Abstract
In polycythemia vera, vascular risk assessment is based on age and thrombotic history, while the role of other potential predictors of this risk is still uncertain. Thus, we exploited the large database collected by the observational study of the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) to investigate the association of hematologic variables and cardiovascular risk factors with the thrombotic risk. Among 1638 polycythemic patients followed for 2.7 ± 1.3 years, there were 205 thromboses. Subjects with hypertension had a mild nonsignificant increase in the risk of arterial thrombosis, while this risk was significantly increased by smoking (hazard ratio [HR], 1.90; 95% confidence interval [CI], 1.15-3.14; P = .012). The time-dependent analysis adjusted for potential confounders showed that patients with a white blood cell count above 15 × 109/L, compared with those with a white blood cell count below 10 × 109/L, had a significant increase in the risk of thrombosis (HR, 1.71; 95% CI, 1.10-2.65; P = .017), mainly deriving from an increased risk of myocardial infarction (HR, 2.84; 95% CI, 1.25-6.46; P = .013). Thus, leukocyte count may help in defining the vascular risk of polycythemic subjects.
Introduction
Polycythemia vera (PV) is a Ph-negative myeloproliferative disorder characterized by a clonal proliferation of hematopoietic precursors leading to an increased production of erythrocytes as well as leukocytes and platelets.1 The red cell expansion causes blood hyperviscosity, and this plays a major role in the pathogenesis of both microcirculatory disturbances and arterial and venous thromboses.2,3 The critical role of hyperviscosity is confirmed by historical data reporting a median survival of about 18 months and a high incidence of fatal cerebrovascular events in untreated PV subjects.4 Additional mechanisms, such as a thromboxane (TX) A2 hyperproduction,5 endothelial dysfunction,6 and platelet and leukocyte activation,7 are likely to be involved in the pathogenesis of thrombotic diathesis in PV. It has been estimated that 30% to 50% of PV patients have minor and major thrombotic complications, and vascular mortality accounts for 35% to 45% of all deaths.8,9 The efficacy of available antithrombotic strategies might be optimized by a proper stratification of the individual risk. This stratification largely relies on age and thrombotic history. The major impact of these 2 factors has been confirmed recently by the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) observational study, which estimated that the incidence rate of major events was approximately doubled in patients aged more than 65 years or with previous thrombotic history, becoming approximately 4-fold higher in subjects with both conditions.8–10 By using the large database of the ECLAP observational study, we analyzed the role of other potential determinants of the individual risk such as the white blood cell count and “classic” cardiovascular risk factors (ie, hypertension, diabetes, smoking, and hypercholesterolemia)
Patients and methods
Patients
All the analyses were performed on the clinical data of the 1638 PV patient population collected within the ECLAP trial. The coordinating center in Italy (Consorzio Mario Negri Sud) received all data from several hematologic centers in 12 European countries and created the largest centralized database existing to date on PV. In all patients included in the study, diagnosis of PV was made according to the criteria established by the Polycythemia Vera Study Group11 or by Pearson et al.12
Treatment strategies had to comply with the recommendation of maintaining the hematocrit below 0.45 and the platelet count below 400 × 109/L. Data regarding clinical outcomes, treatments, and laboratory values during the prospective follow-up were recorded at 12, 24, 36, 48, and 60 months of follow-up. The mean duration of the disease at entry and the duration of the follow-up were 5.0 and 2.7 (SD, 1.3) years, respectively.
Aims of the current study were to determine whether the white blood cell count in the ECLAP study may suggest a “specific target value” to be maintained during the course of the disease or, in other words, if treatment strategies should be more or less aggressive to control the disease also according to the number of white blood cells.
Outcome events
Only thromboses registered during the follow-up period of ECLAP were considered in this analysis. As vascular events we considered cardiovascular death, stroke, transient ischemic attack (TIA), myocardial infarction, (MI) and peripheral arterial thrombosis (PAT) of the legs and abdominal vessels. Venous thromboembolism (VTE) included deep venous thrombosis (DVT) of the extremities and abdominal vein and pulmonary embolism (PE). Deaths from congestive heart failure and intracranial bleedings were not included in cardiovascular deaths. Minor occlusive events such as erythromelalgia and superficial thrombophlebitis of the extremities were not included in the analysis.
The vascular events during follow-up were defined and classified according to the International Classification of Diseases (ninth revision). A diagnosis of stroke required unequivocal signs or symptoms of a neurologic deficit with sudden onset and a duration of more than 24 hours. The diagnosis had to be confirmed with the use of computed tomography (CT), magnetic resonance imaging, or on autopsy. Alternatively, we used the diagnosis reported in the hospital records or on the death certificate. A TIA was defined as the abrupt onset of unilateral motor or sensory disturbance, speech defect, homonymous hemianopsia, constructional apraxia, or transient monocular blindness that resolved completely in less than 24 hours.
MI was defined by at least 2 of the following findings: chest pain of typical intensity and duration; ST segment elevation or depression of 1 mm or more in any limb lead on electrocardiography, of 2 mm or more in any precordial lead, or both; and at least a doubling of the levels of the cardiac enzymes. PAT was diagnosed by angiography.
DVT was diagnosed involving techniques such as phlebography, ultrasonography, impedance plethysmography, or CT scan. PE was defined by a positive pulmonary angiogram, ventilation-perfusion scan, or CT scan or evidence of PE on autopsy.
The validation of the clinical events included in the outcome measures was ensured by an ad hoc committee of expert clinicians. Each event was validated independently by 2 evaluators, and any disagreement was solved by the chairperson of the study.
The vascular risk factors were defined as follows: (1) smokers: patients who smoked during the study or had smoked within 1 month preceding the study period; (2) hypertension: antihypertensive treatment or history of arterial hypertension; (3) hypercholesterolemia: serum total cholesterol higher than 200 mg/dL (5.18 mM); (4) diabetes mellitus: history of diabetes or hypoglycemic treatment.
Statistical analysis
Cox proportional hazards models were used to evaluate risk, with censoring at first event, death, or last follow-up visit through December 2002. Covariates were chosen based on biological plausibility as confounders and associations with exposure and outcome in the present population. Multivariable models were evaluated unadjusted, adjusted for age and sex, and further adjusted for other potential confounding factors.
Data were explored using patients' characteristics at baseline. In addition, multivariable time-dependent analysis was performed to assess whether the level of exposition to a factor that had been recorded in the last clinical visit before the outcome event of interest was associated with the probability of having that event during follow-up. Time-varying covariates were used to update information on white blood cell and platelet count and other risk factors (footnotes to Table 4) at 1, 2, 3, 4, and 5 years. Where appropriate, the substitution of the missing data for incomplete repeated measures was done with the last value carried forward. Indicator variables were used for missing data on baseline covariates; values were otherwise carried forward for missing time-varying covariates. Some covariates (age at recruitment, sex, time from diagnosis to enrollment, thrombotic or hemorrhagic events prior to recruitment, history of hypertension, intermittent claudication, or erythromelalgia) were determined only at baseline, whereas others were updated during follow-up (smoking habits, diabetes mellitus, total blood cholesterol, splenomegaly, immature cells, hematocrit, platelet and leukocyte count, and therapeutic interventions such as phlebotomy, interferon, hydroxyurea, antiplatelets and anticoagulants, 32P, busulfan, chlorambucil, pipobroman). For the variables updated during follow-up visits, the last measurement before an event was considered in the time-dependent analysis. White blood cell count was tested as continuous variables, median values, tertiles, quintiles, and deciles depending on the number of events in each analysis (ie, robustness of the model).
Tests for trend were calculated by assigning the median value of each category and evaluating this as a continuous variable. Stratified analyses were used to assess for effect modification, with significance evaluated using likelihood-ratio testing and multiplicative (exposure times covariate) interaction terms. Analyses were performed with SAS 9.1 software (Cary, NC). All probability values are 2 tailed (< .05).
Results
The main characteristics of ECLAP patients have been described in detail elsewhere and are summarized in Table 1.
Baseline characteristics of the ECLAP population
| . | Data . |
|---|---|
| Women, no. (%) | 696 (42.5) |
| Mean age at diagnosis ± SD, y | 60.4 ± 13.2 |
| Age at recruitment | |
| Mean ± SD, y | 65.4 ± 12.7 |
| Less than 55 y, no. (%) | 316 (19.3) |
| 55-64 y, no. (%) | 387 (23.6) |
| 65-74 y, no. (%) | 561 (34.3) |
| 75 y or more, no. (%) | 374 (22.8) |
| Time from diagnosis of PV to enrollment | |
| Mean ± SD, y | 5.0 ± 5.0 |
| 0-2 y, no. (%) | 581 (35.5) |
| 3-4 y, no. (%) | 306 (18.7) |
| 5-6 y, no. (%) | 233 (14.2) |
| 7-10 y, no. (%) | 282 (17.2) |
| More than 10 y, no. (%) | 236 (14.4) |
| Prior bleeding, no. (%) | 133 (8.1) |
| Prior thrombosis, no. (%) | 587 (35.8) |
| Erythromelalgia, no. (%) | 86 (5.3) |
| Intermittent claudication, no. (%) | 77 (4.7) |
| Smoking, no. (%) | 209 (12.8) |
| Hypertension, no. (%) | 647 (39.5) |
| Diabetes mellitus, no. (%) | 117 (7.1) |
| . | Data . |
|---|---|
| Women, no. (%) | 696 (42.5) |
| Mean age at diagnosis ± SD, y | 60.4 ± 13.2 |
| Age at recruitment | |
| Mean ± SD, y | 65.4 ± 12.7 |
| Less than 55 y, no. (%) | 316 (19.3) |
| 55-64 y, no. (%) | 387 (23.6) |
| 65-74 y, no. (%) | 561 (34.3) |
| 75 y or more, no. (%) | 374 (22.8) |
| Time from diagnosis of PV to enrollment | |
| Mean ± SD, y | 5.0 ± 5.0 |
| 0-2 y, no. (%) | 581 (35.5) |
| 3-4 y, no. (%) | 306 (18.7) |
| 5-6 y, no. (%) | 233 (14.2) |
| 7-10 y, no. (%) | 282 (17.2) |
| More than 10 y, no. (%) | 236 (14.4) |
| Prior bleeding, no. (%) | 133 (8.1) |
| Prior thrombosis, no. (%) | 587 (35.8) |
| Erythromelalgia, no. (%) | 86 (5.3) |
| Intermittent claudication, no. (%) | 77 (4.7) |
| Smoking, no. (%) | 209 (12.8) |
| Hypertension, no. (%) | 647 (39.5) |
| Diabetes mellitus, no. (%) | 117 (7.1) |
A total of 587 of the patients (36%) had a prior history of thrombosis, which was made up of an arterial thrombotic event in three quarters of the cases, whereas 169 patients had thrombotic vascular complications during follow-up. Altogether we collected data on 926 vascular events in 668 PV patients. Of these events, 721 had occurred prior to patient recruitment in the study, and 205 episodes of thrombosis were recorded during follow-up.8 The main characteristics of the thrombotic events during follow-up are summarized in Table 2. Cerebrovascular events (stroke and TIA) constituted half of all arterial events and about one third of all thromboses. Among the 205 thrombotic episodes, 81 were a first vascular event.
Main characteristics of 169 patients with thrombotic events in the ECLAP study
| . | Data . |
|---|---|
| Patients, no. | 169 |
| Men, no. | 103 |
| Mean age at recruitment ± SD, y | 71 ± 10 |
| Age at diagnosis ± SD, y | 66 ± 10 |
| Type of thrombosis, no. | |
| Arterial | 143 |
| TIA | 35 |
| AMI | 46 |
| Stroke | 36 |
| PAT | 26 |
| Venous | 62 |
| DVT | 44 |
| PE | 18 |
| Total thromboses, no. | 205 |
| First vascular event, no. | 81 |
| . | Data . |
|---|---|
| Patients, no. | 169 |
| Men, no. | 103 |
| Mean age at recruitment ± SD, y | 71 ± 10 |
| Age at diagnosis ± SD, y | 66 ± 10 |
| Type of thrombosis, no. | |
| Arterial | 143 |
| TIA | 35 |
| AMI | 46 |
| Stroke | 36 |
| PAT | 26 |
| Venous | 62 |
| DVT | 44 |
| PE | 18 |
| Total thromboses, no. | 205 |
| First vascular event, no. | 81 |
Of 1638 polycythemic patients recruited in the ECLAP study, 587 had a history of thrombosis at baseline, and 169 had thrombosis during follow-up.
AMI indicates acute myocardial infarction.
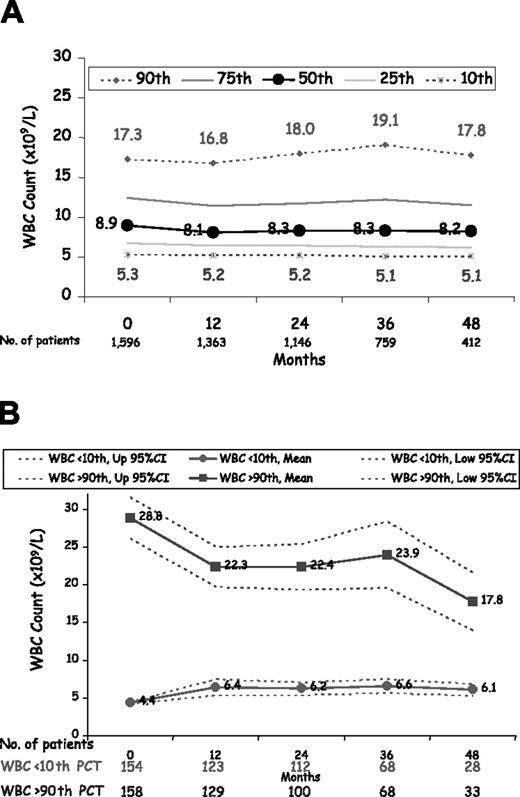
The proportion of patients with a normal white blood cell count (10 × 109/L or below) was approximately 62.0% at baseline, ranging between 59.6% and 68.0% during follow-up (Figure 1A). Only 15.1% and 15.5% of patients had a white blood cell count above 15 × 109/L at baseline and during follow-up, respectively. The white blood cell count, however, seemed not to influence therapeutic decisions to control the disease. In fact, patients belonging to the lowest and highest decile of white blood cell count at baseline were maintained at almost the same number of white blood cells during follow-up (Figure 1B). Median values at baseline of the white blood cell count of patients in the 10th and 90th percentile were 4.5 × 109/L and 21.5 × 109/L, respectively. During follow-up, median values of the white blood cell count of patients in the 10th and 90th percentile were 5.4 × 109/L and 21.0 × 109/L, respectively. Multivariate analysis using patients' white blood cell count at baseline (Table 3) showed that leukocytosis was significantly associated with MI but not with other thrombotic events. In the multivariable, time-dependent analysis (Figure 2A) the risk of thrombosis was clearly increased in patients with a white blood cell count above 10 × 109/L, becoming statistically significant when the white blood cell count was above 15 × 109/L (hazard ratio [HR], 1.71; 95% confidence interval [CI], 1.10-2.65; P = .017.) Table 4 shows the results of time-dependent, multivariable models progressively adjusted for potential confounding factors. Overall, the results of these analyses suggest that high white blood cell count was an independent predictor of vascular risk, the strength of the HR being either unchanged or only slightly increased by using adjustment models progressively more complete and including patient characteristics, clinical history, comorbidity, and time-dependent modification of laboratory values and treatments.
White blood cell count levels during 4 years of follow-up in 1638 patients with polycythemia vera. (A) The 10th, 25th, 50th, 75th, and 90th percentile of white blood cell count at the various time points. (B) The mean value of white blood cell count at the entry visit along with its 95% CI in patients belonging to the 10th and 90th percentile (time 0). Mean (95% CI) values of white blood cell count of these patients during follow-up (time points at 12 to 48 months) are maintained at levels similar to those observed at the entry visit.
White blood cell count levels during 4 years of follow-up in 1638 patients with polycythemia vera. (A) The 10th, 25th, 50th, 75th, and 90th percentile of white blood cell count at the various time points. (B) The mean value of white blood cell count at the entry visit along with its 95% CI in patients belonging to the 10th and 90th percentile (time 0). Mean (95% CI) values of white blood cell count of these patients during follow-up (time points at 12 to 48 months) are maintained at levels similar to those observed at the entry visit.
Multivariate analysis using patients' characteristics at baseline on the relative risk of major thrombosis, arterial thrombosis, venous thrombosis, myocardial infarction, stroke plus TIA, and peripheral arterial thrombosis among men and women with polycythemia vera; n = 1638
| . | No. patients . | 10.1 to 15.0 × 109/L white blood cells*† . | More than 15.0 × 109/L white blood cells*‡ . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Major thrombosis | 169 | 1.05 (0.69-1.61) | .811 | 1.24 (0.78-1.96) | .370 |
| Arterial thrombosis | 121 | 1.07 (0.65-1.76) | .802 | 1.21 (0.69-2.11) | .508 |
| Venous thrombosis | 51 | 1.02 (0.47-2.21) | .963 | 1.28 (0.56-2.92) | .554 |
| Myocardial infarction | 41 | 0.82 (0.31-2.16) | .683 | 2.27 (1.00-5.15) | .049 |
| Stroke/TIA | 54 | 1.32 (0.64-2.70) | .450 | 0.69 (0.26-1.86) | .464 |
| Peripheral arterial thrombosis | 21 | 1.44 (0.44-4.74) | .549 | 1.12 (0.21-6.02) | .892 |
| . | No. patients . | 10.1 to 15.0 × 109/L white blood cells*† . | More than 15.0 × 109/L white blood cells*‡ . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Major thrombosis | 169 | 1.05 (0.69-1.61) | .811 | 1.24 (0.78-1.96) | .370 |
| Arterial thrombosis | 121 | 1.07 (0.65-1.76) | .802 | 1.21 (0.69-2.11) | .508 |
| Venous thrombosis | 51 | 1.02 (0.47-2.21) | .963 | 1.28 (0.56-2.92) | .554 |
| Myocardial infarction | 41 | 0.82 (0.31-2.16) | .683 | 2.27 (1.00-5.15) | .049 |
| Stroke/TIA | 54 | 1.32 (0.64-2.70) | .450 | 0.69 (0.26-1.86) | .464 |
| Peripheral arterial thrombosis | 21 | 1.44 (0.44-4.74) | .549 | 1.12 (0.21-6.02) | .892 |
Multivariable model adjusted for information colleted at baseline, including white blood cells (3 categories), age (2 categories), sex, time from PV diagnosis to recruitment (2 categories), thrombotic or hemorrhagic events prior to recruitment (yes/no), smoking (yes/no), history of diabetes (yes/no), hypertension (yes/no), intermittent claudication (yes/no), erythromelalgia (yes/no), splenomegaly (yes/no), circulating immature cells (yes/no), hematocrit (tertiles), platelet count (tertiles), total blood cholesterol (2 categories), phlebotomy use (yes/no), interferon use (yes/no), hydroxyurea use (yes/no), antiplatelet use (yes/no), anticoagulant use (yes/no), 32P use (yes/no), busulfan use (yes/no), chlorambucil use (yes/no), and pipobroman use (yes/no).
Reference category: ≤ 10.0 × 109/L (n = 990 [62.0%]).
n = 365 (22.9%).
n = 241 (15.1%).
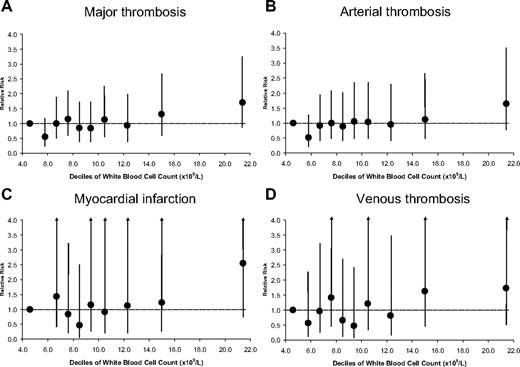
White blood cell count deciles and risk of thrombosis in 1638 patients with polycythemia vera. (A) Major thrombosis. (B) Arterial thrombosis. (C) Myocardial infarction. (D) Venous thrombosis. Circles are HRs, and bars are 95% CIs. The dotted horizontal line indicates null effect (P > .05).
White blood cell count deciles and risk of thrombosis in 1638 patients with polycythemia vera. (A) Major thrombosis. (B) Arterial thrombosis. (C) Myocardial infarction. (D) Venous thrombosis. Circles are HRs, and bars are 95% CIs. The dotted horizontal line indicates null effect (P > .05).
Time-dependent multivariate analysis on the relative risk of major thrombosis, arterial thrombosis, venous thrombosis, myocardial infarction, stroke plus TIA, and peripheral arterial thrombosis among men and women with polycythemia vera (n = 1638)
| . | 10.1 to 15.0 × 109/L white blood cells*† . | More than 15.0 × 109/L white blood cells*‡ . | |||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Patients with major thrombosis (n = 169) | |||||
| Unadjusted§ | 1.00 (0.67-1.48) | .986 | 1.56 (1.05-2.30) | .028 | |
| Adjusted | |||||
| For age and sex‖ | 0.98 (0.66-1.46) | .922 | 1.48 (1.00-2.19) | .052 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.97 (0.65-1.44) | .859 | 1.39 (0.94-2.07) | .103 | |
| For RF and comorbidity# | 1.09 (0.72-1.67) | .677 | 1.70 (1.10-2.63) | .016 | |
| For cytoreductive and antithrombotic treatment** | 1.06 (0.69-1.62) | .803 | 1.71 (1.10-2.65) | .017 | |
| Patients with arterial thrombosis (n = 121) | |||||
| Unadjusted§ | 1.02 (0.64-1.61) | .945 | 1.41 (0.88-2.28) | .155 | |
| Adjusted | |||||
| For age and sex‖ | 1.00 (0.63-1.59) | .998 | 1.34 (0.83-2.16) | .227 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.98 (0.62-1.55) | .928 | 1.27 (0.78-2.05) | .334 | |
| For RF and comorbidity# | 1.15 (0.71-1.89) | .568 | 1.61 (0.96-2.73) | .074 | |
| For cytoreductive and antithrombotic treatment** | 1.11 (0.68-1.82) | .675 | 1.67 (0.98-2.84) | .058 | |
| Patients with venous thrombosis (n = 51) | |||||
| Unadjusted§ | 0.86 (0.39-1.88) | .703 | 1.94 (0.99-3.80) | .052 | |
| Adjusted | |||||
| For age and sex‖ | 0.85 (0.39-1.86) | .683 | 1.86 (0.95-3.64) | .070 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.85 (0.39-1.85) | .675 | 1.75 (0.89-3.45) | .105 | |
| For RF and comorbidity# | 0.90 (0.40-2.05) | .809 | 1.93 (0.91-4.07) | .086 | |
| For cytoreductive and antithrombotic treatment** | 0.87 (0.38-1.98) | .733 | 1.81 (0.84-3.89) | .127 | |
| Patients with myocardial infarction (n = 41) | |||||
| Unadjusted§ | 1.15 (0.51-2.59) | .730 | 2.46 (1.19-5.09) | .015 | |
| Adjusted | |||||
| For age and sex‖ | 1.13 (0.50-2.55) | .763 | 2.28 (1.11-4.72) | .026 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 1.10 (0.49-2.48) | .811 | 2.13 (1.02-4.44) | .045 | |
| For RF and comorbidity# | 1.32 (0.56-3.10) | .522 | 2.65 (1.19-5.93) | .018 | |
| For cytoreductive and antithrombotic treatment** | 1.32 (0.56-3.10) | .531 | 2.84 (1.25-6.46) | .013 | |
| Patients with stroke/TIA (n = 54) | |||||
| Unadjusted§ | 1.08 (0.56-2.07) | .829 | 0.80 (0.34-1.91) | .617 | |
| Adjusted | |||||
| For age and sex‖ | 1.07 (0.55-2.06) | .850 | 0.77 (0.32-1.83) | .554 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 1.04 (0.54-2.01) | .909 | 0.77 (0.32-1.83) | .547 | |
| For RF and comorbidity# | 1.31 (0.65-2.64) | .454 | 0.93 (0.37-2.32) | .870 | |
| For cytoreductive and antithrombotic treatment** | 1.25 (0.62-2.53) | .541 | 0.93 (0.37-2.34) | .870 | |
| Patients with peripheral arterial thrombosis (n = 21) | |||||
| Unadjusted§ | 0.95 (0.31-2.93) | .933 | 1.43 (0.47-4.38) | .535 | |
| Adjusted | |||||
| For age and sex‖ | 0.94 (0.31-2.89) | .915 | 1.36 (0.44-4.17) | .595 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.95 (0.31-2.93) | .932 | 1.17 (0.38-3.64) | .781 | |
| For RF and comorbidity# | 0.94 (0.28-3.19) | .924 | 1.74 (0.47-6.36) | .406 | |
| For cytoreductive and antithrombotic treatment** | 0.86 (0.25-2.92) | .803 | 1.72 (0.46-6.46) | .425 | |
| . | 10.1 to 15.0 × 109/L white blood cells*† . | More than 15.0 × 109/L white blood cells*‡ . | |||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Patients with major thrombosis (n = 169) | |||||
| Unadjusted§ | 1.00 (0.67-1.48) | .986 | 1.56 (1.05-2.30) | .028 | |
| Adjusted | |||||
| For age and sex‖ | 0.98 (0.66-1.46) | .922 | 1.48 (1.00-2.19) | .052 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.97 (0.65-1.44) | .859 | 1.39 (0.94-2.07) | .103 | |
| For RF and comorbidity# | 1.09 (0.72-1.67) | .677 | 1.70 (1.10-2.63) | .016 | |
| For cytoreductive and antithrombotic treatment** | 1.06 (0.69-1.62) | .803 | 1.71 (1.10-2.65) | .017 | |
| Patients with arterial thrombosis (n = 121) | |||||
| Unadjusted§ | 1.02 (0.64-1.61) | .945 | 1.41 (0.88-2.28) | .155 | |
| Adjusted | |||||
| For age and sex‖ | 1.00 (0.63-1.59) | .998 | 1.34 (0.83-2.16) | .227 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.98 (0.62-1.55) | .928 | 1.27 (0.78-2.05) | .334 | |
| For RF and comorbidity# | 1.15 (0.71-1.89) | .568 | 1.61 (0.96-2.73) | .074 | |
| For cytoreductive and antithrombotic treatment** | 1.11 (0.68-1.82) | .675 | 1.67 (0.98-2.84) | .058 | |
| Patients with venous thrombosis (n = 51) | |||||
| Unadjusted§ | 0.86 (0.39-1.88) | .703 | 1.94 (0.99-3.80) | .052 | |
| Adjusted | |||||
| For age and sex‖ | 0.85 (0.39-1.86) | .683 | 1.86 (0.95-3.64) | .070 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.85 (0.39-1.85) | .675 | 1.75 (0.89-3.45) | .105 | |
| For RF and comorbidity# | 0.90 (0.40-2.05) | .809 | 1.93 (0.91-4.07) | .086 | |
| For cytoreductive and antithrombotic treatment** | 0.87 (0.38-1.98) | .733 | 1.81 (0.84-3.89) | .127 | |
| Patients with myocardial infarction (n = 41) | |||||
| Unadjusted§ | 1.15 (0.51-2.59) | .730 | 2.46 (1.19-5.09) | .015 | |
| Adjusted | |||||
| For age and sex‖ | 1.13 (0.50-2.55) | .763 | 2.28 (1.11-4.72) | .026 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 1.10 (0.49-2.48) | .811 | 2.13 (1.02-4.44) | .045 | |
| For RF and comorbidity# | 1.32 (0.56-3.10) | .522 | 2.65 (1.19-5.93) | .018 | |
| For cytoreductive and antithrombotic treatment** | 1.32 (0.56-3.10) | .531 | 2.84 (1.25-6.46) | .013 | |
| Patients with stroke/TIA (n = 54) | |||||
| Unadjusted§ | 1.08 (0.56-2.07) | .829 | 0.80 (0.34-1.91) | .617 | |
| Adjusted | |||||
| For age and sex‖ | 1.07 (0.55-2.06) | .850 | 0.77 (0.32-1.83) | .554 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 1.04 (0.54-2.01) | .909 | 0.77 (0.32-1.83) | .547 | |
| For RF and comorbidity# | 1.31 (0.65-2.64) | .454 | 0.93 (0.37-2.32) | .870 | |
| For cytoreductive and antithrombotic treatment** | 1.25 (0.62-2.53) | .541 | 0.93 (0.37-2.34) | .870 | |
| Patients with peripheral arterial thrombosis (n = 21) | |||||
| Unadjusted§ | 0.95 (0.31-2.93) | .933 | 1.43 (0.47-4.38) | .535 | |
| Adjusted | |||||
| For age and sex‖ | 0.94 (0.31-2.89) | .915 | 1.36 (0.44-4.17) | .595 | |
| For disease duration, prior thrombosis, and prior hemorrhage¶ | 0.95 (0.31-2.93) | .932 | 1.17 (0.38-3.64) | .781 | |
| For RF and comorbidity# | 0.94 (0.28-3.19) | .924 | 1.74 (0.47-6.36) | .406 | |
| For cytoreductive and antithrombotic treatment** | 0.86 (0.25-2.92) | .803 | 1.72 (0.46-6.46) | .425 | |
Reference category: ≤ 10.0 × 109/L (n = 990 [62.0%]).
n = 365 (22.9%).
n = 241 (15.1%).
Model 1: white blood cells (3 categories).
Model 2: model 1 plus age (2 categories), sex.
Model 3: model 2 plus time from PV diagnosis to recruitment (2 categories), thrombotic or hemorrhagic events prior to recruitment (yes/no).
Model 4: model 3 plus smoking (yes/no), history of diabetes (yes/no), hypertension (yes/no), intermittent claudication (yes/no), erythromelalgia (yes/no), splenomegaly, (yes/no), circulating immature cells (yes/no), hematocrit (tertiles), platelet count (tertiles), and total blood cholesterol (2 categories).
Model 5: model 4 plus phlebotomy use (yes/no), interferon use (yes/no), hydroxyurea use (yes/no), antiplatelet use (yes/no), anticoagulant use (yes/no), 32P use (yes/no), busulfan use (yes/no), chlorambucil use (yes/no), and pipobroman use (yes/no).
Leukocytosis was more evidently associated with arterial thrombosis than with venous thromboembolism (Figure 2B,D; Table 5). The various arterial thrombotic events were also differently influenced by white blood cell count, the association being evident for myocardial infarction and nonsignificant for peripheral arterial thrombosis, stroke, and TIA.
Predictors of risk of major thrombosis in 1638 PV patients
| . | Major thrombosis, HR (95% CI) . | Arterial thrombosis, HR (95% CI) . | Venous thrombosis, HR (95% CI) . | MI, HR (95% CI) . | TIA or stroke, HR (95% CI) . | PAT, HR (95% CI) . |
|---|---|---|---|---|---|---|
| Age more than 65 y | 2.89 (1.98-4.22) | 3.14 (1.99-4.96) | 2.03 (1.05-3.90) | 5.96 (2.26-15.7) | 2.47 (1.31-4.65) | 3.13 (1.02-9.55) |
| Male sex | 1.25 (0.90-1.73) | 1.37 (0.92-2.03) | 0.87 (0.48-1.56) | 2.02 (0.99-4.10) | 1.04 (0.58-1.86) | 1.24 (0.48-3.22) |
| PV diagnosis 2 or more y before enrollment | 1.15 (0.81-1.62) | 1.10 (0.74-1.65) | 1.34 (0.70-2.59) | 1.00 (0.49-2.05) | 0.78 (0.44-1.39) | 4.33 (1.21-15.4) |
| Major thrombosis prior to enrollment | 1.69 (1.21-2.36) | 1.76 (1.19-2.61) | 1.62 (0.88-2.98) | 1.70 (0.86-3.35) | 1.60 (0.88-2.86) | 2.25 (0.84-6.02) |
| Arterial thrombosis prior to enrollment | 1.66 (1.20-2.32) | 2.07 (1.40-3.06) | 1.09 (0.57-2.05) | 1.83 (0.93-3.59) | 2.09 (1.17-3.75) | 1.94 (0.76-4.98) |
| Venous thrombosis prior to enrollment | 1.74 (1.06-2.87) | 0.86 (0.43-1.75) | 4.19 (2.01-8.72) | 0.47 (0.10-2.17) | 0.52 (0.15-1.78) | 2.93 (0.80-10.8) |
| AMI prior to enrollment | 1.13 (0.68-1.88) | 1.33 (0.76-2.32) | 0.72 (0.22-2.36) | 2.41 (1.09-5.33) | 0.74 (0.26-2.12) | 0.51 (0.06-4.05) |
| Stroke or TIA prior to enrollment | 1.81 (1.27-2.57) | 2.20 (1.47-3.30) | 1.11 (0.54-2.29) | 1.76 (0.86-3.59) | 2.86 (1.57-5.23) | 1.10 (0.36-3.33) |
| PAT prior to enrollment | 1.61 (0.92-2.83) | 2.13 (1.16-3.92) | 0.72 (0.20-2.60) | 0.51 (0.11-2.36) | 1.59 (0.54-4.69) | 13.02 (4.30-39.4) |
| DVT prior to enrollment | 2.04 (1.22-3.39) | 1.11 (0.55-2.27) | 4.11 (1.90-8.90) | 0.65 (0.14-2.96) | 0.65 (0.19-2.28) | 3.64 (0.91-14.5) |
| PE prior to enrollment | 0.79 (0.30-2.08) | 0.55 (0.12-2.43) | 1.09 (0.29-4.07) | NA | 0.73 (0.09-6.05) | 0.72 (0.06-8.46) |
| Bleeding prior to enrollment | 1.04 (0.61-1.77) | 1.10 (0.60-2.04) | 0.87 (0.31-2.47) | 1.24 (0.46-3.33) | 0.91 (0.32-2.59) | 0.78 (0.16-3.94) |
| Smoking | 1.55 (0.99-2.43) | 1.90 (1.15-3.14) | 0.81 (0.29-2.30) | 1.40 (0.53-3.69) | 1.89 (0.89-4.01) | 2.86 (0.92-8.86) |
| Total blood cholesterol count above 200 mg/dL | 1.16 (0.78-1.72) | 1.24 (0.79-1.94) | 0.83 (0.37-1.87) | 1.50 (0.62-3.65) | 0.95 (0.49-1.85) | 1.41 (0.51-3.91) |
| Diabetes mellitus | 1.10 (0.63-1.91) | 1.38 (0.76-2.53) | 0.48 (0.12-1.98) | 0.94 (0.29-3.08) | 1.28 (0.50-3.26) | 2.07 (0.58-7.39) |
| Hypertension | 1.25 (0.91-1.71) | 1.14 (0.78-1.65) | 1.48 (0.83-2.63) | 1.17 (0.62-2.23) | 0.97 (0.55-1.70) | 1.23 (0.50-3.06) |
| Intermittent claudication | 1.57 (0.90-2.73) | 1.29 (0.66-2.53) | 2.81 (1.14-6.89) | 2.16 (0.80-5.85) | 0.29 (0.04-2.14) | 3.71 (1.15-12.0) |
| Erythromelalgia | 0.54 (0.24-1.24) | 0.67 (0.27-1.68) | 0.30 (0.04-2.18) | 1.25 (0.37-4.23) | 0.80 (0.19-3.37) | NA |
| Splenomegaly | 0.74 (0.52-1.04) | 0.67 (0.44-1.00) | 0.88 (0.48-1.62) | 0.79 (0.39-1.57) | 0.86 (0.48-1.54) | 0.27 (0.08-0.89) |
| . | Major thrombosis, HR (95% CI) . | Arterial thrombosis, HR (95% CI) . | Venous thrombosis, HR (95% CI) . | MI, HR (95% CI) . | TIA or stroke, HR (95% CI) . | PAT, HR (95% CI) . |
|---|---|---|---|---|---|---|
| Age more than 65 y | 2.89 (1.98-4.22) | 3.14 (1.99-4.96) | 2.03 (1.05-3.90) | 5.96 (2.26-15.7) | 2.47 (1.31-4.65) | 3.13 (1.02-9.55) |
| Male sex | 1.25 (0.90-1.73) | 1.37 (0.92-2.03) | 0.87 (0.48-1.56) | 2.02 (0.99-4.10) | 1.04 (0.58-1.86) | 1.24 (0.48-3.22) |
| PV diagnosis 2 or more y before enrollment | 1.15 (0.81-1.62) | 1.10 (0.74-1.65) | 1.34 (0.70-2.59) | 1.00 (0.49-2.05) | 0.78 (0.44-1.39) | 4.33 (1.21-15.4) |
| Major thrombosis prior to enrollment | 1.69 (1.21-2.36) | 1.76 (1.19-2.61) | 1.62 (0.88-2.98) | 1.70 (0.86-3.35) | 1.60 (0.88-2.86) | 2.25 (0.84-6.02) |
| Arterial thrombosis prior to enrollment | 1.66 (1.20-2.32) | 2.07 (1.40-3.06) | 1.09 (0.57-2.05) | 1.83 (0.93-3.59) | 2.09 (1.17-3.75) | 1.94 (0.76-4.98) |
| Venous thrombosis prior to enrollment | 1.74 (1.06-2.87) | 0.86 (0.43-1.75) | 4.19 (2.01-8.72) | 0.47 (0.10-2.17) | 0.52 (0.15-1.78) | 2.93 (0.80-10.8) |
| AMI prior to enrollment | 1.13 (0.68-1.88) | 1.33 (0.76-2.32) | 0.72 (0.22-2.36) | 2.41 (1.09-5.33) | 0.74 (0.26-2.12) | 0.51 (0.06-4.05) |
| Stroke or TIA prior to enrollment | 1.81 (1.27-2.57) | 2.20 (1.47-3.30) | 1.11 (0.54-2.29) | 1.76 (0.86-3.59) | 2.86 (1.57-5.23) | 1.10 (0.36-3.33) |
| PAT prior to enrollment | 1.61 (0.92-2.83) | 2.13 (1.16-3.92) | 0.72 (0.20-2.60) | 0.51 (0.11-2.36) | 1.59 (0.54-4.69) | 13.02 (4.30-39.4) |
| DVT prior to enrollment | 2.04 (1.22-3.39) | 1.11 (0.55-2.27) | 4.11 (1.90-8.90) | 0.65 (0.14-2.96) | 0.65 (0.19-2.28) | 3.64 (0.91-14.5) |
| PE prior to enrollment | 0.79 (0.30-2.08) | 0.55 (0.12-2.43) | 1.09 (0.29-4.07) | NA | 0.73 (0.09-6.05) | 0.72 (0.06-8.46) |
| Bleeding prior to enrollment | 1.04 (0.61-1.77) | 1.10 (0.60-2.04) | 0.87 (0.31-2.47) | 1.24 (0.46-3.33) | 0.91 (0.32-2.59) | 0.78 (0.16-3.94) |
| Smoking | 1.55 (0.99-2.43) | 1.90 (1.15-3.14) | 0.81 (0.29-2.30) | 1.40 (0.53-3.69) | 1.89 (0.89-4.01) | 2.86 (0.92-8.86) |
| Total blood cholesterol count above 200 mg/dL | 1.16 (0.78-1.72) | 1.24 (0.79-1.94) | 0.83 (0.37-1.87) | 1.50 (0.62-3.65) | 0.95 (0.49-1.85) | 1.41 (0.51-3.91) |
| Diabetes mellitus | 1.10 (0.63-1.91) | 1.38 (0.76-2.53) | 0.48 (0.12-1.98) | 0.94 (0.29-3.08) | 1.28 (0.50-3.26) | 2.07 (0.58-7.39) |
| Hypertension | 1.25 (0.91-1.71) | 1.14 (0.78-1.65) | 1.48 (0.83-2.63) | 1.17 (0.62-2.23) | 0.97 (0.55-1.70) | 1.23 (0.50-3.06) |
| Intermittent claudication | 1.57 (0.90-2.73) | 1.29 (0.66-2.53) | 2.81 (1.14-6.89) | 2.16 (0.80-5.85) | 0.29 (0.04-2.14) | 3.71 (1.15-12.0) |
| Erythromelalgia | 0.54 (0.24-1.24) | 0.67 (0.27-1.68) | 0.30 (0.04-2.18) | 1.25 (0.37-4.23) | 0.80 (0.19-3.37) | NA |
| Splenomegaly | 0.74 (0.52-1.04) | 0.67 (0.44-1.00) | 0.88 (0.48-1.62) | 0.79 (0.39-1.57) | 0.86 (0.48-1.54) | 0.27 (0.08-0.89) |
NA indicates not available.
Age was by far the most important predictor of thrombosis in all vascular districts, as previously reported (Figure 3; Table 5). History of venous thrombosis was significantly associated with the risk of venous thromboembolism but not associated with the risk of arterial thrombosis (ie, myocardial infarction, stroke, and TIA). Hematocrit and platelet variations did not influence the overall thrombotic risk (data not shown). History of myocardial infarction, stroke, and PAT predicted the recurrence of the same type of event (Figure 3). Of the 169 patients with thrombotic events during follow-up, 51 patients (30%) had 1 or more cardiovascular risk factors. Hypertension was most prevalent (39.5% of PV patients), followed by smoking (12.8%), diabetes (7.1%), and high blood cholesterol (3.5%). Hypertension was associated with a nonsignificant increase in the thrombotic risk, while smoking was significantly associated with increased risk of arterial thrombotic events (HR, 1.90; 95% CI, 1.15-3.14; P = .012) and was nonsignificantly associated with the risk of peripheral artery disease (HR, 2.86; 95% CI, 0.92-8.86; P = .069) and of stroke/TIA (HR, 1.89; 95% CI, 0.89-4.01; P = .098).
Multivariable analysis assessing major risk factors of thrombosis in 1638 patients with polycythemia vera. Circles are HRs, and bars are 95% CIs. Black circles and bars indicate statistically significant results; gray circles and bars, results that are not statistically significant.
Multivariable analysis assessing major risk factors of thrombosis in 1638 patients with polycythemia vera. Circles are HRs, and bars are 95% CIs. Black circles and bars indicate statistically significant results; gray circles and bars, results that are not statistically significant.
Discussion
In polycythemia vera, thromboses and malignancies are the 2 main causes of morbidity and mortality.1,2 Aspirin has been shown to be an effective and safe antithrombotic agent,10 but high-risk subjects—notwithstanding aspirin and chemotherapy—still have an incidence of vascular events above 5% per year.13,14 In these patients, more aggressive treatments may provide additional benefit, but balancing the thrombotic, neoplastic, and hemorrhagic risk of the various drug combinations remains a major challenge of PV treatment strategy.15 A main issue is the lack of essential knowledge on the long-term efficacy and safety of many treatment options as well as on the main determinants of the vascular risk. These determinants were investigated in the present study, which gathered information from the prospective ECLAP observational study. This, which is the far largest epidemiologic study in polycythemia vera, included 1638 patients and recorded 205 thromboses in 169 patients. The multivariate analysis performed on the various clinical and laboratory parameters showed that, among disease-related parameters, only leukocytosis was significantly associated with the vascular risk. The lack of correlation between platelet number and thrombosis confirms the results of previous studies, while our data on hematocrit should be more cautiously interpreted due to possible discrepancies between hematocrit and red cell mass values in PV. Within the limitations of the relatively wide CIs, the impact of leukocytosis on the vascular risk could be compared with that of age and thrombotic history, which are the main determinants of the vascular risk of PV subjects. As already reported, young subjects with no thrombotic history had a relatively low vascular risk.8–10 In particular, only 5 MIs were recorded in this subgroup. White blood cell count had previously been reported to influence the risk of vascular complications in the general population and this, similar to the association of other inflammatory markers with atherothrombosis, has been interpreted as a reflection of the inflammatory process associated with plaque progression.16 Recently, however, the hypothesis of a direct causative role of leukocytes has led to the consideration of the short-term use of hydroxyurea in subjects with very high vascular risk.17 A prothrombotic role of leukocytes in essential thrombocythemia, which was initially suggested on the basis of an experimental study,18 seems confirmed by the recent report of an increased vascular risk in thrombocythemic subjects with leukocytocis19 and may provide an explanation for the antithrombotic property of hydroxyurea not shared by anagrelide.20 Besides, leukocyte activation and interaction with platelets have recently received much attention after the various reports concerning the presence and clinical significance of the JAK2 mutation in myeloproliferative disorders.21
The multivariate analysis took into consideration and ruled out several potential confounders, including the type of treatment. Despite this analysis we cannot rule out the hypothesis that inadequate treatment and/or the presence of inflammatory stimuli may partially account for the observed linkage between leukocytosis and vascular risk. In particular, our time-dependent analysis could not be adjusted for the dose of hydroxyurea, which was the most frequently used chemotherapeutic agent. On the other hand, the unavailability of other inflammatory markers did not allow us to explore the inflammatory status of our patients. The impact of leukocytosis was mostly observed on MI risk, thus suggesting a more direct causative role of leukocytes on this event. An interaction between leukocyte count and other thrombotic complications might also exist and require larger studies for its detection. In addition, other mechanisms, currently elusive, may have a more prominent role in the pathogenesis of cerebrovascular, peripheral, and venous events. Because thrombotic recurrence has been often observed in the same vascular district, the coexistence of history of MI with leukocytosis could allow us to predict a very high risk of recurrent MI, while a high risk of venous thrombosis was observed among patients with past history of venous thromboembolism. These high-risk conditions are likely to benefit from more effective and/or targeted interventions, and future clinical studies will need to compare the benefit-risk profile of more aggressive antithrombotic and/or chemotherapeutic regimens. Finally, the results of this study highlight the need for an effective intervention on smoking habits due to the very high prevalence of such an important vascular risk factor in our PV population.
Authorship
Contribution: R.L. and R.M. conceived and designed the research and wrote the paper; L.D.G. and R.M.M. collected and analyzed the data; and T.B., G.F., G.T., and V.D.S. discussed the data and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the ECLAP appears as a data supplement to the online version of this article.
Correspondence: Raffaele Landolfi, Catholic University School of Medicine, Institute of Internal Medicine and Geriatrics, Haemostasis Research Center, Largo Agostino Gemelli 8, 00168 Rome, Italy; e-mail: rlandolfi@rm.unicatt.it.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the European Union, BIOMED 2 Program (contract no. ERBBMH4CT961433). The valuable contribution of Carlo Patrono and Luis Garcia Rodriguez is gratefully acknowledged.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal