Abstract
To assess the impact of minimally differentiated acute myeloid leukemia (AML-M0) morphology in children, we analyzed 2 sequential Children's Cancer Group AML clinical trials. We compared presenting characteristics and outcomes of 82 CCG-2891 and CCG-2961 patients with de novo, non–Down syndrome (DS) AML-M0 with those of 1620 patients with non-M0 AML, and of 10 CCG-2891 patients with DS-associated AML-M0 with those of 179 with DS-associated non-M0 AML. Morphology and cytogenetics were centrally reviewed. The non-DS AML-M0 children had a lower white blood cell (WBC) count (P = .001) than their non-M0 counterparts and a higher incidence of chromosome 5 deletions (P = .002), nonconstitutional trisomy 21 (P = .027), and hypodiploidy (P = .002). Outcome analyses considering all children with non-DS AML demonstrated no significant differences between M0 and non-M0 patients. Analyses restricted to intensive-timing CCG-2891 and CCG-2961 demonstrated comparable complete response (CR) rates (79% and 78%) between non-DS M0 and non-M0 patients. Overall survival (OS) from diagnosis (38% ± 14% versus 51% ± 3%; P = .160) was not significantly different between the 2 groups. OS from end of induction (45% ± 17% versus 63% ± 3%; P = .038), event-free survival (EFS; 23% ± 11% versus 41% ± 3%; P = .018), and disease-free survival (DFS; 31% ± 14% versus 52% ± 3%; P = .009) were inferior in the M0 group. There was no significant outcome difference between DS-associated AML-M0 and non-M0 children. This study suggests that intensively treated non–DS-associated AML-M0 children have an inferior outcome compared with children with non-M0 AML.
Introduction
Minimally differentiated acute myeloid leukemia (AML-M0) is a rare AML subtype in both children and adults.1–8 Since its recognition as a distinct entity in 1987,9 the French-American-British (FAB) Cooperative Group has proposed that the criteria for a diagnosis of AML-M0 consist of the presence of less than 3% myeloperoxidase (MPO)– and/or sudan black B (SBB)–positive blasts in the bone marrow (BM) by light microscopy, myeloid-associated antigen positivity (CD13 and/or CD33), and lack of B-/T-cell lineage–associated antigen expression, with the exception of TdT, CD7, and CD4.10 Despite the formulation of this generally accepted definition, the unequivocal recognition of this AML subtype continues to pose certain challenges, with many investigators shaping the FAB guidelines into varying institutional definitions.11
To date, the presenting characteristics and the outcome of patients with AML-M0 have primarily been described in small cohorts of adult patients, where the consistent feature has been a low remission induction rate and a short remission duration.1–3,5–8 Little is known regarding the clinical and biologic significance of the FAB AML-M0 subtype in children.5,7,11 Three identified studies reporting children with AML-M0 are limited by small sample size,5,7 the administration of heterogeneous therapies,11 and a lack of adequate follow-up.7,11 Huang et al5 reported 9 children with FAB AML-M0. Seven were treated using conventional chemotherapy, of whom 4 (57%) achieved a complete remission (CR), and 2 received no chemotherapy. The AML-M0 children did not demonstrate a significantly worse prognosis compared with their non-M0 counterparts. Kotylo et al7 reported 7 children with AML-M0, of which 6 (85.7%) achieved a CR with unspecified therapy. Follow-up in this study, which ranged from 6 to 14 months, was limited. Bene et al11 reported the presenting characteristics of 58 children with AML-M0 and the outcome for 36 (62%). This study, however, is difficult to evaluate as the patients received nonuniform therapies, lymphoid antigen positivity of leukemic blasts was not an exclusion criterion for study entry, and, importantly, the diagnosis of AML-M0 was not centrally reviewed, suggesting that any diagnostic criteria used were likely nonuniform.
We report the largest series of children with minimally differentiated FAB AML-M0. The aim of this report is to ascertain the incidence of this AML subtype in the pediatric population, and to describe the presenting characteristics and determine the treatment outcome for children with FAB AML-M0 enrolled on 2 consecutive Children's Cancer Group (CCG) clinical trials of intensive chemotherapy for AML.
Patients, materials, and methods
Study patients
Between 1989 and 2002, the CCG conducted two phase 3 clinical trials of intensive chemotherapy for AML and myelodysplastic syndrome (MDS), CCG-2891 and CCG-2961.12–14 Eligibility criteria for the 2 studies differed in that children with acute promyelocytic leukemia (APL), treatment-related AML (t-AML), and Down syndrome (DS)–associated MDS/AML were eligible for enrollment on CCG-2891 but not CCG-2961. Patients' data were collected at individual institutions and entered into the CCG database in Arcadia, CA. Local institutional review board approval and written informed consent were required for both studies.
CCG-2891 and CCG-2961 included 2171 eligible children. This report considers 1702 children with de novo, non-DS AML from CCG-2891 and -2961 and 189 children with DS-associated AML from CCG-2891. Patients from CCG-2891 who received both standard and intensive-timing therapy13 were excluded, as were all patients with APL (50 patients), t-AML (19 patients), MDS (149 patients), isolated granulocytic sarcoma (22 patients), and unknown FAB classification (27 patients).
In this report, the presenting clinical and biologic features of all children with FAB AML-M0 are described and compared with those of children with all other FAB subtypes (M1-M7). Outcome analyses were first performed considering all enrolled children with de novo AML and known FAB classification. As CCG-2891 demonstrated a superior outcome for children with non–DS-associated AML who received intensively timed (IT) therapy,12,13 we also performed outcome analyses excluding the 333 non–DS-associated AML patients treated with standard timing (ST) therapy, thereby providing a homogeneously treated patient population. Similarly, as CCG-2891 demonstrated that in DS-associated AML superior outcome was conferred by ST therapy,15,16 analyses in this cohort were performed excluding DS-associated AML patients who received IT therapy.
Treatment protocol
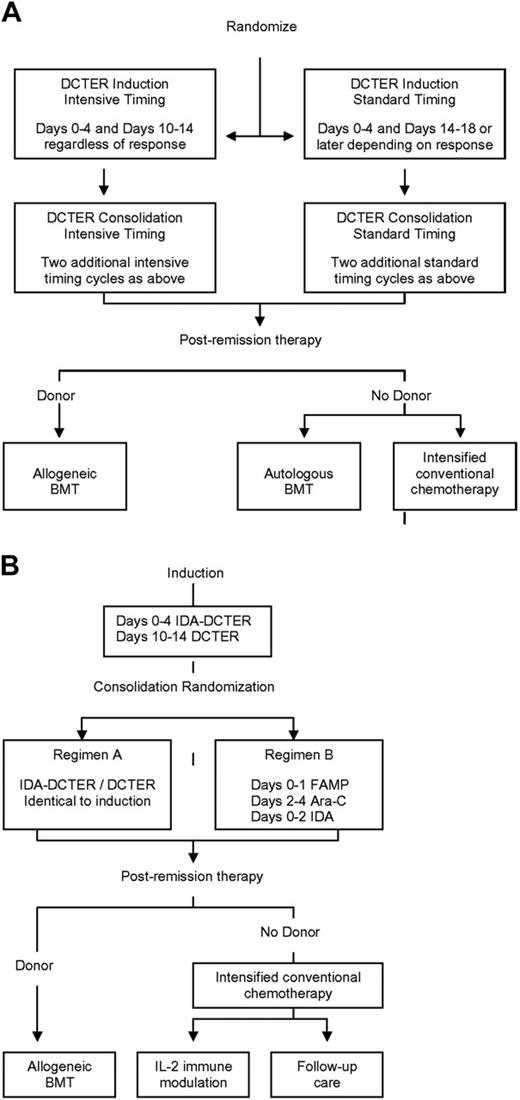
CCG-2891 (Figure 1A) accrued patients between October 1989 and April 1995 for non-DS patients and October 1999 for those with DS.12,13,15,16 Enrolled patients were randomized at diagnosis to receive 1 of 2 induction regimens containing 4-day, 5-drug cycles of therapy (dexamethasone, cytarabine, 6-thioguanine, etoposide, and daunorubicin [DCTER]). The randomization was between a standard-timing (second therapy cycle administered following a day-14 bone marrow [BM] examination; patients in remission received the second DCTER cycle upon blood count recovery; patients with persistent leukemia received it on day 14) or an intensive-timing (second therapy cycle administered on day 10 irrespective of BM status) approach. All patients received 4 induction cycles. Patients in CR with a compatible family donor were then nonrandomly assigned to allogeneic hematopoietic stem cell transplantation (HSCT). All others were randomized to either autologous HSCT or nonmyeloablative chemotherapy. The standard-timing arm was closed for non-DS patients in May 1993, following recommendation by the Data Monitoring Committee, with all non-DS patients subsequently enrolled receiving intensively timed therapy.12,13 For patients with DS, intensive timing and allogeneic HSCT were closed in July 1992, following an interim analysis demonstrating excessive toxicity.15,16 All subsequently enrolled patients with DS nonrandomly received a standard timing induction followed by postremission chemotherapy.
Design of Children's Cancer Group AML Treatment Studies 2891 and 2961. (A) CCG-2891. (B) CCG-2961. DCTER refers to dexamethasone, cytarabine, 6-thioguanine, etoposide, and rubidomycin (daunorubicin). Idarubicin (IDA) replaced daunorubicin in IDA-DCTER. FAMP, fludarabine; Ara-C, cytarabine. Intrathecal cytarabine was administered to all patients.
Design of Children's Cancer Group AML Treatment Studies 2891 and 2961. (A) CCG-2891. (B) CCG-2961. DCTER refers to dexamethasone, cytarabine, 6-thioguanine, etoposide, and rubidomycin (daunorubicin). Idarubicin (IDA) replaced daunorubicin in IDA-DCTER. FAMP, fludarabine; Ara-C, cytarabine. Intrathecal cytarabine was administered to all patients.
CCG-2961 (Figure 1B) accrued patients between August 1996 and December 2002.14 CCG-2961 differed from CCG-2891 in that all patients received intensively timed induction and consolidation, idarubicin (IDA) replaced daunorubicin in the first induction cycle, and a randomized comparison of 2 consolidation and 2 postintensification regimens was evaluated. Briefly, all patients in CR following induction (IDA-DCTER/DCTER) were randomized to 1 of 2 consolidation regimens (A or B). Regimen A was identical to induction therapy. Regimen B consisted of a single 5-day cycle of fludarabine, cytarabine, and IDA. Again, those patients in CR following consolidation and with a compatible family donor were nonrandomly assigned to allogeneic HSCT. All others underwent intensification therapy followed by a postintensification randomization between either interleukin-2 immune therapy17 or no further therapy.
Definition of AML-M0
FAB classification in all patients was initially determined by institutional investigators. Representative pretreatment BM smears were then reviewed by a central hematopathologist (D.R.B.) to confirm study eligibility and assign FAB classification.10,12,18 The criteria for a diagnosis of AML-M0 in both studies were a BM containing more than 30% undifferentiated blasts, with less than 3% of these blasts staining positive for nonspecific esterase (NSE), MPO, and/or SBB as determined by light microscopy. Additional criteria included myeloid-associated antigen (CD13 and/or CD33) positivity, absence of B-/T-cell lineage–associated antigen expression (with the exception of TdT, CD7, and CD4), and lack of megakaryocytic-related antigen expression.
FLT3 mutation analysis
Thirty-six cryopreserved diagnostic BM specimens from FAB AML-M0 patients treated using CCG-2891-IT and CCG-2961 were available for analysis. FLT3 mutation detection was performed using DNA extracted from diagnostic BM according to methods previously described.19
Statistical analysis
Data obtained from CCG-2891 through January 14, 2004, and from CCG-2961 through April 5, 2005, were used to compare patients with and without FAB AML-M0. Patients lost to follow-up were censored at the time of last known contact or at a cutoff date of July 14, 2003, for those on CCG-2891 and October 5, 2004, for those on CCG-2961. CR was defined as an absolute neutrophil count greater than or equal to 1 × 109/L and platelet count greater than or equal to 100 × 109/L, with less than 5% blasts in BM, demonstrating trilineage recovery of hematopoiesis. The significance of observed differences in proportions was tested using the Chi-squared test and Fisher exact test when data were sparse. For continuous data, the Mann-Whitney test was used to compare the medians of distributions.20
Event-free survival (EFS) was defined as the time from study enrollment to failure to achieve CR at the end of 2 courses of induction therapy (EOI), relapse, or death. Disease-free survival (DFS) was defined as the time from EOI for patients in CR to relapse or death. Overall survival (OS) was defined as the time from study enrollment to death from any cause. Estimates of OS and EFS from study entry were calculated using the Kaplan-Meier method.21 The Kaplan-Meier method was also used to calculate estimates of OS and DFS from the end of 2 courses of induction therapy for patients achieving remission. Confidence intervals were calculated using Greenwood's22 estimate of the standard error. Differences in OS, EFS, and DFS were tested for significance using the log-rank statistic.23 The cumulative incidence of relapse (CIR), measured as the time from EOI to relapse or death from progressive disease, was calculated using the competing risk method,24 where deaths from nonprogressive disease were considered competing events. The cumulative incidence of toxicity-related mortality (CITRM), measured as the time from EOI to death from nonprogressive disease, was calculated by considering relapses and deaths from progressive disease as competing events. Differences between CIR and CITRM estimates were tested for significance using Gray's test.25 The Cox proportional hazards model was used to estimate hazard ratios (HRs) for multivariate analyses.26 Cytogenetics were not included in multivariate analyses because of the sparseness of cytogenetic data.
Results
Incidence of AML-M0 in patients with de novo, non–DS-associated AML-M0
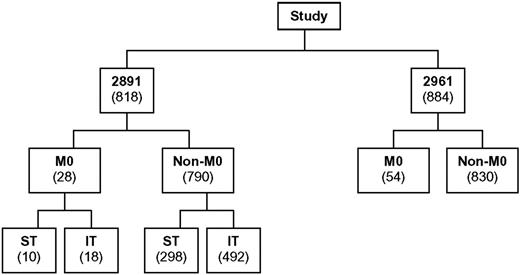
The distribution of patients among CCG-2891 and CCG-2961 is shown in Figure 2. Among the 1702 evaluable de novo, non-DS AML patients, 82 (4.8%) had FAB AML-M0. Interestingly, there was some disagreement between institutional and central pathologists in the diagnosis of AML-M0 for de novo patients, with 10 (12%) institutional AML-M0 diagnoses excluded following central review (9 reclassified as FAB M1-M7 and 1 reclassified as acute undifferentiated leukemia). There were also 7 institutional FAB M1-M7 diagnoses which, following central review, were reclassified as FAB M0 (4 initially classified as FAB M1, 1 as FAB M4, 1 as FAB M6, and 1 as FAB M7). This highlights the value of a centralized review with precise and uniform recognition of different AML subsets and the homogeneous application of FAB criteria.
Distribution of de novo, non-DS AML patients with known FAB classification enrolled on studies CCG-2891 and CCG-2961.
Distribution of de novo, non-DS AML patients with known FAB classification enrolled on studies CCG-2891 and CCG-2961.
Presenting clinical and hematologic characteristics of patients with de novo, non–DS-associated AML-M0
The presenting features of the AML-M0 and non-M0 patients are listed in Tables 1 and 2. The presenting median white blood cell (WBC) count was lower in the AML-M0 group compared with the non-M0 group (11.3 × 109/L versus 21.7 × 109/L; P = .001). The AML-M0 patients also had a lower incidence of granulocytic sarcoma at the time of diagnosis (P = .016) and a higher diagnostic BM blast percentage (P = .014). The majority of AML-M0 patients presented with mild to moderate anemia, whereas the degree of thrombocytopenia was variable. Consistent with AML in general, the incidence of central nervous system (CNS) involvement at diagnosis in the FAB AML-M0 patients was low (3/82 [4%]). No other pretreatment characteristic differed significantly between the de novo, non-DS AML-M0 and non-M0 patients.
Pretreatment clinical and hematologic features of M0 and non-M0 AML patients enrolled on CCG-2891 and CCG-2961
| . | CCG-2891 (ST and IT) and CCG-2961 de novo, non-DS patients . | CCG-2891 Down syndrome patients (ST and IT) . | ||||
|---|---|---|---|---|---|---|
| AML-M0 . | Non-M0 . | P . | AML-M0 . | Non-M0 . | P . | |
| Total no. patients | 82 | 1620 | — | 10 | 179 | — |
| Age, no. (%) | ||||||
| 0-2 y | 28 (34) | 448 (28) | .249 | 10 (100) | 153 (85) | .362 |
| 3-10 y | 19 (23) | 523 (32) | .108 | 0 (0) | 25 (14) | .364 |
| 11-21 y | 35 (43) | 649 (40) | .721 | 0 (0) | 1 (1) | >.999 |
| Median age, y | 8.20 | 8.59 | .601 | 1.50 | 1.88 | .111 |
| Sex, no. (%) | .992 | .529 | ||||
| Male | 42 (51) | 841 (52) | 6 (60) | 86 (48) | ||
| Female | 40 (49) | 779 (48) | 4 (40) | 93 (52) | ||
| Race, no. (%) | .823 | .095 | ||||
| White | 56 (68) | 1066 (67) | 3 (30) | 108 (60) | ||
| Nonwhite | 26 (32) | 538 (33) | 7 (70) | 71 (40) | ||
| Granulocytic sarcoma, no. (%) | 1 (1) | 159 (10) | .016 | 1 (10) | 8 (4) | .395 |
| CNS+ at diagnosis, no. (%) | 3 (4) | 126 (8) | .255 | 0 (0) | 5 (3) | .999 |
| Splenomegaly, no. (%) | 34 (42) | 583 (36) | .383 | 6 (60) | 87 (49) | .532 |
| Hepatomegaly, no. (%) | 33 (40) | 581 (36) | .499 | 7 (70) | 90 (50) | .332 |
| Hepatosplenomegaly, no. (%) | 40 (49) | 737 (46) | .653 | 7 (70) | 103 (58) | .525 |
| Lymphadenopathy, no. (%) | 32 (39) | 712 (44) | .440 | 3 (30) | 47 (26) | .703 |
| WBC count × 109/L, median (range) | 11.3 (1.0-230.0) | 21.7 (0-860.0) | .001 | 10.0 (2.6-108.4) | 7.2 (0.4-121.8) | .478 |
| Platelets, median (range) | 56.5 (6-536) | 51 (1-800) | .378 | 20 (7-48) | 29 (3-541) | .141 |
| Hemoglobin, median (range) | 8.1 (1.8-27.1) | 8.3 (1.8-38.6) | .233 | 7.6 (2.6-9.8) | 8.4 (1.9-17.1) | .216 |
| % BM blasts, median (range) | 83 (10-100) | 70 (0-100) | .014 | 63 (31-99) | 40 (0-96) | .017 |
| . | CCG-2891 (ST and IT) and CCG-2961 de novo, non-DS patients . | CCG-2891 Down syndrome patients (ST and IT) . | ||||
|---|---|---|---|---|---|---|
| AML-M0 . | Non-M0 . | P . | AML-M0 . | Non-M0 . | P . | |
| Total no. patients | 82 | 1620 | — | 10 | 179 | — |
| Age, no. (%) | ||||||
| 0-2 y | 28 (34) | 448 (28) | .249 | 10 (100) | 153 (85) | .362 |
| 3-10 y | 19 (23) | 523 (32) | .108 | 0 (0) | 25 (14) | .364 |
| 11-21 y | 35 (43) | 649 (40) | .721 | 0 (0) | 1 (1) | >.999 |
| Median age, y | 8.20 | 8.59 | .601 | 1.50 | 1.88 | .111 |
| Sex, no. (%) | .992 | .529 | ||||
| Male | 42 (51) | 841 (52) | 6 (60) | 86 (48) | ||
| Female | 40 (49) | 779 (48) | 4 (40) | 93 (52) | ||
| Race, no. (%) | .823 | .095 | ||||
| White | 56 (68) | 1066 (67) | 3 (30) | 108 (60) | ||
| Nonwhite | 26 (32) | 538 (33) | 7 (70) | 71 (40) | ||
| Granulocytic sarcoma, no. (%) | 1 (1) | 159 (10) | .016 | 1 (10) | 8 (4) | .395 |
| CNS+ at diagnosis, no. (%) | 3 (4) | 126 (8) | .255 | 0 (0) | 5 (3) | .999 |
| Splenomegaly, no. (%) | 34 (42) | 583 (36) | .383 | 6 (60) | 87 (49) | .532 |
| Hepatomegaly, no. (%) | 33 (40) | 581 (36) | .499 | 7 (70) | 90 (50) | .332 |
| Hepatosplenomegaly, no. (%) | 40 (49) | 737 (46) | .653 | 7 (70) | 103 (58) | .525 |
| Lymphadenopathy, no. (%) | 32 (39) | 712 (44) | .440 | 3 (30) | 47 (26) | .703 |
| WBC count × 109/L, median (range) | 11.3 (1.0-230.0) | 21.7 (0-860.0) | .001 | 10.0 (2.6-108.4) | 7.2 (0.4-121.8) | .478 |
| Platelets, median (range) | 56.5 (6-536) | 51 (1-800) | .378 | 20 (7-48) | 29 (3-541) | .141 |
| Hemoglobin, median (range) | 8.1 (1.8-27.1) | 8.3 (1.8-38.6) | .233 | 7.6 (2.6-9.8) | 8.4 (1.9-17.1) | .216 |
| % BM blasts, median (range) | 83 (10-100) | 70 (0-100) | .014 | 63 (31-99) | 40 (0-96) | .017 |
— indicates not applicable.
Cytogenetic features of M0 and non-M0 AML patients enrolled on CCG-2891 and CCG-2961
| . | CCG-2891 (ST and IT) and CCG-2961 de novo, non-DS patients . | CCG-2891 Down syndrome patients (ST and IT) . | ||||
|---|---|---|---|---|---|---|
| AML-M0, no. (%) . | Non-M0, no. (%) . | P . | AML-M0, no. (%) . | Non-M0, no. (%) . | P . | |
| Total patients | 39 (100) | 944 (100) | — | 6 (100) | 94 (100) | — |
| Normal/constitutional | 6 (15) | 226 (24) | .298 | 1 (17) | 24 (26) | >.999 |
| t(8;21) | 0 (0) | 144 (15) | .004 | 0 (0) | 1 (1) | >.999 |
| Abnormal 16 | 0 (0) | 83 (9) | .069 | 0 (0) | 0 (0) | >.999 |
| Abnormal 11* | 6 (15) | 197 (21) | .545 | 0 (0) | 3 (3) | >.999 |
| t(6;9) | 0 (0) | 15 (2) | >.999 | 0 (0) | 0 (0) | >.999 |
| −7/7q− | 2 (5) | 40 (4) | .681 | 0 (0) | 8 (9) | >.999 |
| −5/5q− | 4 (10) | 10 (1) | .002 | 1 (17) | 5 (5) | .317 |
| +8 | 5 (13) | 62 (7) | .175 | 1 (17) | 14 (15) | >.999 |
| +21 (not constitutional) | 3 (8) | 14 (1) | .027 | 1 (17) | 9 (10) | .478 |
| Pseudodiploid | 6 (15) | 118 (13) | .620 | 0 (0) | 20 (21) | .597 |
| Hyperdiploid | 3 (8) | 24 (3) | .085 | 2 (33) | 8 (9) | .109 |
| Hypodiploid | 4 (10) | 11 (1) | .002 | 0 (0) | 2 (2) | >.999 |
| . | CCG-2891 (ST and IT) and CCG-2961 de novo, non-DS patients . | CCG-2891 Down syndrome patients (ST and IT) . | ||||
|---|---|---|---|---|---|---|
| AML-M0, no. (%) . | Non-M0, no. (%) . | P . | AML-M0, no. (%) . | Non-M0, no. (%) . | P . | |
| Total patients | 39 (100) | 944 (100) | — | 6 (100) | 94 (100) | — |
| Normal/constitutional | 6 (15) | 226 (24) | .298 | 1 (17) | 24 (26) | >.999 |
| t(8;21) | 0 (0) | 144 (15) | .004 | 0 (0) | 1 (1) | >.999 |
| Abnormal 16 | 0 (0) | 83 (9) | .069 | 0 (0) | 0 (0) | >.999 |
| Abnormal 11* | 6 (15) | 197 (21) | .545 | 0 (0) | 3 (3) | >.999 |
| t(6;9) | 0 (0) | 15 (2) | >.999 | 0 (0) | 0 (0) | >.999 |
| −7/7q− | 2 (5) | 40 (4) | .681 | 0 (0) | 8 (9) | >.999 |
| −5/5q− | 4 (10) | 10 (1) | .002 | 1 (17) | 5 (5) | .317 |
| +8 | 5 (13) | 62 (7) | .175 | 1 (17) | 14 (15) | >.999 |
| +21 (not constitutional) | 3 (8) | 14 (1) | .027 | 1 (17) | 9 (10) | .478 |
| Pseudodiploid | 6 (15) | 118 (13) | .620 | 0 (0) | 20 (21) | .597 |
| Hyperdiploid | 3 (8) | 24 (3) | .085 | 2 (33) | 8 (9) | .109 |
| Hypodiploid | 4 (10) | 11 (1) | .002 | 0 (0) | 2 (2) | >.999 |
A central review of institutional karyotype preparations was available in only 57% of enrolled patients.
— indicates not applicable.
These cases include those with and without MLL rearrangements.
Cytogenetic analyses of patients with de novo, non–DS-associated AML-M0
The available centrally reviewed cytogenetic results are presented in Table 2. There was a higher incidence of whole or partial chromosome 5 deletions (P = .002), nonconstitutional trisomy 21 (P = .027), and hypodiploidy (P = .002), and a lower incidence of core binding factor (CBF) leukemias, including t(8;21) (P = .004), in the FAB AML-M0 subgroup. The incidence of complex karyotypes (defined as 5 or more abnormalities) was similar in M0 and non-M0 patients.
In addition, 6 AML-M0 patients had 11q23 abnormalities. Three of the 6 had ins(10;11)(p11.2-p13;q13-q22q23). All 3 had varying breakpoints. Of the non-M0 patients with an abnormal chromosome 11, only 4 (2%) had an ins(10;11). The breakpoints within these 4 non-M0 patients were more consistent.
Treatment outcome of patients with de novo, non–DS-associated AML-M0
Analyses considering all children enrolled on CCG-2891 (ST and IT) and CCG-2961 demonstrated no significant difference in most outcome measures between the 82 AML-M0 and 1670 non-M0 patients (Table 3). Only the cumulative incidence of relapse differed significantly, being higher in the AML-M0 patients (61% ± 14% versus 43% ± 3%; P = .025).
Treatment outcome of all de novo, non-DS AML-M0 patients enrolled on CCG-2891 and CCG-2961
| . | De novo CCG-2891 (ST and IT) and CCG-2961 . | ||
|---|---|---|---|
| AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | |
| Remission induction rate after 2 courses (4 cycles) | 76 (10) | 77 (2) | .945 |
| Remission induction failures | 17 (8) | 14 (2) | .442 |
| Induction deaths | 7 (6) | 10 (2) | .544 |
| OS from Dx at 8 y | 40 (13) | 48 (3) | .373 |
| EFS from Dx at 8 y | 26 (10) | 37 (3) | .088 |
| OS from EOI at 8 y | 49 (15) | 59 (3) | .241 |
| DFS from EOI at 8 y | 36 (13) | 48 (3) | .107 |
| CIR at 8 y | 61 (14) | 43 (3) | .025 |
| CITRM from EOI at 8 y | 4 (5) | 8 (2) | .264 |
| . | De novo CCG-2891 (ST and IT) and CCG-2961 . | ||
|---|---|---|---|
| AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | |
| Remission induction rate after 2 courses (4 cycles) | 76 (10) | 77 (2) | .945 |
| Remission induction failures | 17 (8) | 14 (2) | .442 |
| Induction deaths | 7 (6) | 10 (2) | .544 |
| OS from Dx at 8 y | 40 (13) | 48 (3) | .373 |
| EFS from Dx at 8 y | 26 (10) | 37 (3) | .088 |
| OS from EOI at 8 y | 49 (15) | 59 (3) | .241 |
| DFS from EOI at 8 y | 36 (13) | 48 (3) | .107 |
| CIR at 8 y | 61 (14) | 43 (3) | .025 |
| CITRM from EOI at 8 y | 4 (5) | 8 (2) | .264 |
For AML-M0, n = 82; for non-M0, n = 1620.
SE indicates Greenwood standard error; EOI, end of induction and is considered to be at the end of 2 courses of induction therapy; CIR, cumulative incidence of relapse; CITRM, cumulative incidence of toxicity-related mortality; and Dx, diagnosis.
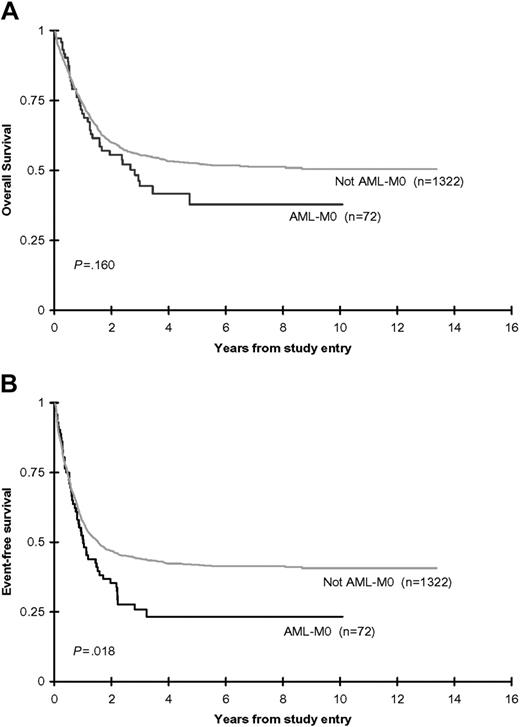
Analyses restricted to the similarly treated CCG-2891-IT and CCG-2961 de novo, non-DS cohort, demonstrated that 79% of AML-M0 and 78% of non-M0 patients achieved remission following 2 courses of induction therapy. The remission induction failure rate was not significantly different between the M0 and non-M0 patients. The induction death rate in the non-M0 patients, although not significantly different, was almost twice that of the M0 patients. Although the 8-year OS from time of study entry (38% ± 14% versus 51% ± 3%; P = .160) did not differ significantly between the AML-M0 and non-M0 patients, the EFS (23% ± 11% versus 41% ± 3%; P = .018), DFS (31% ± 14% versus 52% ± 3%; P = .009), and OS from attainment of remission (45% ± 17% versus 63% ± 3%; P = .038) were all inferior in the AML-M0 children (Table 4; Figure 3). Multivariate analyses adjusted for age (0-2 years, 3-10 years, 11-21 years), WBC count (< 50 × 109/L, ≥ 50 × 109/L), sex, ethnicity (white, nonwhite), and study confirmed the reduced EFS from study entry (HR: 1.48; 95% confidence interval [CI]: 1.12-1.96; P = .006), OS from EOI (HR: 1.64; 95% CI: 1.08-2.49; P = .020), and DFS from EOI (HR: 1.70; 95% CI: 1.19-2.43; P = .003). OS from study entry remained not significantly different on multivariate analysis (HR: 1.31; 95% CI: 0.95-1.82; P = .102).
Treatment outcome of de novo, non-DS AML-M0 patients treated using CCG-2891-IT and CCG-2961
| . | De novo CCG-2891 (IT) and CCG-2961 . | De novo CCG-2891 (IT) . | De novo CCG-2961* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | |
| Remission induction rate after 2 courses (4 cycles) | 79 (10) | 78 (2) | .919 | 72 (21) | 81 (4) | .367 | 81 (0) | 76 (3) | .619 |
| Remission induction failures | 15 (9) | 11 (2) | .373 | 22 (19) | 10 (3) | .124 | 13 (9) | 12 (2) | .955 |
| Induction deaths | 6 (6) | 11 (2) | .325 | 6 (11) | 9 (3) | .973 | 7 (7) | 13 (2) | .346 |
| OS from Dx at 8 y | 38 (14) | 51 (3) | .160 | 42 (24) | 49 (5) | .770 | 43 (15) | 58 (4) | .113 |
| EFS from Dx at 8 y | 23 (11) | 41 (3) | .018 | 33 (22) | 40 (4) | .566 | 23 (12) | 45 (4) | .012 |
| OS from EOI at 8 y | 45 (17) | 63 (3) | .038 | 50 (29) | 60 (5) | .765 | 46 (18) | 70 (4) | .011 |
| DFS from EOI at 8 y | 31 (14) | 52 (3) | .009 | 46 (28) | 51 (5) | .919 | 24 (17) | 56 (4) | .001 |
| CIR at 8 y | 65 (14) | 40 (3) | .002 | 54 (28) | 42 (5) | .506 | 71 (17) | 37 (4) | .001 |
| CITRM from EOI at 8 y | 4 (5) | 7 (2) | .441 | 0 (0) | 7 (3) | .311 | 5 (7) | 7 (2) | .775 |
| . | De novo CCG-2891 (IT) and CCG-2961 . | De novo CCG-2891 (IT) . | De novo CCG-2961* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | AML-M0, % (2SE%) . | Non-M0, % (2SE%) . | P . | |
| Remission induction rate after 2 courses (4 cycles) | 79 (10) | 78 (2) | .919 | 72 (21) | 81 (4) | .367 | 81 (0) | 76 (3) | .619 |
| Remission induction failures | 15 (9) | 11 (2) | .373 | 22 (19) | 10 (3) | .124 | 13 (9) | 12 (2) | .955 |
| Induction deaths | 6 (6) | 11 (2) | .325 | 6 (11) | 9 (3) | .973 | 7 (7) | 13 (2) | .346 |
| OS from Dx at 8 y | 38 (14) | 51 (3) | .160 | 42 (24) | 49 (5) | .770 | 43 (15) | 58 (4) | .113 |
| EFS from Dx at 8 y | 23 (11) | 41 (3) | .018 | 33 (22) | 40 (4) | .566 | 23 (12) | 45 (4) | .012 |
| OS from EOI at 8 y | 45 (17) | 63 (3) | .038 | 50 (29) | 60 (5) | .765 | 46 (18) | 70 (4) | .011 |
| DFS from EOI at 8 y | 31 (14) | 52 (3) | .009 | 46 (28) | 51 (5) | .919 | 24 (17) | 56 (4) | .001 |
| CIR at 8 y | 65 (14) | 40 (3) | .002 | 54 (28) | 42 (5) | .506 | 71 (17) | 37 (4) | .001 |
| CITRM from EOI at 8 y | 4 (5) | 7 (2) | .441 | 0 (0) | 7 (3) | .311 | 5 (7) | 7 (2) | .775 |
For the de novo CCG-2891 and CCG 2961, n = 27 and 1322, respectively, for AML-M0 and non-M0; for de novo CCG-2891, n = 18 and 492, respectively, for AML-M0 and non-M0; and for de novo CCG-2961, n = 54 and 830, respectively, for AML-M0 and non-M0.
SE indicates Greenwood standard error; EOI, end of induction and is considered to be at the end of 2 courses of induction therapy; CIR, cumulative incidence of relapse; CITRM, cumulative incidence of toxicity-related mortality.
Survival figures for CCG-2961 only are reported at 3 years.
Combined CCG-2891 (IT-regimen only) and CCG-2961 OS and EFS rates. OS (A) and EFS (B) from study entry for de novo, non-DS children with and without AML-M0.
Combined CCG-2891 (IT-regimen only) and CCG-2961 OS and EFS rates. OS (A) and EFS (B) from study entry for de novo, non-DS children with and without AML-M0.
To determine the impact of therapy administered, outcomes were also analyzed individually for CCG-2891 and CCG-2961 (Table 4). Within the CCG-2891-IT de novo, non-DS AML group, the remission induction rates were 72% and 81% (P = .367) in the AML-M0 and non-M0 patients, respectively. The 8-year OS from diagnosis (42% ± 24% versus 49% ± 5%; P = .770) and from EOI (50% ± 29% versus 60% ± 5%; P = .765), and the EFS (33% ± 22% versus 40% ± 4%; P = .566) and DFS (46% ± 28% versus 51% ± 5%; P = .919) were also not significantly different between the AML-M0 and non-M0 children. Within CCG-2961–treated patients the remission induction rate also did not differ between the FAB AML-M0 and non-M0 patients. The OS from diagnosis was lower, although not significantly, in the AML-M0 patients (43% ± 15% versus 58% ± 4%; P = .113). All other postremission outcomes were significantly inferior in the AML-M0 group (3-year EFS 23% ± 12% versus 45% ± 4%; P = .012; DFS 24% ± 17% versus 56% ± 4%; P = .001; and OS from attainment of remission 46% ± 18% versus 70% ± 4%; P = .011). Multivariate analyses adjusted for age (0-2 years, 3-10 years, 11-21 years), WBC count (< 50 × 109/L, ≥ 50 × 109/L), sex, and ethnicity (white, nonwhite) confirmed the reduced EFS from study entry (HR: 1.58; 95% CI: 1.14-2.17; P = .006), OS from EOI (HR: 1.94; 95% CI: 1.19-3.18; P = .008), and DFS from EOI (HR: 2.03; 95% CI: 1.35-3.07; P = .001). OS from study entry was not significantly different (HR: 1.40; 95% CI: 0.96-2.05; P = .084).
A comparison of de novo, non-DS AML-M0 patients treated using CCG-2891-IT therapy and CCG-2961 indicated a comparable remission induction rate (72% versus 81%; P = .507) and 3-year OS from time of study entry between the 2 AML-M0 groups (42% ± 24% versus 43% ± 15%; P = .690). However, CCG-2961 AML-M0 patients had an inferior, though not significant, 3-year DFS (24% ± 17% versus 46% ± 28%; P = .215) compared with their CCG-2891-IT counterparts. The CIR (71% ± 17% versus 54% ± 28% at 8 years; P = .393) and CITRM (5% ± 7% versus 0% ± 0% at 8 years from EOI; P = .400) also showed a trend to be inferior in the CCG-2961 patients.
Analyses according to assigned postremission therapy (Figure 1) revealed that a higher percentage of CCG-2961 AML-M0 patients received postinduction nonmyeloablative chemotherapy (68% versus 39%; P = .098). This was a consequence of CCG-2891 randomizing patients without a compatible family donor to either autologous HSCT or nonmyeloablative chemotherapy, and CCG-2961 nonrandomly assigning the same patients to nonmyeloablative chemotherapy (Figure 1). The percentage of AML-M0 patients who underwent allogeneic HSCT was similar for both CCG-2891-IT and CCG-2961 (29% versus 39%; P = .730). There was no significant difference in outcome between any of the postremission regimens for either CCG-2891 or CCG-2961 (data not shown). The number of de novo, non-DS AML-M0 patients, in both studies, within each postremission therapeutic group was small and may have precluded the detection of any significant variation.
The large number of de novo, non-DS AML-M0 patients enrolled on CCG-2891 and CCG-2961 enabled an examination of various factors predicting outcome in this subgroup of patients with AML. Multivariate analyses, however, demonstrated no significant impact of age and WBC count at diagnosis, sex, race, treatment, or hepatosplenomegaly on remission induction rate, OS, and EFS from study entry.
FLT3 analyses of patients with de novo, non–DS-associated AML-M0
Presence of FLT3 internal tandem duplication (FLT3/ITD) has been shown to be a molecular marker for relapse in pediatric AML.19,27 To determine the impact of FLT3/ITD in FAB AML-M0, we evaluated its presence in the AML-M0 cohort. Thirty-six of the 82 AML-M0 patients had diagnostic BM specimens available for FLT3 mutation analysis. Six of the 36 AML-M0 patient specimens tested (17%) were positive for FLT3/ITD, a similar prevalence to that reported for the entire AML population.19,27 The clinical characteristics of the FLT3/ITD-positive AML-M0 cohort were representative of the entire AML-M0 population. There was no significant impact detected of FLT3/ITD positivity on outcome within this cohort of 36 AML-M0 patients tested for FLT3 mutations (EFS from study entry for the FLT3/ITD-positive and -negative patients was 17% ± 30% and 25% ± 16% at 4 years from study entry [P = .995], and DFS from EOI was 20% ± 36% and 42% ± 26%, respectively [P = .510]).
Incidence, presenting characteristics, and treatment outcome of patients with DS-associated AML-M0
There were 10 children (5.3%) enrolled on CCG-2891 who had AML-M0 in association with DS. When these DS-associated AML-M0 children were compared with 179 DS, non-M0 AML counterparts also enrolled on CCG-2891, there was no significant difference detected in the majority of presenting clinicopathologic characteristics (Tables 1 and 2). Only the diagnostic BM blast percentage differed, being higher in the DS AML-M0 children (63% versus 40%; P = .017).
There was no significant difference in any outcome measure between the ST-treated DS-associated AML-M0 and non-M0 patients. In 90% of AML-M0 and 92% (P > .999) of non-M0 DS patients, remission was achieved following 2 courses of induction therapy. Within the AML-M0 and non-M0 patients, the 8-year OS from time of study entry was, respectively, 79% ± 27% versus 77% ± 7%, P = .984; the EFS from study entry was 80% ± 25% versus 75% ± 7%, P = .819; the OS from attainment of remission was 88% ± 23% versus 84% ± 6%, P = .791; and the DFS from attainment of remission was 89% ± 21% versus 82% ± 6%, P = .683. In keeping with results found for all DS children with AML, these results indicate that DS-associated AML-M0 patients appear to have an improved outcome compared with their de novo, non-DS AML-M0 counterparts.
Discussion
This study examines the incidence, pretreatment characteristics, and outcome of the FAB AML-M0 subtype in prospectively enrolled pediatric patients treated on 2 consecutive but similarly intensive treatment protocols. In CCG-2891 and CCG-2961, 4.8% of children with de novo, non-DS AML were documented to have FAB AML-M0, an incidence similar to that previously reported.1–8 The incidence of AML-M0 in children with DS was 5.3%, also comparable to that reported in both adult and pediatric studies of non-DS AML-M0 patients.1–8
An evaluation of the presenting characteristics of the AML-M0 patients demonstrated that children with AML-M0 display few clinical features, physical examination findings, or routine laboratory data that distinguish them from their non-M0 counterparts (Table 1). The finding that presenting WBC count was lower in the AML-M0 patients is interesting in view of previous reports suggesting that the M0 AML subtype is associated with an elevated presenting WBC count, a factor which may contribute to its generally unfavorable outcome.28 Granulocytic sarcomas, reported to occur in up to 10% of children with AML,29 have infrequently been documented in AML-M0 patients,29,30 which is in keeping with our findings.
AML-M0 stands out in previous studies of children and adults for its high incidence of karyotypic abnormalities including complex karyotypes, -5/5q-, -7/7q-, and trisomy 8.2,3,5–7,11,28,31 In this study, no consistent chromosomal abnormality was detected within the AML-M0 cohort, though cytogenetic data were limited. The classic favorable AML translocations (those involving CBF, including t(8;21), and inv16 ), present in more than 20% of non-M0 AML patients, were not present in any AML-M0 patient. However, chromosome 5 deletions, nonconstitutional trisomy 21, and hypodiploidy were significantly more common in the de novo, non-DS FAB AML-M0 children compared with their non-M0 counterparts (Table 2). Whole or partial chromosome 5 losses occur rarely in childhood AML, but are generally considered an unfavorable prognostic risk factor in adult patients.32,33 In our AML-M0 cohort chromosome 5 abnormalities were significantly overrepresented, with 10% of patients having either deletion of 5q or monosomy 5. Nonconstitutional trisomy 21 has also been documented infrequently in the AML-M0 population.1–3,5–8 Its effect on outcome has proved difficult to assess as most cases occur in association with other known recurrent chromosomal abnormalities and it has consequently been ascribed an intermediate cytogenetic risk by the major adult collaborative groups.32,34 Hypodiploidy has been reported in up to 9.8% of pediatric patients with AML.32 Its prognostic import in AML, unlike in ALL, is uncertain.32 The ins(10;11) is an uncommon cytogenetic abnormality in AML, but may be more frequent in AML-M0.35,36
Studies showing an association of AML-M0 with poor outcome have generally been performed in adult patients.1–3,6–8,28 In the subsets of adults treated intensively, these studies report CR rates of 25% to 62%,2,3,5,6,28 with median CR durations of 1 to 12 months3,6,28 and median survivals of 2 to 10 months.2,5,6,28
The reasons underlying the adverse outcome of adult patients with AML-M0 are incompletely understood but may include use of generally less intensive treatment protocols and the frequent convergence of many negative prognostic factors. These include a higher presenting WBC count,2,6,28 higher incidence of complex karyotypes,3,6,28 the blast cells' immaturity as indicated by high expression of CD34 and CD7,3,6 and the frequent expression of the multidrug resistance-associated protein GP-170.3 Investigators have also found, in adult AML-M0 patients, an association between increased expression of bcl-2, a protein involved in the regulation of apoptosis, and lower CR rates.8,37 Recently the incidence of FLT3 mutations has also been evaluated as a potential explanation for the adverse outcome in adult AML-M0 patients. Although the incidence of FLT3 mutations does not appear to differ from that found in adult AML in general,28,38 Roumier et al28 demonstrated in intensively treated adults with AML-M0 that presence of FLT3/ITD correlated with inferior outcome. Two studies examining the incidence of FLT3 mutations in pediatric AML also found no predominance of the AML-M0 subtype in their FLT3/ITD-positive patients.19,27 This study further demonstrates that FLT3/ITD is not overrepresented in patients with AML-M0. However, the small number of FLT3/ITD-positive AML-M0 patients made it difficult to ascertain its clinical significance within this AML subtype.
Our results clarify some of the uncertainty regarding the prognostic significance of the FAB-M0 subtype of AML in the pediatric population. When all enrolled patients (those treated with both ST and IT therapy) with de novo, non-DS AML were included in analyses, no significant difference was found in outcome between the AML-M0 and non-M0 patients. However, ST therapy was the inferior regimen in CCG-2891 and, as has occurred in previous clinical studies, the inferior regimen can fail to discriminate between patient subsets. An analysis restricted to CCG-2891-IT and CCG-2961 demonstrated a high remission induction rate in both M0 and non-M0 children, and a comparable incidence of induction failures and deaths. The AML-M0 children had a significantly inferior EFS, DFS, and OS from attainment of remission compared with their non-M0 counterparts (Table 4; Figure 3). This poorer postremission outcome in the AML-M0 children appeared to be the consequence of a significantly elevated CIR (65% ± 14% versus 40% ± 3%; P = .002). Interestingly, OS from diagnosis, although lower in the M0 patients, was not significantly different between the 2 groups. This may reflect the higher induction death rate in the non-M0 patients.
An analysis of study-specific outcomes found, within the CCG-2891-IT de novo, non-DS AML group, no significant difference in any outcome measure between the FAB AML-M0 and non-M0 patients. In CCG-2961, however, EFS, DFS, and OS from EOI were all inferior in the AML-M0 patients. It is unlikely that the differences in outcome between the 2 studies were a result of differences in pretreatment patient characteristics. CCG-2891 patients had increased CNS disease at diagnosis (11% versus 0%; P = .038) and an increased incidence of splenomegaly (57% versus 33%; P = .038), but the median age (P = .685) and WBC count (P = .699) at diagnosis and the distribution of cytogenetic abnormalities between the 2 studies were not significantly different. In view of their treatment similarities, the differences in outcome likely are a reflection of small M0 sample size within each of the 2 studies.
In CCG-2891, within the de novo, non-DS AML-M0 children, the induction regimen used (ST versus IT) was also found to not significantly impact on outcome. The remission induction rate (P = .677), OS (P = .318), EFS (P = .882), and DFS from EOI (P = .499) were not significantly different between the IT- and ST-treated AML-M0 children. The small sample size within each group (18 IT- versus 10 ST-treated M0 patients) is likely the reason underlying this finding of nonsignificance.
The AML-M0 subtype does not appear to alter the excellent outcome of DS-associated AML. The remission induction rates, OS, EFS, and DFS between the 2 DS groups were not significantly different. This is similar to reports describing DS patients with FAB AML-M7. Lange et al16 demonstrated that megakaryoblastic morphology, although associated with unfavorable outcome in the non-DS population, has no prognostic import in DS patients.
We have demonstrated within a consistently treated pediatric population, with lengthy follow-up, that FAB AML-M0 in children appears to be associated, as it is in adult patients, with an adverse outcome. Although the etiology of this adverse outcome is unknown, as in adult patients with AML-M0, our data suggest it may relate to a lack of favorable AML cytogenetic abnormalities (t(8;21), inv16 ) and a corresponding overrepresentation of high-risk (chromosome 5) abnormalities. FLT3/ITD was not overrepresented in our AML-M0 cohort and its prognostic significance in these patients remains undefined. Thus, though optimal therapy has not yet been defined for patients with AML-M0, it may be necessary to explore alternative therapeutic approaches for these patients.
Authorship
Contribution: D.R.B. performed the bone marrow morphology reviews; N.A.H. performed the bone marrow cytogenetics reviews; S.M. performed the FLT3 analyses; D.B., T.A.A., and F.O.S. contributed to the study concept and design; T.A.A., R.B.G., D.B., and F.O.S. participated in the analysis and interpretation of data; D.B. drafted the article; and all authors contributed critical reviews and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Children's Oncology Group appears as a data supplement to the online version of this article, available at the Blood website; click on the Supplemental Document link at the top of the online article.
Correspondence: Draga Barbaric, Centre for Children's Cancer and Blood Disorders, Sydney Children's Hospital, High St, Randwick, NSW, 2031, Australia; e-mail: dragmatt96@optusnet.com.au; cc: pubs@childrensoncologygroup.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute (CA98 543 and CA98 413; F.O.S.), and a Greg and Laura Norman Research Fellowship of the National Childhood Cancer Foundation (D.B.). W.G.W. is the CCG-2891 Study Chair; B.J.L. is the CCG-2961 Study Chair.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal