Abstract
The present work evaluated antibody-coated liposomes as a new treatment strategy for immune thrombocytopenic purpura (ITP) through the use of a mouse model of the disease. Effects of antimethotrexate antibody (AMI)–coated liposomes and intravenous immunoglobulin (IVIG)–coated liposomes (15, 30, 60 μmol lipid/kg) were compared with the effects of IVIG (0.4, 1, 2 g/kg) and anti–red blood cell (anti-RBC) monoclonal antibody immunotherapy (TER119, 5, 15, 25, and 50 μg/mouse) on MWReg30-induced thrombocytopenia. Each treatment was found to attenuate thrombocytopenia in a dose-dependent manner and, consistent with previous work, IVIG was found to increase antiplatelet antibody clearance in a dose-dependent manner. TER119 demonstrated greater effects on thrombocytopenia relative to other therapies (peak platelet counts: 224% ± 34% of initial platelet counts for 50 μg TER119/mouse versus 160% ± 34% for 2 g/kg IVIG, 88% ± 36% for 60 μmol lipid/kg AMI-coated liposomes, and 80% ± 25% for 60 μmol lipid/kg IVIG-coated liposomes). However, the effects of TER119 were associated with severe hemolysis, as TER119 decreased RBC counts by approximately 50%. The present work demonstrated that antibody-coated liposomes attenuated thrombocytopenia in this model at a much lower immunoglobulin dose than that required for IVIG effects and, in contrast with TER119, antibody-coated liposomes increased platelet counts without altering RBC counts.
Introduction
Immune thrombocytopenia (ITP) is classified as an autoimmune disease in which antibody-coated platelets are phagocytosed by macrophages in the reticuloendothelial system (RES) through Fcγ receptor–mediated or complement-mediated pathways.1 There are about 33 000 new cases of ITP diagnosed in the United States each year.2–4 Platelets play an important role in blood homeostasis and vascular repair; consequently, thrombocytopenic patients are at risk for the development of purpura, petechiae, or even life-threatening bleeding such as intracranial hemorrhage. Corticosteroids, splenectomy, intravenous immunoglobulin (IVIG), anti-D immunotherapy, and plasmapheresis have been used to acutely increase platelet counts in the treatment of ITP.2–4 However, the above therapies are associated with troubling side effects and high cost. In addition, some ITP patients do not respond to any of the existing therapies; therefore, there is substantial need for the development of new strategies to treat this disease.
In 1981, Imbach et al5 reported the therapeutic efficacy of high-dose IVIG in ITP patients. Later, Salama et al6 proposed that IVIG contained anti–red blood cell (anti-RBC) antibodies, which led to the opsonization of RBCs in vivo following IVIG administration. Additionally, Salama et al6 hypothesized that antibody-opsonized RBCs competed for binding to Fcγ receptors, effectively inhibiting the Fcγ receptor–mediated elimination of platelets in ITP patients. Consistent with this hypothesis, anti-D, a polyclonal antibody preparation against the D antigens on the RBC, has been used to treat Rh+ ITP successfully.2,7,8 Although anti-D has been Food and Drug Administration (FDA)–approved to treat ITP, this therapy is rarely associated with intravascular hemolysis, leading to severe anemia and, in very rare cases, death.9,10 Additionally, anti-D has not demonstrated efficacy in D-negative patients or in splenectomized patients.7,8 We have proposed that antibody-coated liposomes may be used in place of anti-D to compete for pathways for platelet elimination in ITP.11
Previous work has shown that antibody-coated liposomes increased platelet counts in a rat model of acute passive ITP.11 A murine model of chronic passive ITP, which may be more similar to human ITP, was developed here. The effects of antibody-coated liposomes were examined and compared with effects observed following treatment with IVIG or treatment with an anti-RBC monoclonal antibody (TER119). Our data showed that antibody-coated liposomes, IVIG, and TER119 increased platelet counts in this model. Antibody-coated liposomes achieved effects at a much lower immunoglobulin dose than that required for IVIG and, in contrast with TER119, antibody-coated liposomes achieved an increase in platelet counts without altering RBC counts.
Materials and methods
Mice
Female Balb/c mice (20 g) were obtained from Harlan (Bar Harbor, ME). Mice were kept under a natural light/dark cycle, maintained at 22 ± 4°C, and fed with standard diet and water ad libitum. All experiments were performed following animal-use protocols that were approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
Reagents
Rat anti–mouse integrin αIIb monoclonal antibody (anti-GPIIB, MWReg30, IgG1) and anti–mouse red blood cell antibody (TER119, IgG1) were purchased from BD PharMingen (San Diego, CA). A murine antimethotrexate IgG1 (AMI) monoclonal antibody was generated and purified in our laboratory.12 IVIG (Gamimune N 10%) was from Bayer (Elkhart, IN). Distearoyl-N-(3-carboxypropionoyl poly (ethylene glycol) succinyl) phosphatidylethanolamine (COOH-PEG2000-PE), cholesterol, and dimyristoylphosphatidylcholine (DMPC) were from Avanti (Alabaster, FL). Methotrexate dimyristoylphosphatidylethanolamine conjugate (MTX-PE) was prepared as previously reported.11 N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-(dimethylamino) propyl) carbodiimide hydrochloride (EDC), Sepharose CL-4B, and other buffer reagents were all from Sigma (St Louis, MO). Buffers were phosphate-buffered saline (PBS), 20 mM Na2HPO4 (PB), and PB plus 0.05% Tween-20 (PB-Tween).
AMI-coated liposomes
AMI-coated liposomes were prepared as previously reported.11 Briefly, liposomes were prepared by the thin-film method.13 MTX-PE, PEG2000-PE, cholesterol, and DMPC were combined in the chloroform at a final lipid concentration of 15 mg/mL. The molar ratio of MTX-PE/PEG2000-PE/cholesterol/PC was 1:10:50:90. Once lipids were thoroughly mixed in the chloroform, the chloroform was evaporated using dry nitrogen gas in a fume hood to form a thin lipid film. Sterile saline was then added to the thin film. The preparation stood for 2 hours at 65°C with agitation every 15 minutes, and then the suspension was downsized by extrusion, in which the lipid suspension was forced twice through 2 polycarbonate filters (100 nm; GE Osmonics Labstore, Minnetonka, MN) under nitrogen gas pressure at 65°C. Liposome suspensions were then incubated with AMI at 37°C for 2 hours (AMI/lipid molar ratio, 1:3000). Following incubation, the liposome mixture was sterilized through a 0.22-μm sterile filter and stored under nitrogen at 4°C.
IVIG-coated liposomes
Briefly, liposomes were prepared by the thin-film method.13 COOH-PEG2000-DSPE, cholesterol, and DMPC were combined in chloroform with a final lipid concentration of 15 mg/mL. The molar ratio of COOH-PEG2000-DSPE/cholesterol/DMPC in liposomes was 10:50:90. Once the lipids were thoroughly mixed in the chloroform, the chloroform was evaporated using dry nitrogen gas in a fume hood to form a thin lipid film. Sterile saline was then added to the thin film. The preparation was incubated for 2 hours at 75°C with agitation every 15 minutes, and then the suspension was downsized by extrusion, in which the lipid suspension was forced twice through 2 polycarbonate filters (100 nm; GE Osmonics Labstore) under nitrogen gas pressure at 75°C. Coupling of IVIG to the plain liposomes was prepared by a well-established method in which the carboxyl group of COOH-PEG2000-DSPE was conjugated with the amine group of immunoglobulin, as catalyzed by EDC.14 Briefly, 357 μL of the plain liposome suspension (6 μmol lipids) in MES buffer, 120 μL of 0.25 M EDC in water, and 120 μL of 0.25M NHS in water were combined. This mixture was incubated for 10 minutes at room temperature and pH was adjusted to 7.5 with 1 M NaOH. IVIG, 23.85 μL (100 mg/mL), was then added to this mixture, and the suspension was incubated for 8 hours at 4°C with gentle stirring (IVIG/lipid molar ratio, 1:375). IVIG-coated liposomes were separated from unbound IVIG on a Sepharose CL-4B column pre-equilibrated with saline. Peak fractions containing IVIG-coated liposomes were collected, pooled, sterilized through a 0.22-μm sterile filter, and stored under nitrogen at 4°C.
Characterization of liposomes
The size distribution of liposomes was determined by dynamic light scattering (submicron particle sizer, NICOMP 380; Omega Scientific, Tarzana, CA). Lipid concentration was determined by the modified Bartlett method described by Rouser et al.15 Protein concentration was determined by a modified Lowry method.16
ELISA
MWReg30 concentrations in mouse plasma were determined through a species-specific enzyme-linked immunosorbent assay (ELISA) modified from that reported by Hansen and Balthasar.17 Briefly, Nunc Maxisorp 96-well microplates (Nunc model no. 4-42404; Roskilde, Denmark) were coated with goat antirat antibody (Sigma R5130, 1:500 in PB, 0.25 mL/well) and incubated at 4°C overnight. Samples and standards (0.25 mL/well) were then added, and the plates were incubated for 2 hours at room temperature. Goat antirat antibody–alkaline phosphatase conjugate (Rockland 602-105-120, Gilbertsville, PA; 1:500 in PB Tween, 0.25 mL/well) was added and allowed to incubate for 1 hour at room temperature. P-nitrophenyl phosphate (Pierce, Rockford, IL; 4 mg/mL in diethanolamine buffer, pH 9.8, 0.2 mL) was placed in each well and the change in absorbance with time (over 10 min) was monitored via a microplate reader at 405 nm (Spectra Max 340; Molecular Devices, Sunnyvale, CA). Standards were made by diluting a stock MWReg30 solution, with PBS, to the appropriate concentration (0, 2.5, 5, 10, 20, and 30 ng/mL) and by adding rat plasma to a final concentration of 1% (vol/vol). Standard curves were linear in this concentration range. Intra-assay and interassay variability was approximately 10% at the limit of quantification (2 ng/mL).
IVIG concentrations in mouse plasma were determined through a species-specific ELISA. Briefly, Nunc Maxisorp 96-well microplates (Nunc model no. 4-42404) were coated with Fc-specific goat antihuman antibody (Sigma I-2136; 1:500 in PB, 0.25 mL/well) and incubated at 4°C overnight. Samples and standards (0.25 mL/well) were then added, and the plates were incubated for 2 hours at room temperature. Fab-specific goat antihuman antibody–alkaline phosphatase conjugate (Sigma A-8542; 1:500 in PB Tween, 0.25 mL/well) was added and allowed to incubate for 1 hour at room temperature. P-nitrophenyl phosphate (Pierce; 4 mg/mL in diethanolamine buffer, pH 9.8, 0.2 mL) was placed in each well and the change in absorbance with time (over 10 min) was monitored via a microplate reader at 405 nm. Standards were made by diluting a stock IVIG solution with PBS to the appropriate concentration (0, 10, 20, 30, 40, and 60 ng/mL) and by adding rat plasma to a final concentration of 1% (vol/vol). Standard curves were linear in this concentration range. Intra-assay and interassay variability was approximately 10% at the limit of quantification (10 ng/mL).
Induction of passive chronic immune thrombocytopenia
Sustained ITP was induced in mice by intraperitoneal infusion of the antiplatelet antibody MWReg30. Briefly, MWReg30 was continuously administered using an osmotic pump implanted into the peritoneal cavity (Alzet micro osmotic pump, model 1009; Alza, Palo Alto, CA). The osmotic pumps were filled with 100 μL MWReg30 (82.5 μg/mL) and bovine serum albumin (1.5 mg/mL) in sterile saline and then surgically inserted into the peritoneal cavity of mice. Following insertion, the pumps released the MWReg30 solution at a rate of 0.5 μL/h for the duration of the study (168 h).
Treatment of passive chronic immune thrombocytopenia
Four days after the initiation of the MWReg30 infusion, animals were treated with IVIG (0.4, 1, or 2 g/kg, via intraperitoneal injection), AMI-coated liposomes (15, 30, or 60 μmol lipid/kg, via intravenous injection), IVIG-coated liposomes (15, 30, or 60 μmol lipid/kg, via intravenous injection), TER119 (5, 15, 25, 50 μg/mouse, via intravenous injection), saline via intraperitoneal injection, or saline via intravenous injection. IVIG was administered intraperitoneally because it was not feasible to administer the required volume (ie, 20 mL/kg for the 2 g/kg dose) by intravenous injection to the mice. Blood samples were taken before pump implantation (time 0) and at 96, 97, 99, 102, 108, 120, 144, 168, 192, 216, 240, and 264 hours after pump implantation. Twenty microliters whole blood was collected from the retro-orbital plexus using Unopette capillary pipette (Becton Dickinson, Franklin Lakes, NJ) prerinsed with 10% ethylenediaminetetraacetic acid in double-distilled water. Ten microliters whole blood was then further diluted with Cell-Dyn diluent buffer to a final dilution of 1:5. Platelet counts, RBC counts, and hemoglobin (HGB) values were determined using a Cell-Dyn 1700 multiparameter hematology analyzer (Abbott Laboratories, Abbott Park, IL). Platelet counts, RBC counts, and HGB values were normalized by the initial counts observed for each mouse and data are presented as a percentage of baseline values. Each group consisted of 5 mice.
Pharmacokinetic analysis
Ten microliters blood was centrifuged at 13 000g for 2 minutes and then plasma was isolated and stored at 4°C until analysis (usually within 3 days). Plasma IVIG and MWReg30 concentrations were determined by ELISA. Due to the low concentrations of MWReg30 observed in this study, plasma samples from different mice in the same group were pooled together in order to allow accurate measurement of MWReg30 concentrations. Therefore, only mean MWReg30 plasma concentrations were obtained. The area under the MWReg30 plasma concentration time curve from 96 hours to 264 hours (AUC96-264h) was calculated by WinNonlin (Pharsight, Mountain View, CA).
Statistical analysis
Differences in time-course data (ie, platelet counts, red blood cell counts, hemoglobin levels) were tested for statistical significance using 2-way, repeated-measures analysis of variance (ANOVA). Differences in peak platelet counts for selected treatments were assessed by unpaired t tests. Statistical analyses were accomplished using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) and the level of significance for each test was α = 0.05.
Results
Characterization of liposomes
The liposome preparations, which are truly unique distributions of liposome particles, demonstrated normal distributions of particle size, with mean liposome diameters of 106 ± 4.5 nm and 116.3 ± 5.2 nm for AMI-coated liposomes and IVIG-coated liposomes, respectively. The protein-lipid ratios for AMI-coated liposomes and IVIG-coated liposomes were 65 ± 6 μg AMI/μmol lipid and 30 ± 5 μg IVIG/μmol lipid.
Murine chronic ITP model
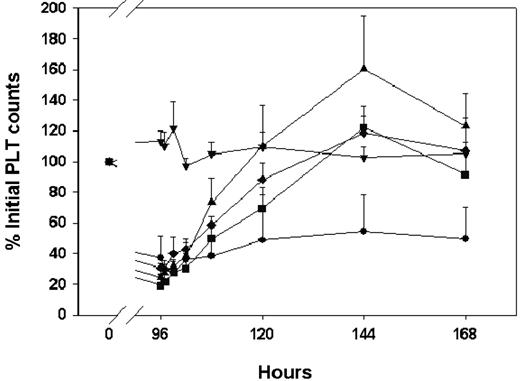
Platelet counts in sham animals remained close to 100% of the pretreatment value throughout the study. In control animals continuously infused with the antiplatelet antibody (MWReg30) and treated with intraperitoneal saline at 96 hours, platelet counts were reduced to 37% ± 14% of the initial values at 96 hours (287 ± 102 k/μL), and platelet counts remained relatively constant from 96 to 168 hours (Figure 1). All animals developed multiple petechiae that were distributed over the skin.
IVIG effects on the time course of MWReg30-mediated thrombocytopenia in mice after continuous intraperitoneal infusion of antiplatelet monoclonal antibody MWReg30. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days). IVIG was given by intraperitoneal bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group and IVIG treatment groups (n = 5 mice/group): sham control group (▾), saline (•), 0.4 g/kg (▪), 1 g/kg (♦), 2 g/kg (▴). Error bars represent the standard deviation. IVIG attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
IVIG effects on the time course of MWReg30-mediated thrombocytopenia in mice after continuous intraperitoneal infusion of antiplatelet monoclonal antibody MWReg30. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days). IVIG was given by intraperitoneal bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group and IVIG treatment groups (n = 5 mice/group): sham control group (▾), saline (•), 0.4 g/kg (▪), 1 g/kg (♦), 2 g/kg (▴). Error bars represent the standard deviation. IVIG attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
IVIG pharmacokinetics and IVIG effects in the mouse model of chronic passive ITP
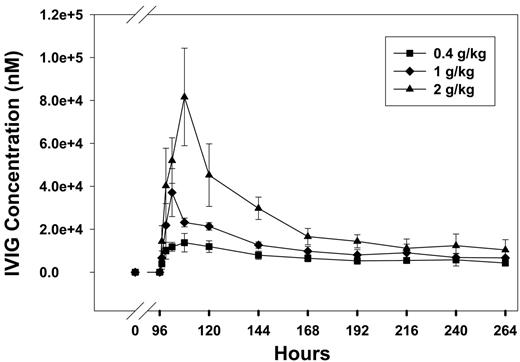
In animals infused with the antiplatelet (anti-PLT) antibody (MWReg30) and treated with an intraperitoneal bolus injection of IVIG at 96 hours, platelet counts increased in a dose-dependent manner (Figure 1). Peak platelet counts were observed at 144 hours with counts of 753 ± 93, 970 ± 92, and 1121 ± 151 k/μL for 0.4, 1, and 2 g/kg, respectively, which was 122% ± 8%, 119% ± 17%, and 160% ± 34% of the initial platelet counts. These results are consistent with the effects of IVIG in patients with ITP.5 In addition, RBC counts and HGB values did not change significantly after IVIG treatment (RBC data shown in Figure 2). IVIG plasma concentrations increased with dose (Figure 3); however, the increase was less than dose proportional. For example, the areas under the mean IVIG plasma concentration versus time curves were 2420, 3710, and 6370 μM · h for IVIG doses of 0.4, 1, and 2 g/kg. As such, the apparent systemic clearance of IVIG, calculated as dose divided by area under the concentration versus time curve, increased with IVIG dose from 1.1 mL kg−1 h−1 (0.4 g/kg) to 2.1 mL kg−1 h−1 (2 g/kg). This finding is consistent with the hypothesis that IVIG therapy leads to competitive inhibition of the IgG transporter FcRn, increasing IgG systemic clearance.
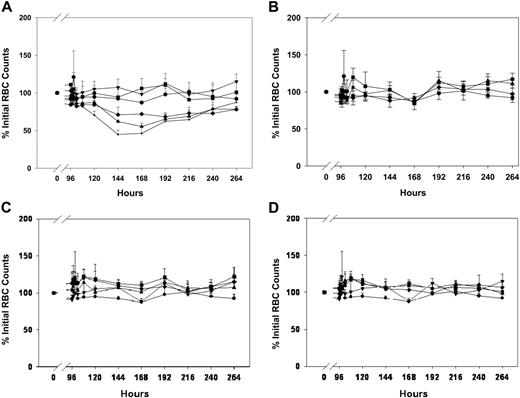
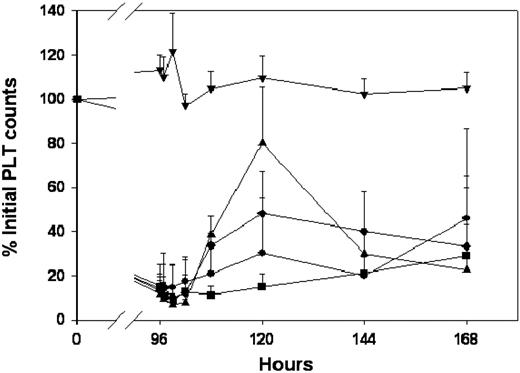
Effects of anti-RBC immunotherapy, antibody-coated liposomes, and IVIG on RBC counts in mice. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and treatments were given by bolus injection at 96 hours. RBC counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. (A) Effects of anti-RBC immunotherapy. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or TER119 at doses of 5 μg/mouse (▪), 15 μg/mouse (♦), 25 μg/mouse (▴), or 50 μg/mouse (+). Error bars represent the standard deviation associated with the RBC counts. TER119 decreased RBC counts in a dose-dependent manner. Treatment differences were statistically significant (P < .001). (B) Effects of IVIG immunotherapy. Symbols represent the sham control group (▾) and animals treated with intraperitoneal saline (•) or IVIG, administered intraperitoneally, at doses of 0.4 g/kg (▪), 1 g/kg (♦), or 2 g/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). (C) Effects of AMI-coated liposomes. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or AMI-coated liposomes at doses of 15 μmol/kg (▪), 30 μmol/kg (♦), or 60 μmol/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). (D) Effects of IVIG-coated liposomes. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or AMI-coated liposomes at doses of 15 μmol/kg (▪), 30 μmol/kg (♦), or 60 μmol/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). Please note that all panels include the same sham control group (▾ in each panel) and panels A, C, and D include the same intravenous saline control group (•). Saline was administered intraperitoneally in the saline control group shown in panel B (also symbolized with •).
Effects of anti-RBC immunotherapy, antibody-coated liposomes, and IVIG on RBC counts in mice. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and treatments were given by bolus injection at 96 hours. RBC counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. (A) Effects of anti-RBC immunotherapy. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or TER119 at doses of 5 μg/mouse (▪), 15 μg/mouse (♦), 25 μg/mouse (▴), or 50 μg/mouse (+). Error bars represent the standard deviation associated with the RBC counts. TER119 decreased RBC counts in a dose-dependent manner. Treatment differences were statistically significant (P < .001). (B) Effects of IVIG immunotherapy. Symbols represent the sham control group (▾) and animals treated with intraperitoneal saline (•) or IVIG, administered intraperitoneally, at doses of 0.4 g/kg (▪), 1 g/kg (♦), or 2 g/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). (C) Effects of AMI-coated liposomes. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or AMI-coated liposomes at doses of 15 μmol/kg (▪), 30 μmol/kg (♦), or 60 μmol/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). (D) Effects of IVIG-coated liposomes. Symbols represent the sham control group (▾) and animals treated with intravenous injections of saline (•) or AMI-coated liposomes at doses of 15 μmol/kg (▪), 30 μmol/kg (♦), or 60 μmol/kg (▴). Error bars represent the standard deviation associated with the RBC counts. RBC counts were not significantly altered (P > .05). Please note that all panels include the same sham control group (▾ in each panel) and panels A, C, and D include the same intravenous saline control group (•). Saline was administered intraperitoneally in the saline control group shown in panel B (also symbolized with •).
IVIG pharmacokinetics. IVIG was administered by intraperitoneal injection at doses of 0.4 g/kg (▪), 1 g/kg (♦), and 2 g/kg (▴). IVIG was injected 96 hours after initiation of the MWReg30 infusion. IVIG concentrations in plasma were assessed via ELISA and were found to increase with dose. Systemic clearance, assessed by the ratio of IVIG dose to the area under the mean IVIG plasma concentration versus time curve, was found to increase with increasing doses of IVIG. This finding is consistent with the hypothesis that high-dose IVIG therapy leads to inhibition of the IgG transporter FcRn. Error bars indicate the standard deviation of assayed IVIG concentrations.
IVIG pharmacokinetics. IVIG was administered by intraperitoneal injection at doses of 0.4 g/kg (▪), 1 g/kg (♦), and 2 g/kg (▴). IVIG was injected 96 hours after initiation of the MWReg30 infusion. IVIG concentrations in plasma were assessed via ELISA and were found to increase with dose. Systemic clearance, assessed by the ratio of IVIG dose to the area under the mean IVIG plasma concentration versus time curve, was found to increase with increasing doses of IVIG. This finding is consistent with the hypothesis that high-dose IVIG therapy leads to inhibition of the IgG transporter FcRn. Error bars indicate the standard deviation of assayed IVIG concentrations.
Effects of antibody-coated liposomes in the murine chronic ITP model
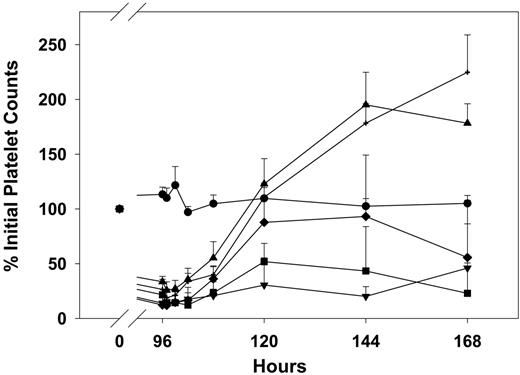
In animals infused with MWReg30 and treated with an intravenous bolus injection of AMI-coated liposomes or IVIG-coated liposomes at 96 hours, platelet counts also increased in a dose-dependent manner. AMI-coated liposomes at doses of 30 and 60 μmol lipid/kg attenuated thrombocytopenia induced by MWReg30, and peak platelet counts were observed at 24 hours after antibody-coated liposome administration with counts of 69% ± 30%, 88% ± 36% of the initial values (Figure 4). IVIG-coated liposomes achieved similar effects on platelet counts as compared with AMI-coated liposomes; peak platelet counts were also observed 24 hours after administration of IVIG-coated liposomes, with counts of 48% ± 19%, 80% ± 25% of initial values (Figure 5). Following antibody-coated liposome administration, RBC counts (Figure 2) and HGB values did not change significantly during the entire experiment period (data not shown). Comparison of results observed following administration of AMI-coated liposomes and IVIG-coated liposomes revealed no significant differences (ie, with respect to peak platelet counts at 30 μmol lipid/kg [P = .254] and at 60 μmol lipid/kg [P = .689]; with respect to the time course of platelet counts at 15 μmol lipid/kg [P = .231], 30 μmol lipid/kg [P = .309], and 60 μmol lipid/kg [P = .132]; or with respect to the time course of RBC counts at 15 μmol lipid/kg [P = .158], 30 μmol lipid/kg [P = .496], and at 60 μmol lipid/kg [P = .733]).
Effects of AMI-coated liposomes on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days). AMI-coated liposomes were given by intravenous bolus injection at 96 hours, and platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (▾) and animals treated with saline (•), 15 μmol lipid/kg (▪), 30 μmol lipid/kg (♦), or 60 μmol lipid/kg (▴) (n = 5 mice/group). Error bars represent the standard deviation of platelet count values. AMI-coated liposomes attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
Effects of AMI-coated liposomes on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days). AMI-coated liposomes were given by intravenous bolus injection at 96 hours, and platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (▾) and animals treated with saline (•), 15 μmol lipid/kg (▪), 30 μmol lipid/kg (♦), or 60 μmol lipid/kg (▴) (n = 5 mice/group). Error bars represent the standard deviation of platelet count values. AMI-coated liposomes attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
Effects of IVIG-coated liposomes on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and IVIG-coated liposomes were given by intravenous bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (▾) and animals treated with saline (•), 15 μmol lipid/kg (▪), 30 μmol lipid/kg (♦), or 60 μmol lipid/kg (▴) (n = 5 mice/group). Error bars represent the standard deviation. IVIG-coated liposomes attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
Effects of IVIG-coated liposomes on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and IVIG-coated liposomes were given by intravenous bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (▾) and animals treated with saline (•), 15 μmol lipid/kg (▪), 30 μmol lipid/kg (♦), or 60 μmol lipid/kg (▴) (n = 5 mice/group). Error bars represent the standard deviation. IVIG-coated liposomes attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
Effects of anti-RBC monoclonal antibody in the murine chronic ITP model
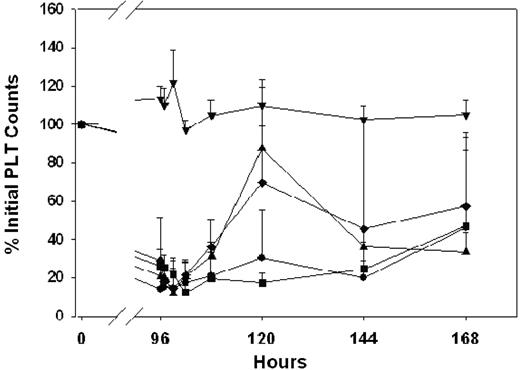
TER119, an anti-RBC antibody, attenuated thrombocytopenia in a dose-dependent manner (Figure 6). Administration of 5 μg/mouse increased platelet counts slightly to 51% ± 16% of initial values, with peak counts observed 24 hours after TER119 dosing. However, 15 and 25 μg TER119/mouse significantly increased platelet counts; maximum counts were observed at 48 hours after treatment, with platelet counts of 93% ± 56%, 195% ± 29% of initial platelet counts. Administration of 50 μg TER119/mouse achieved maximum effects at 72 hours after dosing, with platelet counts reaching 224% ± 34% of initial values. However, the effects of TER119 were associated with severe hemolysis. RBC counts were reduced to 68% ± 4%, 55% ± 4%, and 44% ± 6% of the initial RBC counts following administration of 15, 25, and 50 μg TER119/mouse, respectively (Figure 2). HGB values decreased in parallel with decreases in RBC counts (data not shown).
Effects of anti-RBC monoclonal antibody therapy on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered to mice via intraperitoneal infusion (0.99 μg/d × 7 days), and TER119 was given by intravenous bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (•) and animals treated with saline (▾), 5 μg/mouse (▪), 15 μg/mouse (♦), 25 μg/mouse (▴), or 50 μg/mouse (+). Error bars represent the standard deviation associated with the platelet counts. TER119 attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
Effects of anti-RBC monoclonal antibody therapy on the time course of MWReg30-mediated thrombocytopenia. MWReg30 was administered to mice via intraperitoneal infusion (0.99 μg/d × 7 days), and TER119 was given by intravenous bolus injection at 96 hours. Platelet counts were obtained using a Cell-Dyn 1700 multiparameter hematology analyzer. Symbols represent the sham control group (•) and animals treated with saline (▾), 5 μg/mouse (▪), 15 μg/mouse (♦), 25 μg/mouse (▴), or 50 μg/mouse (+). Error bars represent the standard deviation associated with the platelet counts. TER119 attenuated MWReg30-mediated thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P < .001).
MWReg30 pharmacokinetics
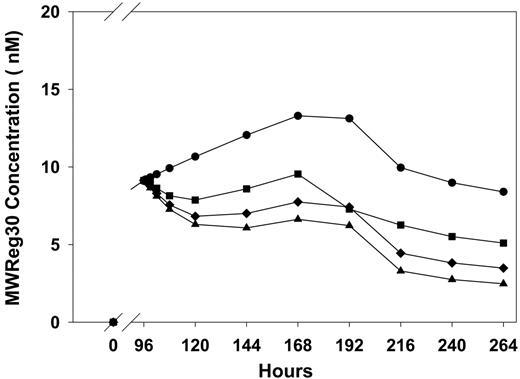
IVIG treatment altered the pharmacokinetics of MWReg30 (Figure 7); however, antibody-coated liposomes did not show effects on MWReg30 disposition (data not shown). Consistent with previous reports18,19 that suggest that IVIG competitively inhibits the IgG transport protein FcRn, we found that IVIG led to an apparent increase in MWReg30 elimination, decreasing MWReg30 AUC96-264h values from 1795 nM×h (control) to 1247, 992, and 835 nM×h, following IVIG at doses of 0.4, 1, and 2 g/kg.
Plasma MWReg30 pharmacokinetics following IVIG treatment. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and IVIG was given by intraperitoneal bolus injection at 96 hours. MWReg30 concentrations were determined via ELISA. Symbols represent treatment groups (n = 5 mice/group) receiving saline (•) or IVIG at doses of 0.4 g/kg (▪), 1 g/kg (♦), or 2 g/kg (▴). IVIG treatment altered the pharmacokinetics of MWReg30, decreasing MWReg30 exposure. This finding is consistent with the hypothesis that high-dose IVIG therapy leads to inhibition of the IgG transporter FcRn.
Plasma MWReg30 pharmacokinetics following IVIG treatment. MWReg30 was administered by intraperitoneal infusion (0.99 μg/d × 7 days), and IVIG was given by intraperitoneal bolus injection at 96 hours. MWReg30 concentrations were determined via ELISA. Symbols represent treatment groups (n = 5 mice/group) receiving saline (•) or IVIG at doses of 0.4 g/kg (▪), 1 g/kg (♦), or 2 g/kg (▴). IVIG treatment altered the pharmacokinetics of MWReg30, decreasing MWReg30 exposure. This finding is consistent with the hypothesis that high-dose IVIG therapy leads to inhibition of the IgG transporter FcRn.
Discussion
In the present study, a chronic murine model for ITP was established and used to compare the effects of antibody-coated liposomes, IVIG, and anti-RBC monoclonal antibodies on the time course of platelet counts, RBC counts, and antiplatelet antibody plasma concentrations. Two antibody-coated liposome preparations were evaluated in this work, including a preparation where a murine monoclonal antibody is reversibly bound to liposomes via antigen-antibody binding (using AMI and liposomes that include methotrexate-phosphatidyl ethanolamine) and a preparation where polyclonal human antibody (ie, IVIG) is covalently bound to liposomes. It is expected that AMI binds to the surface of liposomes in a manner that is very similar to the binding of antiplatelet antibodies to the surface of platelets; that is, in each case, it is anticipated that antibody binds via Fab domains to antigenic sites on the particle surface. The procedure for attachment of IVIG to the liposomes (ie, covalent binding to polyethylene glycol on the liposome surface) is expected to lead to a random orientation of IVIG with respect to the liposome surface. Additionally, in comparison to the IVIG-coated liposomes, the AMI-coated liposome preparation was found to have a greater ratio of antibody to lipid (ie, 65 ± 6 μg AMI/μmol lipid vs 30 ± 5 μg IVIG/μmol lipid). Although the liposome preparations differed in their composition and structure, we found little difference in the effects of the liposome preparations in the mouse model of ITP. We found that IVIG, anti-RBC immunotherapy, and both antibody-coated liposome formulations attenuated thrombocytopenia in the murine ITP model in a dose-dependent manner. Administration of anti-RBC antibodies led to severe hemolysis, whereas IVIG and the antibody-coated liposomes did not lead to significant alterations in RBC counts. IVIG treatment led to an apparent dose-dependent increase in the elimination of the antiplatelet antibody MWReg30, whereas MWReg30 disposition was not altered significantly by treatment with antibody-coated liposomes.
The mouse model of ITP used in this study is a passive model of sustained thrombocytopenia, where MWReg30, a rat monoclonal antibody directed against murine GPIIb/IIIa, is administered via a constant-rate, continuous intraperitoneal infusion. Following 4 days of infusion, significant thrombocytopenia was achieved as platelet counts had dropped to approximately 30% of initial values (Figure 1), and all animals demonstrated petechiae. In comparison to our earlier work using a rat model of an antiplatelet antibody (7E3)–induced thrombocytopenia,11,18,20 and in comparison with animal models presented by other groups,21,22 the present model provides several advantages. For example, in contrast to other passive models where antiplatelet antibody was administered by intravenous or intraperitoneal injection, we have used osmotic pumps to deliver antiplatelet antibody via constant-rate infusion.23 The continuous infusion of antiplatelet antibody may more closely resemble sustained production of antiplatelet antibodies in chronic cases of human ITP, and this may allow a more meaningful evaluation of experimental therapies. That is, in contrast to the treatment of thrombocytopenic patients, the majority of prior work with animal models of ITP has evaluated the effect of pretreatment (eg, with IVIG, anti-RBC antibodies, and antibody-coated liposomes) on prevention of thrombocytopenia following subsequent challenge with an injection of antiplatelet antibody. Evaluation of therapy in the present model is much more similar to the evaluation of therapies in human ITP, as our model assesses the ability of therapy to increase platelet counts in animals that are in a state of sustained thrombocytopenia.
In the present work, we found that TER119 demonstrated greater increases in platelet counts relative to other therapies (peak platelet counts were 224% ± 34% of initial platelet values for 50 μg TER119/mouse versus 160% ± 34% for 2 g/kg IVIG, 88% ± 36% for 60 μmol lipid/kg AMI-coated liposomes, and 80% ± 25% for 60 μmol lipid/kg IVIG-coated liposomes). The effects of these therapies in this model did not correlate directly with the administered dose of immunoglobulin, as the maximum immunoglobulin doses used for TER119, IVIG-coated liposomes, and IVIG were 2.5 mg/kg, 1.8 mg/kg, and 2 g/kg, respectively. Of note, dose-response relationships for each treatment have not yet been fully defined, and it is quite possible that the dose ranges that we have tested have not led to the maximum achievable effect for any of the treatments that we evaluated. The maximum tested dose of IVIG, 2 g/kg, is the highest dose used in the clinical treatment of ITP patients, and the maximum administered dose of TER119 was associated with severe hemolysis. As such, larger doses of IVIG and TER119 may not be clinically relevant or tolerable. It is quite possible that larger doses of antibody-coated liposomes may feasible, and it is possible that larger doses may lead to larger increases in platelet counts in this model; however, this remains to be evaluated.
The time course of platelet counts was found to be substantially different following treatment with TER119, IVIG, and antibody-coated liposomes. An immediate increase in platelet counts was observed for each treatment; however, peak platelet counts were achieved within 1 day for the antibody-coated liposome therapies and at 2 days for IVIG therapy. Interestingly, the time of peak platelet count increased with the dose of TER119 from 1 day at 5 μg/mouse to at least 3 days at 50 μg/mouse. It is important to note that IVIG was administered via intraperitoneal injection and, consequently, it is very likely that the time course of IVIG effect was influenced (ie, delayed) by the time course of IVIG absorption into the systemic circulation. Nonetheless, it is quite possible that the time courses of effect are largely reflective of the rate of elimination of each therapy, as the biologic half-lives may be expected to follow the rank order as follows: antibody-coated liposomes < IVIG < TER119-coated RBCs; however, this hypothesis has not yet been investigated.
Antiplatelet antibodies mediate increased destruction of platelets in ITP.1 Previous work conducted by this laboratory has demonstrated that high-dose IVIG increases the clearance of antiplatelet antibodies, presumably by saturation of FcRn, which protects IgG from intracellular catabolism.18,19 Mathematic modeling has suggested that this mechanism is responsible for approximately 50% of the effect of IVIG on platelet counts in the 7E3 rat model of ITP.24 The present study clearly showed that IVIG altered the systemic exposure of MWReg30 (AUC96-264h values decreased from 1795 nM×h in the control group to 835 nM×h in 2 g/kg IVIG treatment group; Figure 7), consistent with an effect of IVIG on MWReg30 elimination. The significance of the effect of IVIG on MWReg30 disposition has not yet been quantitatively evaluated; however, given the magnitude of the change in MWReg30 exposure, it is likely that IVIG-mediated increases in MWReg30 clearance contributes to the observed effects of IVIG on MWReg30-induced thrombocytopenia.
Multiple mechanisms for IVIG and anti-RBC action in ITP have been proposed,8,25–29 and the exact mechanisms of action of anti-RBC antibody, IVIG, and antibody-coated liposomes are not clear and will be a focus of future work in this laboratory. Recently, Siragam et al30 suggested that IVIG and anti-RBC antibody achieve effects via different mechanisms, either through effects of small, soluble immune complexes on FcγRIIB (eg, IVIG) or through effects of large, particulate immune complexes on FcγRIII (eg, antibody-coated RBCs). Since antibody-coated liposomes are particulate immune complexes that are quite similar to antibody-coated RBCs, it is plausible that antibody-coated liposomes achieve their effects primarily by direct inhibition of FcγR (eg, FcγRIIa, FcγRIII). Mechanistic studies conducted with knockout mice (eg, FcγRIIB knock-outs) will be performed in future work to rigorously evaluate this hypothesis.
In summary, our results demonstrated that antibody-coated liposomes, IVIG, and anti-RBC antibody were all effective in reversing thrombocytopenia in the mouse model of chronic ITP. Antibody-coated liposomes did not alter RBC counts, whereas anti-RBC therapy was associated with a profound decrease in RBC counts. As such, there is some promise that formulations of antibody-coated liposomes may be engineered to provide similar benefit to anti-RBC therapies (eg, anti-D) without precipitating the major toxicities associated with anti-RBC therapies (eg, intravascular hemolysis, anemia, etc). Based on these promising results, the continued investigation of antibody-coated liposomes for ITP appears to be warranted.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph P. Balthasar, Department of Pharmaceutical Sciences, 457B Cooke Hall, University at Buffalo, The State University of New York, Buffalo, NY 14260; e-mail: jb@acsu.buffalo.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant HL67347-01 from the National Heart, Lung, and Blood Institute and by grant AI60687 from the National Institute of Allergy and Infectious Diseases.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal