− B cells in both the BM and the spleen. Surface levels of CD62L were clearly greater on splenic T2 cells compared to AA4+ sIgMhigh CD23+ BM cells (Figure 1B). However, in both the BM and spleen levels of CD62L were significantly enhanced on AA4+ CD23+ B cells compared to their AA4+ CD23− counterparts (Figure 3A). Moreover, whereas steady-state message levels for CD62L were similar for AA4+ sIgMhigh CD23+ B cells in both the BM and spleen, we observed only low to negative evidence for mRNA expression of the CD62L locus among AA4+ CD23− B cells in both compartments (Figure 3B). We conclude that CD62L expression initiates among immature CD23+ B cells in the BM and spleen.
CD62L expression among developing B cells in the BM and spleen. (A) BM and spleen cells were stained with FL-anti-CD62L, PE-anti-CD23, PE-Cy5.5-anti-B220, APC-anti-AA4, and APC-Cy7-anti-IgM before collection of 200 000 events on an LSR2 flow cytometer. AA4+ B220+ sIgMhigh BM and splenic B cells were further gated as follows using the criteria illustrated in Figure 1A: CD23−, black line, CD23+, red line. Background staining with an isotype-matched control antibody for all BM and splenic AA4+ B220+ sIgMhigh cells is shown with the filled gray histograms. Data are representative of 3 separate experiments. (B) cDNA prepared from the indicated sorted cell populations was subjected to semiquantitative RT-PCR as described in “Materials and methods.” Specific BM populations were AA4+ B220+ sIgMhigh CD23− and AA4+ B220+ sIgMhigh CD23+ as shown in Figure 1.
CD62L expression among developing B cells in the BM and spleen. (A) BM and spleen cells were stained with FL-anti-CD62L, PE-anti-CD23, PE-Cy5.5-anti-B220, APC-anti-AA4, and APC-Cy7-anti-IgM before collection of 200 000 events on an LSR2 flow cytometer. AA4+ B220+ sIgMhigh BM and splenic B cells were further gated as follows using the criteria illustrated in Figure 1A: CD23−, black line, CD23+, red line. Background staining with an isotype-matched control antibody for all BM and splenic AA4+ B220+ sIgMhigh cells is shown with the filled gray histograms. Data are representative of 3 separate experiments. (B) cDNA prepared from the indicated sorted cell populations was subjected to semiquantitative RT-PCR as described in “Materials and methods.” Specific BM populations were AA4+ B220+ sIgMhigh CD23− and AA4+ B220+ sIgMhigh CD23+ as shown in Figure 1.
Survival of AA4+ CD23+ BM B cells is Btk- and BLyS-dependent
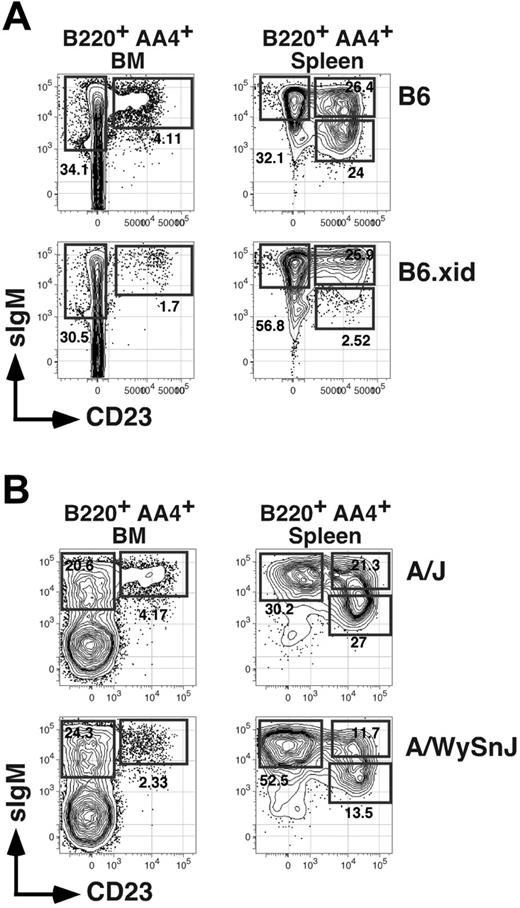
A recent report describing V-gene usage for BM AA4+ CD23+ B cells showed that numbers of these cells are reduced in xid mice bearing a spontaneous point mutation in the Btk gene.30 Consistent with this result, we found that whereas frequencies of CD23− AA4+ BM B cells were unaffected in C57BL/6 mice congenic for the xid mutation (termed B6.xid), frequencies of CD23+ AA4+ BM B cells were reduced 2- to 3-fold in homozygous B6.xid females (Figure 4A). Similarly, analysis of A/WySnJ mice, characterized by a null mutation in the locus encoding the antiapoptotic BLyS receptor BR-3, showed that frequencies and absolute numbers of both AA4+ sIgMhigh CD23+ BM cells and splenic T2 (and T3) B cells declined at least 2-fold compared to age-matched control A/J mice (Figure 4B; Table 1). These data suggest that maturation and survival of AA4+ CD23+ BM B cells requires optimal activity of both Btk and BR-3.
Impact of xid and BR-3 mutation on BM and splenic B-cell subsets. (A) Flow cytometric analysis of BM or spleen cells from an 8-week-old C57BL/6 (B6) or homozygous B6.xid female. Antibody combinations are as described for Figure 1. Data are representative of 4 separate experiments. (B) Separate analysis of 8-week-old A/J and A/WySnJ mice. Data are representative of 3 mice per group (Table 1).
Impact of xid and BR-3 mutation on BM and splenic B-cell subsets. (A) Flow cytometric analysis of BM or spleen cells from an 8-week-old C57BL/6 (B6) or homozygous B6.xid female. Antibody combinations are as described for Figure 1. Data are representative of 4 separate experiments. (B) Separate analysis of 8-week-old A/J and A/WySnJ mice. Data are representative of 3 mice per group (Table 1).
Impact of BR-3 mutation on B-cell subsets in the BM and spleen
| Tissue subset* . | A/J . | A/WySnJ . | ||
|---|---|---|---|---|
| %† . | No.‡ (× 10−6) . | %† . | No.‡ (× 10−6) . | |
| BM | ||||
| CD19+ | 26.4 (1.1) | 84.5 (3.6) | 15.3 (3.1) | 49.0 (10.1) |
| Fr E, CD23− | 3.77 (0.41) | 13.7 (2.0) | 4.52 (0.9) | 11.7 (2.8) |
| Fr E, CD23+ | 1.12 (0.13) | 4.13 (0.5) | 0.59 (0.06) | 1.53 (0.3) |
| Spleen | ||||
| CD19+ | 54.5 (2.2) | 44.4 (0.6) | 13.6 (1.1) | 5.8 (0.3) |
| T1 | 0.26 (0.02) | 0.22 (0.01) | 0.09 (0.13) | 0.04 (0.004) |
| T2 | 0.31 (0.28) | 0.30 (0.03) | 0.03 (0.003) | 0.01 (0.002) |
| T3 | 0.32 (0.04) | 0.30 (0.02) | 0.05 (0.01) | 0.03 (0.006) |
| FOL | 41.7 (1.1) | 33.3 (1.0) | 5.28 (1.3) | 2.17 (0.44) |
| Tissue subset* . | A/J . | A/WySnJ . | ||
|---|---|---|---|---|
| %† . | No.‡ (× 10−6) . | %† . | No.‡ (× 10−6) . | |
| BM | ||||
| CD19+ | 26.4 (1.1) | 84.5 (3.6) | 15.3 (3.1) | 49.0 (10.1) |
| Fr E, CD23− | 3.77 (0.41) | 13.7 (2.0) | 4.52 (0.9) | 11.7 (2.8) |
| Fr E, CD23+ | 1.12 (0.13) | 4.13 (0.5) | 0.59 (0.06) | 1.53 (0.3) |
| Spleen | ||||
| CD19+ | 54.5 (2.2) | 44.4 (0.6) | 13.6 (1.1) | 5.8 (0.3) |
| T1 | 0.26 (0.02) | 0.22 (0.01) | 0.09 (0.13) | 0.04 (0.004) |
| T2 | 0.31 (0.28) | 0.30 (0.03) | 0.03 (0.003) | 0.01 (0.002) |
| T3 | 0.32 (0.04) | 0.30 (0.02) | 0.05 (0.01) | 0.03 (0.006) |
| FOL | 41.7 (1.1) | 33.3 (1.0) | 5.28 (1.3) | 2.17 (0.44) |
B-cell subsets in the indicated 8-week-old mice were defined as follows and as illustrated in Figure 1A: Fr E, B220+ sIgM+ AA4+/− CD23+/−; T1, B220+ AA4+ CD23− sIgMhigh; T2, B220+ AA4+ CD23+ sIgMhigh; T3, B220+ AA4+ CD23+ sIgMlow; FOL, B220+ AA4− CD23+ sIgMlow.
Mean percent of all viable BM or spleen cells derived from 3 (A/J) and 4 (A/WySnJ) mice per group.
Absolute numbers were calculated by multiplying the total cell numbers by the percent cells within each population.
Absolute numbers of BM cells within each gate were calculated using the previously described estimate for the total number of BM cells per adult mouse of 3.2 × 108 (see “Materials and methods”).25
Double BM chimeras reveal Btk-sensitive checkpoints in the BM and periphery
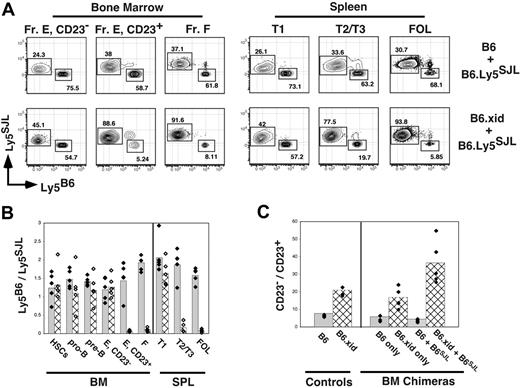
Because frequencies of splenic T2 cells were not reduced in xid mice (Figure 4A), one interpretation of these data is that AA4+ CD23+ BM B cells do not contribute significantly to the mature B-cell pool. However, the relevance of surviving peripheral B cells in xid mice to normal B-cell development is uncertain. Indeed, Sprent and Bruce showed that xid B cells fail to thrive when forced to compete against wild-type B cells in double BM chimeras.22 Because it was unclear at what stage in development Btk-deficient B-lineage cells fail to compete against wild-type cells, we established double BM chimeras in which C57BL/6- or B6.xid B220-depleted BM cells were mixed with equal numbers of wild-type B6.Ly5SJL B220-depleted BM cells before transplantation into irradiated B6.Ly5SJL recipients. Two months later recipients were assessed for the representation of C57BL/6 or B6.xid-derived (both Ly5B6+) versus B6.Ly5SJL-derived (Ly5SJL+) B-lineage cells including BM pro-B and pre-B cells, CD23− and CD23+ AA4+ B cells in the BM and spleen, and mature follicular B cells. To control for engraftment of progenitors from each donor we also assessed the relative contribution of C57BL/6 versus B6.Ly5SJL progenitors to the hematopoietic stem-cell (HSC) pool.
As shown in Figure 5, in the BM Btk-deficient (xid) B lineage, cells effectively competed against wild-type cells within the pro-B, pre-B, and immature sIgMhigh AA4+ CD23− B-cell populations because Ly5B6+ cells ranged from 45% to 55% within each of these populations. Likewise, C57BL/6 and B6.xid progenitors contributed equally to the lineage-negative c-Kithigh Sca-1+ HSC pool (Figure 5B). Remarkably, frequencies of xid B cells in the CD23− splenic T1 population also ranged from 45% to 55% of all B cells in this compartment (Figure 5A-B). In contrast, xid-derived cells failed to effectively contribute to all CD23+ B-cell populations including sIgMhigh AA4+ CD23+ B cells in the BM and splenic T2 B cells despite the striking persistence of xid-derived B cells in the BM AA4+ CD23−sIgMhigh and splenic T1 populations. Significantly, the number of xid-derived B cells in the BM sIgMhigh AA4+ CD23+ population was less in double BM chimeras than in single chimeras generated with xid progenitors only (Figure 5C), demonstrating that Btk activity is required for effective competition of CD23+ immature B cells in the BM as well as the periphery. Together, these data show that development of immature B cells into Btk-dependent B cells correlates with surface CD23 expression in both the BM and periphery.
Diminished competitive fitness of developing xid B cells in the BM and spleen. BM chimeras were established via transplantation of a total of 2 × 106 B220-depleted BM cells into irradiated B6.Ly5SJL hosts. Eight weeks later BM and spleen cells were stained for expression of the indicated markers as illustrated in Figure 1A and allele-specific antibodies to Ly5. Donor BM cells were C57BL/6 (B6), B6.xid (both Ly5B6+), or wild-type B6.Ly5SJL (Ly5SJL+) and double chimeras were established by transferring BM cells from B6.Ly5SJL mice mixed an equal number of BM cells from either C57Bl/6 or B6.xid mice. (A) Representative flow cytometric plots from the indicated double BM chimeras. Gates for each population are as in Figure 1A. Fr E indicates sIgM+ AA4+ BM B cells. (B) Double BM chimeras were assessed for ratios of wild-type C57BL/6 (solid bars with black diamonds) versus B6.xid (hatched bars with white diamonds) BM or splenic B-cell frequencies within the indicated gate divided by the frequency of B6.Ly5SJL-derived (Ly5SJL+) cells in the identical gates. (C) Comparison of the ratios of CD23− to CD23+ immature B cells defined as B220+ AA4+ sIgM+ in normal B6 and B6.xid adults and the indicated single and double chimeras. Data are representative of 2 separate experiments.
Diminished competitive fitness of developing xid B cells in the BM and spleen. BM chimeras were established via transplantation of a total of 2 × 106 B220-depleted BM cells into irradiated B6.Ly5SJL hosts. Eight weeks later BM and spleen cells were stained for expression of the indicated markers as illustrated in Figure 1A and allele-specific antibodies to Ly5. Donor BM cells were C57BL/6 (B6), B6.xid (both Ly5B6+), or wild-type B6.Ly5SJL (Ly5SJL+) and double chimeras were established by transferring BM cells from B6.Ly5SJL mice mixed an equal number of BM cells from either C57Bl/6 or B6.xid mice. (A) Representative flow cytometric plots from the indicated double BM chimeras. Gates for each population are as in Figure 1A. Fr E indicates sIgM+ AA4+ BM B cells. (B) Double BM chimeras were assessed for ratios of wild-type C57BL/6 (solid bars with black diamonds) versus B6.xid (hatched bars with white diamonds) BM or splenic B-cell frequencies within the indicated gate divided by the frequency of B6.Ly5SJL-derived (Ly5SJL+) cells in the identical gates. (C) Comparison of the ratios of CD23− to CD23+ immature B cells defined as B220+ AA4+ sIgM+ in normal B6 and B6.xid adults and the indicated single and double chimeras. Data are representative of 2 separate experiments.
Response to IL-4 plus CD40 stimulation
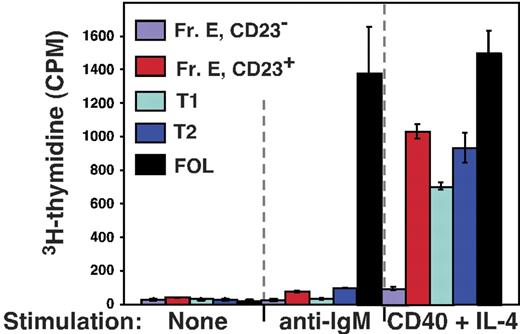
Next we assessed responsiveness of cells within the BM sIgMhigh AA4+ CD23+ population to various B-cell mitogens. Previous work has shown that whereas both splenic T1 and T2 cells are refractory to BCR cross-linking, cells within the T2 population are distinguished from the T1 subset by their capacity to proliferate in response to simultaneous stimulation with IL-4 and antibodies to CD40.31 Accordingly, we sorted cells from CD23− and CD23+ immature (AA4+) B-cell subsets in the BM and spleen as well as mature follicular B cells from 8-week-old C57BL/6 mice and assessed their capacity to enter cell cycle following stimulation with anti-IgM antibodies or IL-4 plus anti-CD40 antibodies. As shown in Figure 6, whereas all AA4+ B cells in the BM and spleen failed to proliferate in response to IgM cross-linking, BM sIgMhigh AA4+ CD23+ B cells exhibited a robust proliferative response to IL-4 plus CD40 stimulation that paralleled the response of splenic T2 cells and exceeded the response of AA4+ CD23− B cells from both the BM and spleen. We conclude that the response of BM sIgMhigh AA4+ CD23+ B cells to BCR or IL-4 plus CD40 stimulation mirrors that of cells within the splenic T2 population.
CD23 expression correlates with responsiveness to IL-4 plus CD40 stimulation in the BM and the periphery. The indicated BM and splenic B-cell subsets were sorted and cultured in triplicate with the indicated stimuli as described in “Materials and methods.” Fr E indicates sIgM+ AA4+ BM B cells. Data represent the mean and error bars indicate the SEM for each group.
CD23 expression correlates with responsiveness to IL-4 plus CD40 stimulation in the BM and the periphery. The indicated BM and splenic B-cell subsets were sorted and cultured in triplicate with the indicated stimuli as described in “Materials and methods.” Fr E indicates sIgM+ AA4+ BM B cells. Data represent the mean and error bars indicate the SEM for each group.
A shared light-chain repertoire selection step
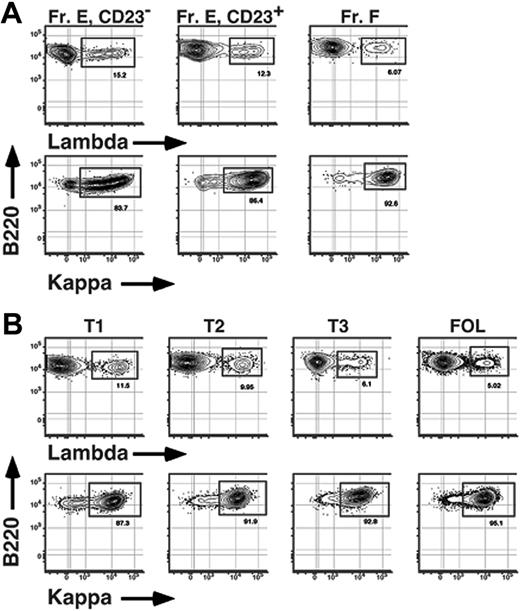
Finally, we sought to determine whether the BCR repertoire is modified as B cells mature into Btk-dependent cells in the BM and periphery. One previous report showed that as many as 16% of immature BM B cells express λ light chains.21 Given that no more than 5% of mature B cells are λ+,32,33 we reasoned that a disproportionate number of newly formed λ+ B cells may fail to enter the mature B-cell pool. To address this issue and test whether any selective loss of λ+ B cells correlated with up-regulation of surface CD23 levels in both the BM and periphery, we determined frequencies of λ+ (λ1 and λ2) and κ+ B cells among subpopulations of sIgM+ B cells in both the BM and spleen of conventional C57BL/6 adults. As shown in Figure 7, among AA4+ sIgM+ cells in the BM, frequencies of λ+ B cells were greater among CD23− B cells compared to AA4+ CD23+ BM cells, ranging from 14% to 16% and 11% to 13% λ+ cells for CD23− and CD23+ B cells, respectively (Figure 7; Table 2). Significantly, a similar trend was observed in the adult spleen; the frequency of λ+ B cells was also greater among the least mature (T1) transitional B cells in the spleen compared to splenic T2 cells, with 11% to 13% of B cells within the CD23− T1 subset and 9% to 10% of CD23+ T2 cells using λ light chains (Figure 7; Table 2). These data demonstrate that functional maturation of Btk-dependent CD23+ immature B cells in the BM and spleen is associated with a light-chain repertoire selection step revealed by a shift in κ/λ ratios.
Selective loss of λ+ B cells during BM and peripheral B-cell maturation. BM (A) and spleen (B) cells from an 8-week-old C57BL/6 mouse were stained with FL-anti-IgM, BI-anti-CD23 (revealed with SA-PECy5.5), APC-anti-AA4, PE-Cy7-anti-B220, and either PE-anti-κ or PE-anti-λ (λ1 + λ2) before collection of 200 000 events on an LSR2 flow cytometer. Fr E indicates sIgM+ AA4+ BM B cells. Data are representative of 5 separate experiments.
Selective loss of λ+ B cells during BM and peripheral B-cell maturation. BM (A) and spleen (B) cells from an 8-week-old C57BL/6 mouse were stained with FL-anti-IgM, BI-anti-CD23 (revealed with SA-PECy5.5), APC-anti-AA4, PE-Cy7-anti-B220, and either PE-anti-κ or PE-anti-λ (λ1 + λ2) before collection of 200 000 events on an LSR2 flow cytometer. Fr E indicates sIgM+ AA4+ BM B cells. Data are representative of 5 separate experiments.
Representation of κ+ and λ+ B cells within BM and splenic B-cell subsets
| Tissue subset* . | % λ+† . | No. λ+‡ (× 10−6) . | % κ+† . | No. κ+‡ (× 10−6) . | Ratio κ/λ§ . |
|---|---|---|---|---|---|
| BM | |||||
| Fr E, CD23− | 15.6 (0.5) | 6.12 (0.18) | 83.2 (1.2) | 32.7 (0.7) | 5.3 |
| Fr E, CD23+ | 12.1 (0.5) | 0.87 (0.08) | 86.5 (0.7) | 6.25 (0.37) | 7.2 |
| Spleen | |||||
| T1 | 12.0 (0.8) | 0.87 (0.5) | 86.5 (1.1) | 5.88 (0.15) | 7.2 |
| T2 | 9.60 (0.4) | 0.65 (0.03) | 91.2 (0.6) | 6.14 (0.4) | 9.5 |
| T3 | 6.01 (0.3) | 0.25 (0.03) | 93.6 (0.7) | 4.14 (0.4) | 15.6 |
| FOL | 4.63 (0.2) | 0.97 (0.04) | 94.8 (0.3) | 21.2 (0.1) | 20.5 |
| Tissue subset* . | % λ+† . | No. λ+‡ (× 10−6) . | % κ+† . | No. κ+‡ (× 10−6) . | Ratio κ/λ§ . |
|---|---|---|---|---|---|
| BM | |||||
| Fr E, CD23− | 15.6 (0.5) | 6.12 (0.18) | 83.2 (1.2) | 32.7 (0.7) | 5.3 |
| Fr E, CD23+ | 12.1 (0.5) | 0.87 (0.08) | 86.5 (0.7) | 6.25 (0.37) | 7.2 |
| Spleen | |||||
| T1 | 12.0 (0.8) | 0.87 (0.5) | 86.5 (1.1) | 5.88 (0.15) | 7.2 |
| T2 | 9.60 (0.4) | 0.65 (0.03) | 91.2 (0.6) | 6.14 (0.4) | 9.5 |
| T3 | 6.01 (0.3) | 0.25 (0.03) | 93.6 (0.7) | 4.14 (0.4) | 15.6 |
| FOL | 4.63 (0.2) | 0.97 (0.04) | 94.8 (0.3) | 21.2 (0.1) | 20.5 |
†Mean percent of gated population of three 8-week-old C57BL/6 mice. SDs are indicated with parentheses.
‡Mean absolute numbers derived from 3 mice were calculated by multiplying the total cell numbers by the percent cells within each population. SDs are indicated with parentheses. Absolute numbers of BM cells within each gate were calculated using the previously described estimate for the total number of BM cells per adult mouse of 3.2 × 108 (see “Materials and methods”).25
§Ratios were calculated by dividing the frequency of κ+ B cells by the frequency of λ+ B cells within each population.
Discussion
With this report we propose a nonlinear model in which peripheral B cells derive from both immature and semimature BM B cells. Five lines of evidence support this model. First, surface CD23 expression can be used to define immature subpopulations of AA4+ sIgMhigh B cells in the BM and the spleen15,29 (Figure 1). Second, the developmental kinetics of AA4+ sIgMhigh CD23+ BM B cells, as determined by both reconstitution experiments and steady-state BrdU labeling, indicate that AA4+ sIgMhigh CD23+ B cells in the BM arise immediately after their CD23− counterparts in the BM yet before CD23+ splenic transitional (T2) B cells (Figure 2 and data not shown). Third, expression of the lymph node homing receptor CD62L initiates among AA4+ CD23+ B cells in both the BM and spleen (Figure 3). Fourth, similar to B cells within the splenic T2 subset, the differentiation and survival of AA4+ sIgMhigh CD23+ BM B cells relies on efficiently synthesizing signals from Btk and the BLyS receptor BR-3 (Figures 4–5; Table 1), and cells within both pools are receptive to proliferative signals derived from costimulation of CD40 and the IL-4 receptor (Figure 6). Finally, frequencies of λ+ B cells decline among CD23+ immature B cells relative to less mature AA4+ sIgMhigh CD23− B cells in both the BM and spleen (Figure 7; Table 2). Together these data illustrate that up-regulation of surface CD23 expression correlates with V-gene repertoire selection and maturation into a Btk-dependent state in both the BM and the periphery.
One previous study used mathematical modeling of steady-state BrdU labeling data to evaluate precursor product relationships among splenic transitional B cells.24 One main conclusion derived from this study was that transitional B cells in the adult spleen derive from more than one source of BM B cells. Our data provide experimental evidence for this model and further suggest that both the BM and periphery provide suitable microenvironments for the generation of BLyS/BAFF- and Btk-dependent cells. This finding raises the question of what role if any unique microenvironments play in the earliest phases of sIgM+ B-cell maturation. Although the final stages of maturation are typically thought to occur uniquely in the periphery,16,34 our data suggest that certain signaling cascades required for B-cell maturation are enacted before many newly formed B cells exit the BM. Testing this idea will require a more detailed understanding of the extracellular and intracellular molecular events required for the initial maturation of newly formed BM B cells.
Our data also add a layer of complexity to studies attempting to estimate the degree of cell loss during B-cell maturation. Previous studies of this issue, including our work,13,15 were based on the assumption that all splenic “T2” transitional B cells derived from the splenic “T1” population. The characterization of a T2-like B-cell subset in the BM raises the possibility that a significant fraction of splenic T2 B cells derive directly from recently formed sIgMhigh CD23+ AA4+ BM cells. Indeed, many recently formed CD23+ BM B cells may contribute directly to the long-lived mature B-cell pool. Consequently, estimations of the fraction of immature splenic B cells that fail to gain entry into the long-lived B-cell pool must be called into question. Perhaps a more comprehensive analysis of the cellular dynamics of all sIgM+ B-cell subsets in the BM and periphery including sIgMhigh CD23+ AA4+ BM cells would shed additional light on this issue.
Why do Btk-deficient B cells fail to survive when forced to compete against wild-type cells in mixed BM chimeras, yet survive and colonize the follicular and MZ B-cell compartments in single BM chimeras and in intact xid mice? Since ablation of the BCR results in the rapid death of mature B cells without imposing competition with wild-type cells,9,11 one interpretation of these data is that Btk is a component of one of several BCR-mediated signaling pathways that together promote B-cell longevity. In this view, less competitive environments, such as in intact xid mice, may decrease the requirement for tonic BCR-mediated signaling events and as a result allow Btk-independent signaling pathways to effectively compensate for the lack of optimal Btk activity. Alternatively, Btk may promote B-cell longevity through a BCR-independent mechanism. Although this possibility may appear remote, it should be emphasized that Btk also regulates cytokine production in mast cells by coupling Fcϵ receptor-mediated activation of Src kinases to both calcium mobilization and NFκB activation.35 Therefore it remains conceivable that Btk mediates the competitive fitness of developing and mature B cells by regulating the expression or function (or both) of a BCR-independent antiapoptotic signaling cascade.
Interestingly, mutation of the BAFF/BLyS receptor BR-3 also leads to reduced competitive fitness of peripheral B cells in a manner reminiscent of the xid mutation.12 Given that BR-3 ligation and Btk activation both lead to NFκB activation,36,37 and BCR cross-linking promotes BR-3 expression,38 it is tempting to speculate that Btk activation via tonic BCR signaling promotes BR-3 expression and hence B-cell longevity. Alternatively, BR-3 ligation might instead directly activate Btk. However, a connection between Btk activity and optimal BR-3 expression appears to conflict with data showing that Btk and BR-3 mutants exhibit rather distinct phenotypes, as Btk-mutant xid mice exhibit near normal absolute numbers of MZ B cells, yet MZ B cells are severely reduced in BR-3– and BLyS-deficient mice.15,39,40 Additional studies into the role of Btk activity in BCR- and BR-3–mediated antiapoptotic pathways are clearly warranted.
Previous work from Chung et al suggested that receptiveness to TH-derived signals such as IL-4 and CD40 ligand first occurs in the periphery among late transitional B cells.31 The capacity of BM AA4+ CD23+ B cells to respond to such signals suggests that a cohort of newly formed BM B cells may be recruited into the T-dependent humoral immune response. Given that BM AA4+ CD23+ B cells are the least mature B-cell subset with the potential to respond to such signals, understanding the extent to which these cells have been subjected to negative and positive selection mechanisms may have important implications for understanding the origin of long-lived self-reactive B-cell clones.
Although κ/λ ratios are mainly set within the pre-B cell pool,41–45 our data (Figure 7) together with data from Dingjan et al21 suggest that a disproportionate number of immature λ+ B cells fail to mature. Interestingly, the emergence of λ+ B cells in self-reactive BCR transgenic mice is often interpreted as evidence for receptor editing via secondary light-chain rearrangements.46,47 Thus, assuming that receptor editing is driven by self-reactivity, it is tempting to speculate that immature λ+ B cells in conventional inbred mice are enriched for cells that have been subjected to one or more self-antigen mediated editing events. If so, κ/λ ratios may shift during B-cell maturation because many immature λ+ B cells remain self-reactive due to a dominant influence of the expressed heavy chain. This possibility is supported by previous work with immunoglobulin transgenic mice in which the 3H9 IgH chain encodes DNA reactivity with a variety of light chains.48 Thus, once self-reactive clones rearrange and express λ light chains, they may become less likely to evade clonal deletion via receptor editing. Alternatively, immature λ+ B cells may be less likely to undergo positive selection due to the failure of tonic BCR stimulation to effectively up-regulate BR-3 expression as proposed by Smith et al.38
Finally, whether qualitatively or quantitatively distinct selection events govern the maturation of AA4+ CD23+ BM and splenic B cells remains to be assessed. In this regard, it is notable that immature CD23+ BM B cells are more dependent on Btk signaling than splenic T2 cells because only the BM subset was noticeably reduced in xid mice without forcing competition with wild-type B cells (Figures 4–5). In contrast, BLyS-binding capacity and dependency on the BLyS receptor BR-3 is increased among CD23+ versus CD23− immature B cells in both the BM and the spleen.17 These observations, together with the positioning of these cells as developmental intermediates in B-cell maturation, lend credence to the notion that initial activation of signaling networks required for positive selection and enhanced B-cell longevity occurs in an asynchronous manner. Consequently, certain clones may bypass the splenic T1 subset and be recruited directly into the late-transitional or mature B-cell pool. Further studies will be required to examine both how Btk and related kinases regulate this developmental transition and the extent to which self-antigens either promote or inhibit B-cell maturation in the BM and periphery.
Authorship
Contribution: R.C.L. designed research, performed research, and analyzed data; M.T. performed research; B.S. performed research; and D.A. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Allman, University of Pennsylvania School of Medicine, 230 John Morgan Bldg, 36th and Hamilton Walk; Philadelphia, PA 19104-6082; e-mail: dallman@mail.med.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AI052861 and AI058066. B.S. was supported by NIH training grant AI055428. D.A. and M.T. are recipients of Career Development Awards from the Leukemia and Lymphoma Society.
We thank Drs Michael Cancro, Juli Miller, and Nina Luning Prak for helpful discussions and critically reviewing this manuscript. We also thank Charles Pletcher and Ryan Wychowanec of the Abramson Cancer Center Flow Cytometry Cell Sorting Facility.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal