Abstract
RhoH is a small GTPase expressed only in the hematopoietic system. With the use of mice with targeted disruption of the RhoH gene, we demonstrated that RhoH is crucial for thymocyte maturation during DN3 to DN4 transition and during positive selection. Furthermore, the differentiation and expansion of DN3 and DN4 thymocytes in vitro were severely impaired. These defects corresponded to defective TCR signaling. Although RhoH is not required for TCR-induced activation of ZAP70 and ZAP70-mediated activation of p38, it is crucial for the tyrosine phosphorylation of LAT, PLCγ1, and Vav1 and for the activation of Erk and calcium influx. These data suggest that RhoH is important for pre–TCR and TCR signaling because it allows the efficient interaction of ZAP70 with the LAT signalosome, thus regulating thymocyte development.
Introduction
RhoH is a member of the Rho GTPase family, expressed only in the hematopoietic system. Rho GTPases are small GTPases that regulate cytoskeletal organization, proliferation, survival, and cell polarization.1 They are present in an active, GTP-bound form and an inactive, GDP-bound form. Only in the GTP-bound conformation can they interact with a wide range of effector molecules, including serine-threonine kinases, lipid kinases, and cytoskeletal proteins.
RhoH was shown to be expressed in hematopoietic progenitor cells (HPCs), lymphocytes, and neutrophils.2,3 Because RhoH has no functional intrinsic GTPase activity, it is thought to be constitutively active and controlled only at the transcriptional level. Indeed, RhoH expression is regulated in lymphocytes.2 In T cells, activation of the T-cell receptor reduced the RhoH message within a few hours, whereas PMA treatment decreased RhoH mRNA levels in Jurkat cells within 60 minutes.2 In diffuse large B-cell lymphoma, RhoH is frequently mutated in the noncoding region, which might affect mRNA stability or translation efficiency.4,5 Functionally, RhoH was proposed to be a negative regulator of Rho GTPases and integrins. RhoH inhibits Rac1-mediated activation of p38 MAPK and NFκB in Jurkat cells but has no effect on JNK or Erk or on the activation of Rac1 in these cells.2 In hematopoietic stem cell (HSC)/HPC preparations, RhoH negatively regulates the proliferation, survival, and migration and reduces the SCF-induced activation of Rac1.3 Finally, RhoH expression maintains lymphocytes in a nonadhesive state by decreasing integrin-mediated attachment.6
These observations suggested an important role of RhoH in the maintenance of HSCs, in leukocyte migration, and possibly in the development of B-cell lymphoma. To test these hypotheses in vivo, we generated mice that lacked a functional RhoH gene and analyzed the development of different hematopoietic lineages. RhoH-null mice did not develop lymphoma and had no obvious defects in HSC maintenance, but they showed impaired T-cell differentiation attributed to defective T-cell receptor (TCR) signaling.
Materials and methods
Mice
RhoH-deficient mice were generated using procedures described previously.7 As a targeting vector, a 6.9-kb EcoRI-EcoRI genomic DNA fragment was used in which a 0.9-kb HindIII-AatII fragment encoding the first 42 amino acids of the RhoH protein was replaced by a neomycin resistance expression cassette. Wild-type and heterozygous knockout mice were used as controls, with indistinguishable results in all assays. If not stated otherwise, mice were kept as 129Sv/C57BL6 outbreds. β2-integrin8 –deficient mice and RhoH-null mice were intercrossed to obtain RhoH-β2-null double-knockouts. RhoH mutant mice backcrossed for 6 generations to C57BL6 were mated with OT-II transgenic mice9 to obtain RhoH-null/OT-II mice. All mice were kept in a barrier facility in accordance with the German policies on animal welfare.
Flow cytometry
Single-cell suspensions were prepared by gentle disaggregation of the dissected organs through 70-μm cell strainers. Cells were stained with antibodies (all BD PharMingen [San Diego, CA] unless otherwise specified) conjugated to FITC, PE, APC, or biotin in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and analyzed on a FACSCalibur with CellQuest software. Biotinylated antibodies were detected by streptavidin-Cy-5 (Jackson ImmunoResearch, West Grove, PA) or streptavidin-Cy-Chrom. Dead cells were excluded by forward-scatter and side-scatter profiles and by 1 μg/mL propidium iodide counterstaining. The following antibodies were used: anti-B220 (RA3-6B2), anti-IgM (R6-60.2), anti-IgD (11-26c.2a), anti–Gr-1 (RB6-8C5), anti-Mac1 (M1-70), anti-Ter119, anti–NK1.1 (PK136), anti-CD4 (H129.19) (GK1.5; eBioscience, San Diego, CA), anti-CD8 (53-6.7), anti-CD5 (53-7.3), anti-TCRβ (H57-597), anti-CD69 (H1.2F3), anti-Vα2 (B20.1), anti-TCRγδ (GL3), and anti-CD62L (MEL-14; eBioscience). For the characterization of double-negative (DN; Lin− CD4−CD8−) thymocytes, cells were gated for lineage-negative (Lin−) (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 (7D4) and CD44 (IM7).
For proliferation assays, mice were given intraperitoneal injections of 1 mg BrdU and were killed 2 hours later. BrdU incorporation of thymocytes was determined using the BrdU Flow kit. AnnexinV staining was carried out in binding buffer (10 mM Hepes, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) using Annexin V–Alexa 488 (kindly provided by Dr E. Pöschl, Erlangen, Germany).
T-cell development in vitro
In vitro T-cell development on OP9-DL1 stromal cells was carried out as described previously.10 OP9-DL1 cells were maintained in α-MEM medium (Invitrogen, Carlsbad, CA) containing 20% FCS and penicillin/streptomycin. Control and RhoH−/− DN thymocytes enriched through MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) were sorted for DN3 and DN4 populations with FACSAria. DN3 (5 × 104) and DN4 (2.5 × 104) thymocytes were plated onto a subconfluent OP9-DL1 monolayer in a 24-well plate and cultured in the presence of 5 ng/mL Flt3 ligand (Peprotech, Rocky Hill, NU) and 1 ng/mL IL-7 (Peprotech) for 8 days. The medium with cytokines was replaced after 4 days. Developmental progression of cultured cells was assessed by flow cytometry after 4 and 8 days. Cells were harvested by forceful pipetting and filtered through a 70-μM cell strainer to remove OP9-DL1 cells.
TCR signaling
Freshly isolated thymocytes (0.5 × 107 to 1 × 107) were incubated with 5 μg/mL biotinylated anti-CD3ϵ (145-2C11) and anti-CD4 or anti-CD3ϵ alone for 20 minutes on ice and washed. Cross-linking of the bound antibodies was carried out with the addition of prewarmed streptavidin (5-10 μg/mL; Sigma, St Louis, MO) and incubation of the samples for 5 minutes at 37°C. After stimulation, 5 × 106 cells were directly fixed in an equal volume of 4% paraformaldehyde in PBS for 10 minutes at 37°C, washed with PBS, and permeabilized in ice-cold 90% methanol for 30 minutes on ice. Cells were then washed twice in PBS, incubated with fluorescence-labeled anti-CD4, anti-CD8, and anti–phospho-ZAP70 (Y319)/Syk (Y352), anti–phospho-Lck (Y505), anti–phospho-Erk1/2 (T202/Y204), or anti–phospho-p38 MAPK (T180/Y182) for 1 hour at room temperature, and analyzed by FACS.
Double-positive (DP) thymocytes (0.5 × 107 to 1 × 107) sorted by FACSVantage were stimulated as described, washed with cold PBS, and lysed in 100 μL 50 mM Tris pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 10% glycerol, 2 mM MgCl2, 1 mM Na3VO4, and 100 mM NaF containing a protease inhibitor cocktail (Complete Mini, EDTA free; Roche, Basel, Switzerland) for 20 minutes on ice. Lysates were cleared by centrifugation at 20 800 g for 15 minutes at 4°C and immunoprecipitated with anti-LAT (Upstate Biotechnology, Lake Placid, NY) or anti-Vav1 (C-14; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies bound to protein A agarose (Sigma-Aldrich, St Louis, MO). Phosphorylation of the immunoprecipitated proteins was detected by Western blotting using anti–phosphotyrosine PY-7E1 (Zymed, San Francisco, CA) antibody. Total levels of the immunoprecipitated proteins were determined by reprobing the blots with anti-LAT (11B.12; Upstate Biotechnology) or anti-Vav1 (D-7; Santa Cruz Biotechnology) antibodies. Aliquots (20 μL) of cell lysates were analyzed by Western blotting using the following antibodies: anti–phospho-ZAP70(T319) (Cell Signaling, Beverly, MA), anti-ZAP70 (99F2; Cell Signaling), anti–phospho-Vav2(T172) (Santa Cruz Biotechnology), anti-Vav2 (H-200; Santa Cruz), anti–phospho-LAT(T191; Cell Signaling), anti-LAT (11B.12; Upstate Biotechnology), anti–phospho-PLCγ1(T783) (Cell Signaling), anti-PLCγ1 (Cell Signaling), anti–phospho-p44/p42 MAPK (T202/Y204) (New England BioLabs, Ipswich, MA), anti–p44/p42 MAPK (New England BioLabs). Pull-downs for active Rac1 and Rac2 were performed as previously described11 with 0.5 × 107 to 1 × 107 DP sorted thymocytes stimulated as indicated by cross-linking of biotinylated CD3 and CD4 antibodies with streptavidin for 30 seconds or 1 minute and 5 minutes at 37°C. Total lysates and precipitates were analyzed by Western blotting with the use of antibodies against Rac1 (Transduction Laboratories, Lexington, KY) and Rac2 (Upstate Biotechnology).
For the detection of phosphorylated Vav2 and active Rac2, DP thymocytes were sorted by positive selection using anti-CD8-FITC and MACS anti-FITC microbeads (Miltenyi). The purity of obtained DP thymocytes was 93% to 97%, as determined by flow cytometry.
For measurement of calcium influx, 2 × 106 thymocytes were loaded with 2.5 μM Fluo-4 (Molecular Probes, Eugene, OR) for 25 minutes at 37°C and washed twice with RPMI 1640, 25 mM Hepes, without phenol red (Gibco, Grand Island, NY). Cells were stained on ice with 5 μg/mL anti–CD8α-APC (53-6.7; eBioscience), anti–CD4-PE-Cy5.5 (RM4-5; Caltag, Burlingame, CA), and biotinylated anti-CD3ϵ. Cells were warmed to 37°C and analyzed for 20 seconds to establish baseline calcium levels. Then CD3ϵ was cross-linked by the addition of 10 μg/mL streptavidin. Flow cytometric analysis was performed with FACSCalibur using FlowJo software (TreeStar, Ashland, OR).
Adhesion assays
Statistical analysis
All mean values are shown with standard deviation. Student t test was performed to assess the significance of observed differences.
Results
Generation of RhoH-deficient mice
To generate mice with targeted disruption of the RhoH gene, we replaced the genomic sequence encoding the translation start and the switch1 region, which is crucial for the interaction with GEFs or GAPs14 effectors, by a neomycin expression cassette using homologous recombination (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Mutation of the RhoH gene was confirmed by Southern blot analysis and genomic PCR (Figure S1B-D). Northern blot analysis indicated the expression of a long transcript in RhoH mutant mice that contained the sequence of the inverted neomycin resistance gene, with at least 6 Kozak box ATGs followed by an in-frame stop codon and the truncated RhoH coding region (Figure S1E). These open-reading frames should prevent any expression of a truncated RhoH molecule. RhoH mRNA levels in heterozygous mutant mice were approximately 50% those of homozygous wild-type mice.
Because of the lack of functional antibodies, confirmation of the loss of RhoH on protein level was not possible. Homozygous mutant mice were born at Mendelian ratio (+/+, 23.2%; +/−, 50.8%; −/−, 26.0%; n = 214), indicating no embryonic lethality. RhoH-null mice were fertile and were of normal weight and life span.
Hematopoiesis
RhoH is expressed in HPCs, lymphoid cells, and myeloid cells.2,3 We analyzed the development of different blood lineages in mutant and control mice. In 2-month-old mice, BM cellularity was not significantly different between RhoH-null and control mice, but splenocyte counts were lower in RhoH-deficient mice (Figure S2). Lymph node cellularity varied but tended to be lower in mutant mice (Figure S2). Population sizes of granulocytes (Mac1+ Gr-1+), monocytes (Mac1+ Gr-1−), erythroblasts (Ter119+), and different stages of B cells (proB + preB [B220+IgM−], immature [B220+IgM+], mature [B220+IgM+IgD+]) in BM were similar in mutant and control mice (Table 1), indicating that RhoH is not essential for the differentiation of myeloid, erythroid, or B cells. Splenic granulocyte counts were unperturbed, whereas splenic monocyte counts were slightly increased in RhoH-deficient mice. B-cell counts (IgD+) in spleen and lymph nodes were similar in mutant and control mice (Table 1). In contrast, T-cell counts were strongly decreased in BM, spleen, and lymph nodes. CD8+ T-cell counts were reduced by approximately 75%, and CD4+ T-cell counts were reduced by 50% (Table 1). Heterozygous mice showed a normal phenotype and were included in the control group. These data suggest a defect in the production, survival, or migration of T cells in the absence of RhoH.
Absolute sizes of myeloid, erythroid, and lymphoid populations in hematopoietic organs
| Hematopoietic organ . | Control, × 106 cells . | RhoH−/−, × 106 cells . | P . | ||
|---|---|---|---|---|---|
| Mean . | SD . | Mean . | SD . | ||
| Bone marrow | |||||
| Mac1+ Gr-1+ | 14.849 | 2.357 | 14.889 | 1.501 | — |
| Mac1+ Gr-1− | 1.679 | 0.594 | 1.360 | 0.466 | — |
| Ter119+ | 7.131 | 4.622 | 6.733 | 4.560 | — |
| NK1.1+ | 0.151 | 0.100 | 0.355 | 0.092 | < .01 |
| CD4+ | 0.218 | 0.123 | 0.101 | 0.021 | < .05 |
| CD8+ | 0.225 | 0.159 | 0.054 | 0.007 | < .05 |
| B220+IgM− | 2.377 | 1.110 | 2.620 | 0.745 | — |
| B220+IgM+ | 1.602 | 0.645 | 2.084 | 0.591 | — |
| B220+IgM+IgD+ | 0.740 | 0.183 | 0.971 | 0.422 | — |
| Spleen | |||||
| Mac1+ Gr-1+ | 10.192 | 3.666 | 9.721 | 4.169 | — |
| Mac1+ Gr-1− | 5.879 | 1.351 | 8.971 | 2.366 | < .05 |
| Ter119+ | 24.934 | 5.423 | 26.361 | 8.124 | — |
| NK1.1+ | 2.791 | 1.337 | 7.816 | 2.617 | < .01 |
| CD4+ | 64.082 | 7.282 | 23.741 | 5.426 | < .001 |
| CD8+ | 27.418 | 3.342 | 7.500 | 0.743 | < .001 |
| IgD+ | 97.934 | 12.041 | 89.384 | 7.611 | — |
| Lymph nodes | |||||
| CD4+ | 4.163 | 3.649 | 1.855 | 1.105 | — |
| CD8+ | 1.603 | 1.506 | 0.281 | 0.226 | — |
| IgM+IgD+ | 1.077 | 0.646 | 0.959 | 0.434 | — |
| Hematopoietic organ . | Control, × 106 cells . | RhoH−/−, × 106 cells . | P . | ||
|---|---|---|---|---|---|
| Mean . | SD . | Mean . | SD . | ||
| Bone marrow | |||||
| Mac1+ Gr-1+ | 14.849 | 2.357 | 14.889 | 1.501 | — |
| Mac1+ Gr-1− | 1.679 | 0.594 | 1.360 | 0.466 | — |
| Ter119+ | 7.131 | 4.622 | 6.733 | 4.560 | — |
| NK1.1+ | 0.151 | 0.100 | 0.355 | 0.092 | < .01 |
| CD4+ | 0.218 | 0.123 | 0.101 | 0.021 | < .05 |
| CD8+ | 0.225 | 0.159 | 0.054 | 0.007 | < .05 |
| B220+IgM− | 2.377 | 1.110 | 2.620 | 0.745 | — |
| B220+IgM+ | 1.602 | 0.645 | 2.084 | 0.591 | — |
| B220+IgM+IgD+ | 0.740 | 0.183 | 0.971 | 0.422 | — |
| Spleen | |||||
| Mac1+ Gr-1+ | 10.192 | 3.666 | 9.721 | 4.169 | — |
| Mac1+ Gr-1− | 5.879 | 1.351 | 8.971 | 2.366 | < .05 |
| Ter119+ | 24.934 | 5.423 | 26.361 | 8.124 | — |
| NK1.1+ | 2.791 | 1.337 | 7.816 | 2.617 | < .01 |
| CD4+ | 64.082 | 7.282 | 23.741 | 5.426 | < .001 |
| CD8+ | 27.418 | 3.342 | 7.500 | 0.743 | < .001 |
| IgD+ | 97.934 | 12.041 | 89.384 | 7.611 | — |
| Lymph nodes | |||||
| CD4+ | 4.163 | 3.649 | 1.855 | 1.105 | — |
| CD8+ | 1.603 | 1.506 | 0.281 | 0.226 | — |
| IgM+IgD+ | 1.077 | 0.646 | 0.959 | 0.434 | — |
Data shown are the averages (means and SD) of the absolute sizes of the cell populations carrying indicated surface markers in single-cell suspensions of bone marrow, spleen, and lymph nodes of 2-month-old control and RhoH-null mice. n [control]/[RhoH−/−]: 5/5.
— indicates not significant (P > .05).
NK1.1+ cell counts, including those of NK and NKT cells, were elevated in BM and spleen (Table 1). Six-month-old mutant mice still had normal numbers of myeloid, erythroid, and B cells in the BM, indicating that RhoH is not crucial for the maintenance of HSCs (Table S1). Splenic granulocyte, monocyte, and B-cell populations were similar in 6-month-old control and RhoH-deficient mice, whereas the number of splenic Ter119+ erythroblasts was greater in mutant mice. CD4+ and CD8+ T cells were reduced in BM, spleen, and lymph nodes. In contrast to counts in 2-month-old mutant mice, NK1.1+ cell counts were not significantly changed in BM and spleen (Table S1).
Defective T-cell development in vivo
To test whether the production of T cells is impaired in the absence of RhoH, we analyzed thymocytes of 2-month-old mice. Thymus cellularity of RhoH-null mice was approximately 60% lower than in control animals (Figure S2). In mutant mice, the absolute number of DN (CD4−CD8−) thymocytes was increased more than twofold, whereas the number of DP (CD4+CD8+) thymocytes was reduced by 60%, indicating an incomplete developmental block between the DN and the DP stages (Figure 1A, lower panel). Absolute amounts of CD4SP and CD8SP thymocytes were reduced by 80% and 85%, respectively, in RhoH-deficient mice, implying an additional defect during the development from DP to SP cells, where positive selection takes place (Figure 1A).
Impaired thymocyte development in the absence of RhoH. (A) Thymocytes of 2-month-old mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell number of each population. **P < .01; ***:P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 11/11). (B) Thymocytes of 2-month-old mice were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−. Bar graph presents the absolute cell number of each population. *P < .05. Error bars show the standard deviation (n [control]/[RhoH−/−]: 4/4).
Impaired thymocyte development in the absence of RhoH. (A) Thymocytes of 2-month-old mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell number of each population. **P < .01; ***:P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 11/11). (B) Thymocytes of 2-month-old mice were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−. Bar graph presents the absolute cell number of each population. *P < .05. Error bars show the standard deviation (n [control]/[RhoH−/−]: 4/4).
To define the block during DN to DP development in more detail, the population of DN cells was further divided into DN1 (CD25−CD44+), DN2 (CD25+CD44+), DN3 (CD25+CD44−), and DN4 (CD25−CD44−) cells. The number of DN1 cells was not significantly different between control and mutant mice, suggesting normal migration of T-cell precursors from the BM to the thymus (Figure 1B). The very small population of DN2 cells was slightly, but not significantly, increased in the absence of RhoH. The total number of DN3 cells, however, was elevated nearly 3-fold in the absence of RhoH. DN4 cell counts were not significantly different between control and mutant mice, whereas DP cell counts were reduced in the absence of RhoH. In addition, 6-month-old mutant mice showed a decrease in CD4SP and CD8SP cells and an increase in DN3 thymocytes (Figure S3A-B). These differences, however, were less pronounced than in 2-month-old mice. DP cell numbers were only slightly decreased in mutant mice (Figure 3A-B).
This T-cell phenotype was indistinguishable in 129Sv/C57BL6 outbred and backcrossed C57BL6 mice (data not shown). Total amounts of thymocytes with TCRγδ were normal in the absence of RhoH (Figure S4A), but the very small population of DN3 cells expressing TCRγδ was increased (Figure S4B). These data indicate that RhoH is only required for the development of T cells carrying αβ TCR. γδ T-cell counts were slightly increased in spleen and significantly increased in lymph nodes (Figure S4A).
Heterozygous mice showed a normal T-cell phenotype and were included in the control group. Quantitative RT-PCR revealed that RhoH is expressed at all stages of thymocyte development, with relative peaks at the DN3 and DP stages (Figure S5). These data indicate defects in the DN3 to DN4 transition and in the differentiation of DN4 to DP cells in RhoH-deficient mice.
Aberrant thymocyte proliferation and survival
To characterize whether impaired thymocyte development was caused by defective proliferation or increased apoptosis, we assessed cell proliferation by measuring the incorporation of BrdU and apoptosis by determining the percentage of AnnexinV binding to thymocyte subpopulations.
The percentage of BrdU-incorporating, proliferating DN1 and DN2 thymocytes was not significantly different between control and mutant mice (Figure 2A). However, DN3 and DN4 cells showed significantly decreased proliferation in the absence of RhoH (Figure 2A). Proliferation of DP cells was unchanged, whereas CD4SP and CD8SP cells showed increased proliferation (Figure 2A).
Impaired proliferation and survival and decreased expression of maturation markers in RhoH-null thymocytes. (A-B) Thymocytes of 2-month-old mice were analyzed for proliferating BrdU-incorporating cells and apoptotic AnnexinV+ cells. Different thymocyte populations were distinguished (see Figure 1A-B). *P < .05; **P < .01; ***P < .001; BrdU, DN1-DN4, n [control]/[RhoH−/−]: 6/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/10; Annexin V, DN1 to DN4, n [control]/[RhoH−/−]: 10/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/9. (C-E) Thymocytes of 2-month-old mice were analyzed for the expression of CD4, CD8, and CD5 (C; n [control]/[RhoH−/−]: 9/9) or TCRβ (D; n [control]/[RhoH−/−]: 4/4) or CD69 (E; n [control]/[RhoH−/−]: 6/6) by FACS. Percentages of cells marked in histograms are shown in graph. **P < .01; ***P <.001. Error bars show standard deviation.
Impaired proliferation and survival and decreased expression of maturation markers in RhoH-null thymocytes. (A-B) Thymocytes of 2-month-old mice were analyzed for proliferating BrdU-incorporating cells and apoptotic AnnexinV+ cells. Different thymocyte populations were distinguished (see Figure 1A-B). *P < .05; **P < .01; ***P < .001; BrdU, DN1-DN4, n [control]/[RhoH−/−]: 6/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/10; Annexin V, DN1 to DN4, n [control]/[RhoH−/−]: 10/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/9. (C-E) Thymocytes of 2-month-old mice were analyzed for the expression of CD4, CD8, and CD5 (C; n [control]/[RhoH−/−]: 9/9) or TCRβ (D; n [control]/[RhoH−/−]: 4/4) or CD69 (E; n [control]/[RhoH−/−]: 6/6) by FACS. Percentages of cells marked in histograms are shown in graph. **P < .01; ***P <.001. Error bars show standard deviation.
In DN1, DN2, and DN3 cells, apoptosis was not significantly different between RhoH-null and control mice (Figure 2B). In contrast, RhoH-deficient DN4 cells displayed increased apoptosis (Figure 2B). In addition, DP, CD4SP, and CD8SP thymocytes showed a higher percentage of AnnexinV+ apoptotic cells, possibly indicating that fewer cells were positively selected (Figure 2B).
Defective TCR signaling suggested by altered expression of maturation markers
During thymocyte development, the expression of CD5, TCRβ, and CD69 is tightly regulated by pre–TCR and TCR signaling. At the DN stage, pre–TCR signaling induces the expression of CD5.15 On DP cells, CD5 expression is maintained because of low-affinity TCR–major histocompatibility complex interactions. Finally, CD5 is up-regulated during DP to SP transition in response to TCR signaling by positive- or negative-selecting ligands.
In all thymocyte populations tested (DN, DP, CD4SP, CD8SP, and γδ T cells), the percentage of cells with low CD5 expression was significantly increased in RhoH-deficient mice (Figures 2C, S4C). In addition, RhoH-null DP cells expressed significantly lower levels of CD5 than control cells. In spleen, the percentage of CD5low T cells was increased in the absence of RhoH (Figure S6A). Among the CD4+ splenocytes, the amount of CD5low cells increased from 1.5% to 24.6%, and among CD8+ cells it increased from 3.7% to 18.5%. These data suggest defects in pre–TCR and TCR signaling.
The expression of TCRβ and CD69 becomes up-regulated during positive selection in response to TCR signaling.16,17 In RhoH-null mice, the number of more mature, TCRβhigh, and CD69high thymocytes was significantly decreased among DP, CD4SP, and CD8SP cells, suggesting impaired positive selection and decreased TCR signaling (Figure 2D-E). No difference was found in the number of CD69high CD4+ and CD8+ cells in the spleen (Figure S6B). Interestingly, peripheral RhoH-null T cells in the spleen and lymph nodes showed a significantly increased amount of cells with cell surface characteristics of activated effector (CD62LlowCD44high) T cells, and more CD8+ T cells demonstrated the memory (CD62LhighCD44high) phenotype (Figure S7A-B).
Defective thymocyte development in vitro
To directly assess the differentiation potential of DN3 and DN4 thymocytes in the absence of RhoH, thymocyte populations were sorted and differentiated in vitro on OP9-DL1 cells. RhoH-deficient DN3 cells showed significantly less ability than controls to differentiate into DN4 and DP cells after 4 days and 8 days in culture (Figures 3A, S8A). In addition, the total cell number was severely lower in the absence of RhoH, indicating an impaired expansion potential (Figures 3A, S8A). In addition, RhoH-null DN4 cells showed a strongly reduced ability to develop into DP cells compared with controls and did not expand as well as the controls (Figures 3B, S8B).
Impaired differentiation of RhoH-null DN3 and DN4 cells in vitro. (A) Sorted DN3 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. Differentiation from DN3 to DN4 was tested by FACS analysis of lineage-negative cells for the expression of CD44 and CD25. RhoH-null cells showed a significantly higher percentage of DN3 and a lower percentage of DN4. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately 5-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01; ***P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 2/3). (B) Sorted DN4 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately15-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01. Error bars show the standard deviation (n [control]/RhoH−/−]: 3/4.
Impaired differentiation of RhoH-null DN3 and DN4 cells in vitro. (A) Sorted DN3 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. Differentiation from DN3 to DN4 was tested by FACS analysis of lineage-negative cells for the expression of CD44 and CD25. RhoH-null cells showed a significantly higher percentage of DN3 and a lower percentage of DN4. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately 5-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01; ***P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 2/3). (B) Sorted DN4 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately15-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01. Error bars show the standard deviation (n [control]/RhoH−/−]: 3/4.
These data demonstrate that in the absence of RhoH, the differentiation and expansion potential of DN3 and DN4 are decreased, confirming the in vivo data. Furthermore, they prove that this is a cell-autonomous defect of RhoH-null thymocytes.
Defect in positive selection
To test the positive selection of thymocytes into the CD4 lineage, we intercrossed RhoH-deficient mice backcrossed to C57BL6 with OT-II transgenic mice, which express an ovalbumin-specific TCR restricted to MHC class I-Ab as present in the RhoH-null mice. FACS analysis for the transgenic TCRα chain Vα2 indicated that nearly all DP and CD4SP thymocytes expressed the ovalbumin-specific TCR in control and mutant mice, though within the RhoH-deficient CD4SP cells a population expressing lower Vα2 levels could be detected (Figure 4C). Among the DN cells, which up-regulate Vα2 expression during β-selection, RhoH-null mice showed a reduced amount of Va2+ cells compared with controls (Figure 4C).
Defective positive selection in the absence of RhoH. (A) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were analyzed for the expression of CD4 and CD8 by FACS (upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel). *P < .05; **P < .01; ***P < .001. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (B) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−; upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel; *P < .05. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (C) Decreased amount of Vα2+ DN thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (D) Increased amount of CD5low thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. **P < .01; ***P < .001; Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (E) Decreased amount of CD69high thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4).
Defective positive selection in the absence of RhoH. (A) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were analyzed for the expression of CD4 and CD8 by FACS (upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel). *P < .05; **P < .01; ***P < .001. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (B) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−; upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel; *P < .05. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (C) Decreased amount of Vα2+ DN thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (D) Increased amount of CD5low thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. **P < .01; ***P < .001; Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (E) Decreased amount of CD69high thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4).
Expression of ovalbumin TCR in control mice led to a reduced DP thymocyte count and an increased CD4SP cell count (Figures 1A, 4A). Furthermore, the percentage of DN3 cells was decreased, and the DN4 population was increased (Figures 1B, 4B). In contrast, RhoH deficiency severely impaired the generation of CD4SP cells in mice expressing the ovalbumin TCR (Figures 1A, 4A). No increase in DN4 population was observed (Figures 1B, 4B). In addition, in the presence of the ovalbumin TCR, the percentage of CD5low cells was higher in RhoH-null mice than in controls (Figure 4D), and the relative amount of CD69high cells was lower (Figure 4E).
These data indicate an impaired positive selection of DP cells into the CD4 lineage in the absence of RhoH and conceivably a defective β-selection, which controls the DN3 to DN4 transition.
Defect in TCR signaling
In the absence of RhoH, the defects observed in thymocyte development and positive selection were consistent with impaired TCR signaling. To test this possibility directly, we analyzed TCR signaling in vitro. We induced TCR signaling in FACS-enriched preparations of DP cells by cross-linking biotinylated antibodies against CD3 or CD3 and CD4 with streptavidin and investigated the activation of different steps of the TCR signaling cascade. In addition, we measured the phosphorylation of different signaling molecules by intracellular FACS staining of stimulated thymocyte preparations.
TCR cross-linking triggers the activation of the tyrosine kinases lck and ZAP70. ZAP70-mediated phosphorylation of the scaffold protein LAT and associated molecules such as SLP-76 and PLCγ1, which together form the LAT signalosome, is then crucial to initiate downstream events such as calcium influx or Erk activation.
In the absence of RhoH, TCR-induced autophosphorylation of ZAP70 in DP cells was not altered (Figures 5A, S9A). Also, in CD4SP cells, TCR-dependent activation of ZAP70 did not require RhoH (Figure 6A). Furthermore, the phosphorylation of lck at Y505 was not altered in the absence of RhoH in DP and CD4SP cells (Figure 6B). Lck is negatively regulated by the phosphorylation of Y505, though this inhibition is overruled by the activating autophosphorylation of Y394.18 These data indicate that early events in TCR signaling are not strongly affected by the loss of RhoH. In CD4+ T cells, ZAP70 activates p38 MAPK independently of LAT.19 In RhoH-null CD4SP thymocytes, p38 was weakly, but significantly, activated as in controls (Figure 6D), suggesting that this downstream pathway of ZAP70 is normally intact in the absence of RhoH.
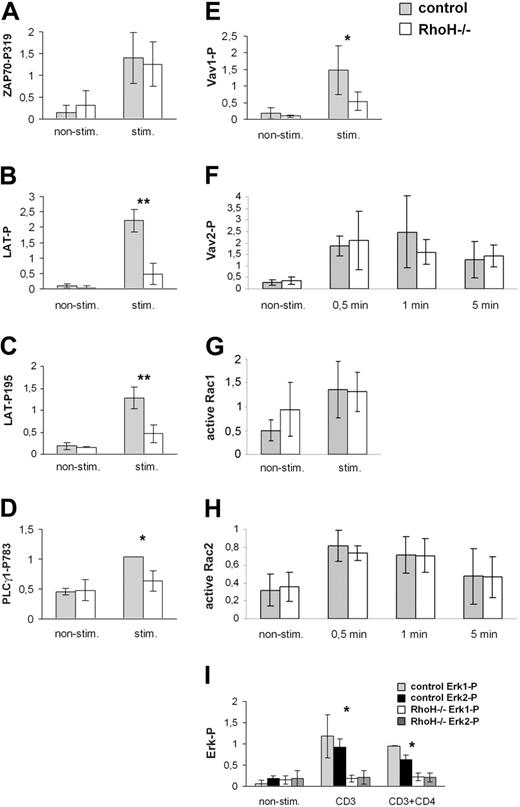
Impaired TCR signaling in RhoH-null DP thymocytes. DP thymocytes of 4- to 8-week-old mutant mice were sorted by FACS or MACS microbeads. TCR signaling was induced as indicated by cross-linking of biotinylated CD3 and CD4 antibodies with streptavidin for 5 minutes (A-E, I), 30 seconds (G), or indicated times (F, H) at 37°C. Total lysates were analyzed by Western blot for ZAP70-P319 (A), LAT-P195 (C), PLCγ1-P783 (D), Vav2-P (F), and Erk-P (I). Immunoprecipitations (IPs) of LAT (B) and Vav1 (E) were blotted with antiphosphotyrosine antibodies and reprobed with LAT (B) or Vav1 (E). Amounts of active Rac1 and Rac2 were determined by pull-down assays (G-H). Bar graphs represent quantifications of 5 (A), 3 (B-D,I), 4 (E), 5 (F), 10 (G), or 4 (H) independent experiments. *P < .05; **P < .01. All values are normalized to equal total amounts of the corresponding protein determined by Western blot. Representative examples of the Western blots are shown in Figure S9. Error bars show the standard deviation.
Impaired TCR signaling in RhoH-null DP thymocytes. DP thymocytes of 4- to 8-week-old mutant mice were sorted by FACS or MACS microbeads. TCR signaling was induced as indicated by cross-linking of biotinylated CD3 and CD4 antibodies with streptavidin for 5 minutes (A-E, I), 30 seconds (G), or indicated times (F, H) at 37°C. Total lysates were analyzed by Western blot for ZAP70-P319 (A), LAT-P195 (C), PLCγ1-P783 (D), Vav2-P (F), and Erk-P (I). Immunoprecipitations (IPs) of LAT (B) and Vav1 (E) were blotted with antiphosphotyrosine antibodies and reprobed with LAT (B) or Vav1 (E). Amounts of active Rac1 and Rac2 were determined by pull-down assays (G-H). Bar graphs represent quantifications of 5 (A), 3 (B-D,I), 4 (E), 5 (F), 10 (G), or 4 (H) independent experiments. *P < .05; **P < .01. All values are normalized to equal total amounts of the corresponding protein determined by Western blot. Representative examples of the Western blots are shown in Figure S9. Error bars show the standard deviation.
However, ZAP70-mediated total tyrosine phosphorylation of LAT and tyrosine phosphorylation of LAT at Y195, which is important for the interaction of LAT with Gads and SLP-76 and which indirectly supports the interaction of LAT with PLCγ1,20,21 were reduced in RhoH-null DP cells (Figures 5B-C, S9B-C). Phosphorylation of PLCγ1 at Y783, which is important for the activation of PLCγ1,22 was reduced in DP cells of RhoH-null mice (Figures 5D, S9D). In addition, the TCR-dependent phosphorylation of Vav1, which is required for the GEF activity of Vav1,23 was decreased in DP thymocytes in the absence of RhoH (Figures 5E, S9E). Interestingly, the phosphorylation of Vav2 after TCR ligation was similar in RhoH-null and control mice (Figures 5F, S9F).
Despite the decreased TCR-induced activation of Vav1, Rac1 and Rac2 activity after TCR activation were normal in RhoH-deficient DP cells (Figures 5G-H, S9G-H). However, the basal level of Rac1 activity appeared to be higher in RhoH-null DP cells than in controls, suggesting compensatory up-regulation of Rac1 activity in the absence of RhoH or inhibition of basal Rac1 activity by RhoH (Figures 5G, S9G). Basal levels of active Rac2 were not significantly different between RhoH-null and control mice (Figures 5H, S9H).
We then tested TCR-dependent activation of Erk and calcium influx, each of which requires PLCγ1 activation. In the absence of RhoH, TCR-induced Erk phosphorylation was severely reduced in DP, CD4SP, and CD8SP cells (Figures 5I, 6C, S9I). To assess TCR-induced calcium influx, thymocytes and splenocytes were loaded with the calcium-sensitive dye Fluo-4 and were incubated with biotinylated antibodies against CD3. After the induction of TCR signaling with streptavidin, the level of intracellular calcium was determined by FACS. Costaining for CD4 and CD8 allowed us to distinguish DN, DP, CD4SP, and CD8SP cells. No strong calcium influx was detected in DN control and mutant cells (Figure 6E). In DP, CD4SP, and CD8SP cells, however, RhoH-deficient cells exhibited a significantly decreased percentage of cells responding to stimulation and a decreased mean fluorescence of all cells, indicating a partially impaired TCR-dependent calcium influx (Figure 6E). In addition, RhoH-deficient CD4+ and CD8+ splenocytes showed a reduced stimulation of calcium influx after CD3 cross-linking, suggesting that RhoH is also important for TCR signaling in peripheral T cells (Figure 6F).
Defective TCR signaling and calcium influx in RhoH-null thymocytes. Thymocytes of 2-month-old mice were stimulated with biotinylated antibodies CD3 (C) or CD3 and CD4 (A-B, D) and streptavidin for 5 minutes at 37°C. Phosphorylation of ZAP70(Y319)/Syk(Y352) (A), lck(Y505) (B), Erk1/2(T202/Y204) (C), and p38 MAPK (T180/Y182) (D) was measured by FACS. Different thymocyte populations were distinguished (filled, nonstimulated; line, stimulated). Differences between the mean of stimulated and nonstimulated (specific mean) cells are shown. *P < .05; **P < .01. n [control]/RhoH−/−]: 5/5 (A-B, D). n [control]/RhoH−/−]: 4/5 (C). Thymocytes (E) or splenocytes (F) of 2-month-old mice were loaded with Fluo-4 and stained on ice for CD4, CD8, and CD3. After warming to 37°C, baseline Fluo-4 fluorescence was determined, and TCR signaling was induced by cross-linking CD3 with streptavidin. Presented is the percentage of cells above a threshold fluorescence (responding cells; left panel) and the mean fluorescence of all cells (right). Shown are representative results of 5 (E) or 2 (F) independent experiments. Error bars indicate the standard deviation.
Defective TCR signaling and calcium influx in RhoH-null thymocytes. Thymocytes of 2-month-old mice were stimulated with biotinylated antibodies CD3 (C) or CD3 and CD4 (A-B, D) and streptavidin for 5 minutes at 37°C. Phosphorylation of ZAP70(Y319)/Syk(Y352) (A), lck(Y505) (B), Erk1/2(T202/Y204) (C), and p38 MAPK (T180/Y182) (D) was measured by FACS. Different thymocyte populations were distinguished (filled, nonstimulated; line, stimulated). Differences between the mean of stimulated and nonstimulated (specific mean) cells are shown. *P < .05; **P < .01. n [control]/RhoH−/−]: 5/5 (A-B, D). n [control]/RhoH−/−]: 4/5 (C). Thymocytes (E) or splenocytes (F) of 2-month-old mice were loaded with Fluo-4 and stained on ice for CD4, CD8, and CD3. After warming to 37°C, baseline Fluo-4 fluorescence was determined, and TCR signaling was induced by cross-linking CD3 with streptavidin. Presented is the percentage of cells above a threshold fluorescence (responding cells; left panel) and the mean fluorescence of all cells (right). Shown are representative results of 5 (E) or 2 (F) independent experiments. Error bars indicate the standard deviation.
These data show that RhoH is required for TCR signaling downstream of ZAP70 in a cell-autonomous manner in thymocytes and in mature T cells.
RhoH is not crucial for the regulation of β2 integrin–mediated adhesion on thymocytes
Previously, it was suggested that RhoH is required to maintain integrin LFA-1 (αLβ2) in a nonadhesive state on lymphocytes.6 To test whether this function could contribute to the defect observed in thymocyte development, we measured the adhesion of RhoH-null thymocytes to the immobilized LFA-1 ligand ICAM-1. Manganese (Mn2+; 2 mM) or PMA (100 ng/mL) activated RhoH-null thymocytes bound to ICAM-1 with an efficiency equal to that of control cells (Figure 7A). At lower concentrations of Mn2+ (1 mM) or PMA (20 ng/mL), the combination of Mn2+ and PMA or, in the presence of Mg2+, the binding to ICAM-1 was similar in RhoH-null and control thymocytes (Figure S10). This demonstrated that LFA-1–mediated adhesion of thymocytes is normally regulatable in the absence of RhoH, at least under the conditions tested. Adhesion to the α4β1 integrin substrate VCAM-1, carried out as an additional control, was indistinguishable between control and mutant thymocytes, even when stimulated with Mg2+, Mn2+, or PMA (Figures 7A, S10).
RhoH does not affect thymocyte development through regulation of αLβ2 integrin-mediated adhesion. (A) Relative adhesion of thymocytes to the immobilized αLβ2 ligand ICAM-1 and to the α4β1 ligand VCAM-1. Integrins were activated by treatment with 2 mM Mn2+ or 100 ng/mL PMA as indicated. Adhesion of wild-type (RhoH +/+), heterozygous (RhoH+/−), and homozygous RhoH-null thymocytes (RhoH −/−) was indistinguishable at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 1/2/3. (B) Relative adhesion of T-cell blasts to the endothelial cell line bEnd5 and the ICAM-1–deficient endothelial cell line bEndI1.1 (ICAM1−/−). Adhesion was stimulated by treating the endothelial cells with TNF. Antibodies against α4-integrin and LFA-1 were used to determine the specific contribution of these adhesion receptors to the attachment. An unrelated antibody was added as a control for nonspecific effects. Wild-type (RhoH+/+), heterozygous (RhoH+/−), and homozygous (RhoH−/− ) RhoH-null T cells showed indistinguishable adhesion at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 2/2/4. (C)Thymocytes of 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell numbers of each population. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3. (D) Expression of CD5 and amount of CD5low thymocytes in 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3.
RhoH does not affect thymocyte development through regulation of αLβ2 integrin-mediated adhesion. (A) Relative adhesion of thymocytes to the immobilized αLβ2 ligand ICAM-1 and to the α4β1 ligand VCAM-1. Integrins were activated by treatment with 2 mM Mn2+ or 100 ng/mL PMA as indicated. Adhesion of wild-type (RhoH +/+), heterozygous (RhoH+/−), and homozygous RhoH-null thymocytes (RhoH −/−) was indistinguishable at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 1/2/3. (B) Relative adhesion of T-cell blasts to the endothelial cell line bEnd5 and the ICAM-1–deficient endothelial cell line bEndI1.1 (ICAM1−/−). Adhesion was stimulated by treating the endothelial cells with TNF. Antibodies against α4-integrin and LFA-1 were used to determine the specific contribution of these adhesion receptors to the attachment. An unrelated antibody was added as a control for nonspecific effects. Wild-type (RhoH+/+), heterozygous (RhoH+/−), and homozygous (RhoH−/− ) RhoH-null T cells showed indistinguishable adhesion at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 2/2/4. (C)Thymocytes of 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell numbers of each population. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3. (D) Expression of CD5 and amount of CD5low thymocytes in 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3.
In a more physiological setting, we investigated the adhesion of T-cell blasts to the endothelial cell line bEnd5, which up-regulates the expression of ICAM-1 and VCAM-1 on treatment with TNF. Both RhoH-null and control T cells showed TNF-stimulated binding to these endothelial cells, which was partially inhibited by antibodies against LFA-1 and α4-integrin, respectively (Figure 7B). TNF-induced binding to the ICAM-1–deficient cell line bEndI1.1 was low for control and RhoH-null T cells and was completely inhibited by antibodies against α4-integrin but was insensitive to LFA-1 antibody inhibition (Figure 7B).
To investigate, by an alternative approach, whether RhoH-dependent modulation of β2-integrin function was involved in the defective thymocyte development in RhoH-null mice, we crossed RhoH-deficient mice with mice lacking β2-integrin and analyzed T-cell development in the absence of both RhoH and β2-integrin. If loss of RhoH indeed constitutively up-regulated αLβ2 activity and this contributed to the thymocyte phenotype, ablation of the β2-integrin gene would logically have rescued the defect. However, we found that population sizes of DN, DP, CD4SP, and CD8SP thymocytes were identical in RhoH-null and RhoH-β2-integrin double-knockout mice (Figure 7C). Furthermore, the increase of CD5low cells in different RhoH-null thymocyte populations was unaffected by the additional loss of β2-integrin (Figure 7D). These data indicate that the impaired thymocyte development of the RhoH-null mice is independent of β2-integrin function.
Discussion
Previous work has shown that RhoH is a negative regulator of Rac1-dependent signaling, HPC proliferation and survival, and LFA-1–mediated adhesion.2,3,6 We demonstrated that during thymocyte development, RhoH is a positive regulator of thymocyte differentiation and TCR signaling and that it is crucial for β-selection and positive selection. Furthermore, we report that RhoH also contributes to the TCR signaling of mature T cells.
In the absence of RhoH, thymocyte development was partially blocked at the DN3 to DN4 transition. β-Selection takes place at this transition, which ensures that only thymocytes that have generated a functional TCRβ chain can differentiate to DP cells. A second partial block occurred at the transition from DP to CD4SP and CD8SP cells, where positive selection allows only MHC-restricted, self-tolerant thymocytes to develop further. In line with defects in β-selection and positive selection, the proliferation of RhoH-null DN3 and DN4 cells was decreased, whereas apoptosis of DN4, DP, CD4SP, and CD8SP cells was significantly increased. Furthermore, RhoH-null DN3 and DN4 cells showed a clearly reduced ability to differentiate and expand in vitro. Previously, it was documented that decreased RhoH expression increased the proliferation and survival of HPCs.3,24 Our data suggest that RhoH regulates proliferation and cell survival independently and in a cell type– and differentiation stage–specific manner.
While β-selection is dependent on pre–TCR signaling, positive selection is thought to require weak TCR signaling.25 CD5 expression correlates with pre–TCR and TCR signaling strength.15 Reduced expression of CD5 on DP cells and an increased number of CD5low cells among DN, CD4SP, and CD8SP thymocytes of RhoH-null mice suggests defective pre–TCR signaling in DN and impaired TCR signaling in DP, CD4SP, and CD8SP cells. Furthermore, reduced numbers of TCRβhigh and CD69high cells among DP, CD4SP, and CD8SP thymocytes indicated defective positive selection. Analysis of RhoH-null mice expressing an ovalbumin-specific, MHC class II–restricted TCR confirmed that RhoH is important for positive selection because no significant increase in positively selected CD4SP thymocytes was observed, in contrast to controls. Among DN cells, RhoH-null/OT-II mice did not display the obvious reduction of DN3 or the increase of DN4 thymocytes observed in controls, suggesting impaired β-selection. Interestingly, an increased proportion of peripheral T cells displayed surface characteristics of activated and memory cells in the absence of RhoH. We will test in the future whether this activation might be attributed to an increased amount of autoreactive T cells escaping negative selection that are activated in the periphery by high amounts of self-antigen. Such a phenotype has been observed in mice with a mutation in the ZAP70 gene; these mice also have partially impaired TCR signaling.26
Our data indicated that RhoH is important for pre–TCR and TCR signaling by facilitating the phosphorylation of the LAT signalosome by ZAP70. TCR activation by cross-linking of CD3 leads to activation of the lck tyrosine kinase, which phosphorylates the CD3 complex. Docking of ZAP70 to the phosphorylated ITAMs of the TCR and phosphorylation by lck stimulates its tyrosine kinase activity and results in ZAP70-dependent phosphorylation of the membrane-anchored LAT protein and of LAT-associated molecules, forming together the “LAT signalosome.” Normal autophosphorylation of ZAP70 at Y319 in RhoH-null thymocytes after cross-linking of CD3 suggests that TCR signaling is not impaired at the level of ZAP70 activation. This notion is strengthened by the normal TCR-dependent activation of p38 in CD4SP cells because in T cells this activation is mediated by ZAP70 independently of LAT.19 However, the phosphorylation of LAT and the LAT-associated proteins PLCγ1 and Vav1 and the activation of Erk and calcium influx, which are downstream of PLCγ1, were dramatically reduced in RhoH-deficient thymocytes. RhoH, therefore, seems to specifically interfere with the LAT branch of ZAP70 signaling. The impaired TCR-dependent calcium influx in peripheral CD4+ or CD8+ T cells indicates that RhoH is also important for TCR signaling in mature T cells and that loss of RhoH is not compensated during development.
When comparing the RhoH-null phenotype with other mouse mutants with defects in thymocyte development, striking similarities with Vav1-deficient mice become obvious27–29 and make it tempting to speculate that RhoH function is closely linked to Vav1. Given that Vav1 is a GEF binding to Rho GTPases, the simplest link would be a direct interaction of Vav1 with RhoH. This could lead to the recruitment of a constitutively active RhoH to the TCR complex, enabling further protein–protein interactions through RhoH. As a second possibility, Vav1 might activate RhoH by catalyzing the exchange of GDP to GTP. This would imply that, in thymocytes, RhoH activity is regulated by GEFs and GAPs and that RhoH is not constitutively active, as presently assumed.2 Like RhoH, the small GTPase Rap1 does not have detectable intrinsic GTPase activity because of the mutation of a catalytic glutamine.30 However, Rap1 can hydrolyze GTP with the help of Rap1GAP, which provides a catalytic asparagine.31 It remains to be tested whether such a GAP exists for RhoH. Interestingly, we did not observe any defect in hematopoiesis in heterozygous RhoH-mutant mice, though mRNA levels of RhoH were reduced by half.
Our data indicate that RhoH is upstream of Vav1 because TCR-dependent activation of Vav1, as determined by tyrosine phosphorylation, is reduced in RhoH-null mice, whereas total levels of Vav1 are unchanged. A conceivable scenario might be that though RhoH is weakly associated in the resting state with Vav1 or other members of the LAT signalosome, this interaction becomes strengthened on TCR stimulation, resulting in the stabilization of the entire LAT signalosome complex. Thus, RhoH can be involved in a positive feedback loop downstream of Vav1, which increases the tyrosine phosphorylation of LAT, PLCγ1, and Vav1 itself. However, thus far we have been unsuccessful in coimmunoprecipitation of recombinant RhoH with Vav1, ZAP70, or LAT from lysates of resting and stimulated thymocytes or Jurkat cells transfected with tagged RhoH, suggesting that such interactions, if existent, are weak or transient (data not shown).
Several functions have previously been assigned to RhoH. First, RhoH was described as a negative regulator of p38 MAPK in Jurkat and 293 cells.2 In thymocytes lacking RhoH, we did not detect increased activation of p38 MAPK, suggesting a cell type–specific function of RhoH in this respect.
Second, RhoH was reported to decrease LFA-1–mediated adhesion in Jurkat cells and human peripheral blood lymphocytes.6 We showed here that LFA-1–mediated adhesion of thymocytes and T cells to ICAM-1 is not affected by the absence of RhoH. Our results do not rule out a more subtle role for RhoH in cell adhesion and cell–cell contact in vivo.
Finally, RhoH inhibited SCF-induced Rac1 activation in HPCs.3 Indeed, basal Rac1 activity was increased in RhoH-null DP thymocytes, suggesting that RhoH is a negative regulator of Rac1 activity in the resting state. Increased Rac1 activity could be a compensatory change in response to the loss of RhoH. Other compensatory changes might not yet have been found, and future studies will address this search. It is unlikely that the increased basal activity of Rac1 in RhoH-null DP thymocytes is the reason for the impaired thymocyte development. Constitutive activation of Rac1 should rather result in “augmented” TCR signaling because constitutively active Rac1 can rescue the defective DN3 to DN4 transition in Vav1-null thymocytes and can increase the expression of TCRβ, CD5, and CD69 on DP and CD4SP cells, in contrast to the phenotype of RhoH-deficient cells.32,33 Conceivably, the different phenotypes are explained by the fact that the level of active Rac1 is lower in RhoH-deficient thymocytes than in thymocytes overexpressing the constitutively active mutant form (L61Rac1).32,33
In conclusion, our data suggest that RhoH is required for efficient β-selection and positive selection because it promotes the ZAP70-dependent phosphorylation of the LAT signalosome during pre–TCR and TCR signaling.
Authorship
Contribution: T.D. designed and performed the research, collected and analyzed the data, and wrote the paper. U.K. performed the research. G.B. collected the data. S.S contributed analytical tools. M.B. collected the data. J.E. collected the data. T.P. contributed vital new reagents. K.S.-K. contributed vital new reagents. M.S. contributed vital new reagents. M.L. performed the research. B.H. contributed vital new reagents. W.E.F.K. collected the data. P.T.S. contributed vital reagents. T.K. collected the data. M.S. collected the data. C.B. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cord Brakebusch, Department of Molecular Pathology, University of Copenhagen, Frederik V Vej 11, 2100 Copenhagen, Denmark; e-mail: cord@pai.ku.dk.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the German Research Council (DFG), the Friis Foundation, the Lundbeck Foundation, and the Max Planck Society.
We thank Dr Cinthia Farina for help with the calcium measurements, Bianka Ksienzyk for her support with cell sorting, Dr Michael Bösl for generation of chimeric mice, Dr Ernst Pöschl for Annexin V, Dr Jürgen Wehland for antibodies, Dr Juan Carlos Zuniga-Pfluecker and Dr Britta Engelhardt for cell lines, Dr Hans-Reimer Rodewald and Dr Klaus-Dieter Fischer for advice, Dr Werner Müller, Dr Kathryn Rodgers, and Tine Lefever for critically reading the manuscript, and Dr Reinhard Fässler for his comments and his continuous and generous support.

![Figure 1. Impaired thymocyte development in the absence of RhoH. (A) Thymocytes of 2-month-old mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell number of each population. **P < .01; ***:P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 11/11). (B) Thymocytes of 2-month-old mice were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−. Bar graph presents the absolute cell number of each population. *P < .05. Error bars show the standard deviation (n [control]/[RhoH−/−]: 4/4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420001.jpeg?Expires=1768573407&Signature=SWl2ysERHUe-pxzr1IOZIe-A1aGYfedUgNmq0GCTVED1HkqABZ-aoKljn2C1UR6-1GUeoMEJrCMVxNUVWQndFvTwmqNzm6kRFAxQbXB8Er6qdm9m50W0nGaHMezg7bGanTMvjN5hhoY0j8muN4r6qkUxUrqEaa1kD7zWEDuBZu4S4iHqRGBDTHfQvuU~Be9AacgwrRRVgY~gv2w05L-cL4JyzbXS61d4NQlD1EufLnI2ZEbq8k1tBbNNjmRGdaJIbx2sJ~zXQPp-AeUjiO8y13PqfHjcewse3R-5vmocWrNa65lnnRLrDP1nlh1iCK6VJjGcQvJfJKKzNR-Dmp0UVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Impaired proliferation and survival and decreased expression of maturation markers in RhoH-null thymocytes. (A-B) Thymocytes of 2-month-old mice were analyzed for proliferating BrdU-incorporating cells and apoptotic AnnexinV+ cells. Different thymocyte populations were distinguished (see Figure 1A-B). *P < .05; **P < .01; ***P < .001; BrdU, DN1-DN4, n [control]/[RhoH−/−]: 6/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/10; Annexin V, DN1 to DN4, n [control]/[RhoH−/−]: 10/10; DP, CD4SP, CD8SP, n [control]/[RhoH−/−]: 8/9. (C-E) Thymocytes of 2-month-old mice were analyzed for the expression of CD4, CD8, and CD5 (C; n [control]/[RhoH−/−]: 9/9) or TCRβ (D; n [control]/[RhoH−/−]: 4/4) or CD69 (E; n [control]/[RhoH−/−]: 6/6) by FACS. Percentages of cells marked in histograms are shown in graph. **P < .01; ***P <.001. Error bars show standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420002.jpeg?Expires=1768573407&Signature=GlW2zff2l8AiiYGcExM4cAdO4Vz3tqsOvR0z4X6xQKCkD5pyV41UfVY4BQ7Lp~ktlgKGMMmuUDvV6kInCYF0-meui66A9MdyOgEWWxKlF1bKLlE9UGV74vs0SPc25zmVn8QCBzOcB6Zvbgpf5ZgYlQ3Qp9i09IlPxjy8wmglp2u3~LIqqauVVuAeviudJEkHI5WizGAYhRdP8t21AcTiBJuIO1r3Mw9cf2JnDikIBkLDXdcZocmdnwo3HYAlXvacl7tKqjlySX0MPGjwS1cHARH4wcPbbmTw-O8HwEE-Osss86TwDfBO-cKVwE-DeUngc9fuM~b1AkOX8Ca~E81dOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Impaired differentiation of RhoH-null DN3 and DN4 cells in vitro. (A) Sorted DN3 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. Differentiation from DN3 to DN4 was tested by FACS analysis of lineage-negative cells for the expression of CD44 and CD25. RhoH-null cells showed a significantly higher percentage of DN3 and a lower percentage of DN4. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately 5-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01; ***P < .001. Error bars show the standard deviation (n [control]/[RhoH−/−]: 2/3). (B) Sorted DN4 cells were cultured for 4 days on OP9-DL1 cells in the presence of IL-7 and Flt3 ligand. FACS staining for CD4 and CD8 revealed significantly increased levels of DN and reduced levels of DP in the absence of RhoH. Furthermore, cellularity of the RhoH-null cultures was approximately15-fold decreased, indicating defective expansion in the absence of RhoH. **P < .01. Error bars show the standard deviation (n [control]/RhoH−/−]: 3/4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420003.jpeg?Expires=1768573407&Signature=Rtv1GmODYxx1qx4XE73vdsLE41VRdxyU1WmExhs4uNJscDmEn9Op5zAKtKGaWGPpf5e53VsSHzdbUfZwH1OKYtpH65yR2dw1EDIB9vqfyp7DjUKgFAl23lJCCwxAxNIxPRbmd7C0qFTUgg1i06Tj0Jf9DaKPYuIuGMnjBBNs-U0-UuGvUBHIzTeOIg9BfcZrv99q8Tl8VnzZsSzWl8WQBvgyC4QjmAnzMME3pjvKTqypgM4ReLZ1kkiIUR-7ywr5QrK6DsfDKl4cdC~1d4pYszL8-R-LG~J~JobSi6WNCZeb315fGsdPixba0oKpSlrRr6JUiCR8mVenCt7xix2XNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Defective positive selection in the absence of RhoH. (A) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were analyzed for the expression of CD4 and CD8 by FACS (upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel). *P < .05; **P < .01; ***P < .001. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (B) Thymocytes of 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR, either expressing or not expressing RhoH, were gated for lineage-negative (B220, CD4, CD8, NK1.1, Mac1, Gr-1, Ter119) cells and analyzed for the expression of CD25 and CD44. DN1, CD25−CD44+; DN2, CD25+CD44+; DN3, CD25+CD44−; DN4, CD25−CD44−; upper panel). Quantification of absolute numbers of thymocyte subpopulations (lower panel; *P < .05. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 3/4). (C) Decreased amount of Vα2+ DN thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (D) Increased amount of CD5low thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. **P < .01; ***P < .001; Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4). (E) Decreased amount of CD69high thymocytes in 4- to 7-week-old OT-II mice transgenic for an ovalbumin-specific TCR in the absence of RhoH. *P < .05; **P < .01. Error bars indicate the standard deviation (n [control]/RhoH−/−]: 4/4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420004.jpeg?Expires=1768573407&Signature=dgkQudO4~YbmK7akQxmKDdk1rTnSU3w-WW9rXpSoXloUuk227xkoWde66LSlGZ5tBwCHGlDaJcICJzNvY7uS~fFAg4BTo3ml8uVOAFfLgiMbDQ-KN62FFRkM60esE2EGFn7mXrZ1Wp9-k6YRQJXD1iSCyLrr6haETsI4BCu6stZBiBx41WjHWr2dGwfSAvu~HXn8z~ZZr3Aarplj2Eo-np8Bwuyx3Z51p1p0oXJ0zmiv8RDSo47RSE31ekaLu4lnYFoY98Bs-XOjyhEsql3NyreIcSggX~-AC--aGOLpghBwCVVVka9v6vGXgLH4WdLViT6pLqXsrVgrZEZhjxNXFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Defective TCR signaling and calcium influx in RhoH-null thymocytes. Thymocytes of 2-month-old mice were stimulated with biotinylated antibodies CD3 (C) or CD3 and CD4 (A-B, D) and streptavidin for 5 minutes at 37°C. Phosphorylation of ZAP70(Y319)/Syk(Y352) (A), lck(Y505) (B), Erk1/2(T202/Y204) (C), and p38 MAPK (T180/Y182) (D) was measured by FACS. Different thymocyte populations were distinguished (filled, nonstimulated; line, stimulated). Differences between the mean of stimulated and nonstimulated (specific mean) cells are shown. *P < .05; **P < .01. n [control]/RhoH−/−]: 5/5 (A-B, D). n [control]/RhoH−/−]: 4/5 (C). Thymocytes (E) or splenocytes (F) of 2-month-old mice were loaded with Fluo-4 and stained on ice for CD4, CD8, and CD3. After warming to 37°C, baseline Fluo-4 fluorescence was determined, and TCR signaling was induced by cross-linking CD3 with streptavidin. Presented is the percentage of cells above a threshold fluorescence (responding cells; left panel) and the mean fluorescence of all cells (right). Shown are representative results of 5 (E) or 2 (F) independent experiments. Error bars indicate the standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420006.jpeg?Expires=1768573407&Signature=NaKYPC~8JWX7uH-jktPlTimOaZrRWhW6QhiULEOLxKcO4i1apYJ7qcWyNhgCw~~sgRcdyz65y98tONnMeojGIWG9qekA27hSUIIZbVzdFsAkv3IWAPkIj2i6yMBa21hH9eX4FghzS-WUjTT1ghYGBZdJsx~0TE1YaguJprihiyvK-66Q7KNwNSkaNCNjdiW1rx7sVDPzkULPVPu5dQnFMjX0VJ1eAWTDaZZVVgAi-38qhnT7Ne2l2sDRQKciH5TpAFzVhiEB4D2GKuR80sUSBuqWB0xScDo5sQPfxA5487WiZ-je8H6wvKAo24X-DlEK-l-Gq-aPY2o5JU2mqj1T1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. RhoH does not affect thymocyte development through regulation of αLβ2 integrin-mediated adhesion. (A) Relative adhesion of thymocytes to the immobilized αLβ2 ligand ICAM-1 and to the α4β1 ligand VCAM-1. Integrins were activated by treatment with 2 mM Mn2+ or 100 ng/mL PMA as indicated. Adhesion of wild-type (RhoH +/+), heterozygous (RhoH+/−), and homozygous RhoH-null thymocytes (RhoH −/−) was indistinguishable at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 1/2/3. (B) Relative adhesion of T-cell blasts to the endothelial cell line bEnd5 and the ICAM-1–deficient endothelial cell line bEndI1.1 (ICAM1−/−). Adhesion was stimulated by treating the endothelial cells with TNF. Antibodies against α4-integrin and LFA-1 were used to determine the specific contribution of these adhesion receptors to the attachment. An unrelated antibody was added as a control for nonspecific effects. Wild-type (RhoH+/+), heterozygous (RhoH+/−), and homozygous (RhoH−/− ) RhoH-null T cells showed indistinguishable adhesion at all conditions tested. Error bars show the standard deviation (n [RhoH+/+]/[RhoH+/−]/[RhoH−/−]: 2/2/4. (C)Thymocytes of 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice were analyzed for the expression of CD4 and CD8 by FACS. Bar graph presents the absolute cell numbers of each population. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3. (D) Expression of CD5 and amount of CD5low thymocytes in 6- to 10-week-old β2-integrin-null, RhoH-null, and β2-integrin-RhoH double-knockout mice. Error bars indicate the standard deviation (n [β2−/−]/[RhoH−/−]/[β2−/−RhoH−/−]: 3/3/3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/6/10.1182_blood-2006-04-019034/4/m_zh80060709420007.jpeg?Expires=1768573407&Signature=vV5uc0y4c0rBaDyQ1nxNBpV1qx8PwDsemfQONYmV-LAvxgUa0CEG0s4gxOc35fxWk4qc0iqUMSUE7oRorOXNU7hoo4fJi20Q9W29mL2bC7~AGCcO8rNamke2lfy6cRUelLyJtGtMEM6XgsePZ~iIJ0CnH8sZzKYTYQhEYxcEoTGuJU1oSfhnc7jsAXUSFW2gObGi6-eRTT1OdD17XFVOiaNH8C~nynWcQswzdsqtNEzJ3qZDGc2xVtwQR5DSiWy~4u13XXsEhCzVtrWfWC6nasCb6rI9msnLG-gEtK5VByaPCrxW8sLv-HlWI99CKETGn4E7-vONQN5NR0-8gg3drw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal