Abstract
Whether resveratrol, a component of red grapes, berries, and peanuts, could suppress the proliferation of multiple myeloma (MM) cells by interfering with NF-κB and STAT3 pathways, was investigated. Resveratrol inhibited the proliferation of human multiple myeloma cell lines regardless of whether they were sensitive or resistant to the conventional chemotherapy agents. This stilbene also potentiated the apoptotic effects of bortezomib and thalidomide. Resveratrol induced apoptosis as indicated by accumulation of sub-G1 population, increase in Bax release, and activation of caspase-3. This correlated with down-regulation of various proliferative and antiapoptotic gene products, including cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2. In addition, resveratrol down-regulated the constitutive activation of AKT. These effects of resveratrol are mediated through suppression of constitutively active NF-κB through inhibition of IκBα kinase and the phosphorylation of IκBα and of p65. Resveratrol inhibited both the constitutive and the interleukin 6–induced activation of STAT3. When we examined CD138+ plasma cells from patients with MM, resveratrol inhibited constitutive activation of both NF-κB and STAT3, leading to down-regulation of cell proliferation and potentiation of apoptosis induced by bortezomib and thalidomide. These mechanistic findings suggest that resveratrol may have a potential in the treatment of multiple myeloma.
Introduction
Multiple myeloma (MM) or plasma cell myeloma is characterized by latent accumulation of secretory plasma cells with a low proliferative index and an extended life span in the bone marrow.1 The second most prevalent hematologic cancer after non-Hodgkin lymphoma, multiple myeloma accounts for 10% of all hematologic cancers and approximately 2% of all cancer deaths. Conventional therapy for multiple myeloma involves combinations of vincristine, carmustine (bischloroethylnitrosourea), melphalan, cyclophosphamide, doxorubicin (Adriamycin), and prednisone or dexamethasone.2 Patients younger than 65 years are usually given high-dose melphalan with autologous stem cell support, and older patients or those who cannot tolerate such intensive treatment are given standard-dose oral melphalan and dexamethasone. Shortcomings of these treatments are low remission rates (about 5%), short survival times (median, 30-36 months), and the development of drug resistance.3,4
Chemoresistance remains a major therapeutic challenge in MM. The precise mechanism underlying chemoresistance in multiple myeloma is not clear, but one of the main contributors to both chemoresistance and pathogenesis is thought to be activation of NF-κB and STAT3 and dysregulation of apoptosis.4–8 Overexpression of antiapoptotic molecules has been linked to chemoresistance in MM; in one study, expression of the antiapoptotic protein Bcl-xL correlated with chemoresistance, with chemoresponse rates of 83% to 87% among non–Bcl-xL–expressing cases but only 20% to 31% among Bcl-xL–expressing cases.9
Chemoresistance in several types of cancer has been linked to activation of NF-κB, a transcription factor with central roles in the regulation of cell growth, survival, angiogenesis, cell adhesion, and apoptosis.10 Progression and chemoresistance are also thought to involve interleukin 6 (IL-6), expression of which is induced by NF-κB, through its regulation of the growth and survival of tumor cells.11,12 IL-6 leads to constitutive activation of STAT3, which in turn results in expression of high levels of Bcl-xL.6 Bcl-2 overexpression, another important characteristic of many multiple myeloma cell lines,13 can rescue cells from glucocorticoid-induced apoptosis.4 Cell lines resistant to doxorubicin (eg, RPMI 8226–Dox-40) have been shown to overexpress Bcl-xL.9 Thus, both constitutive activation of NF-κB and STAT3 play an important role in chemoresistance, and inhibition of NF-κB and STAT3 may overcome this chemoresistance.
The use of natural agents may be able to overcome resistance without some of the debilitating side effects of conventional chemotherapy. One such agent is resveratrol, a polyphenol (trans-3, 4′, 5-trihydroxystilbene) abundant in red grapes, berries, and peanuts. As early as 1997, resveratrol was found to be a potent chemopreventive agent, blocking the initiation, promotion, and progression of tumors induced by the aryl hydrocarbon dimethylbenz(a)anthracene (DMBA).14 Since that time, resveratrol has been shown to inhibit the growth of a wide variety of tumor cells, including lymphoid and myeloid; cancers of breast, prostate, and thyroid; melanoma; head and neck squamous cell carcinomas; and ovarian and cervical carcinomas.15 Putative mechanisms of growth inhibition include cell-cycle arrest, apoptosis, suppression of transcription factors such as NF-κB,16,17 and down-regulation of various inflammatory gene products such as cyclooxygenase-2 (COX-2) or IL-6.18 Our laboratory has also previously reported that resveratrol suppresses DMBA-induced mammary carcinogenesis in rats, through the down-regulation of NF-κB, COX-2, and matrix metalloproteinase-9 expression.19
Whether resveratrol can modulate proliferation of human MM cells or overcome the resistance of such cells to chemotherapy is not known. In exploring these questions, we found that resveratrol can indeed inhibit the proliferation and overcome the chemoresistance of MM cells, and that these effects occur through the suppression of NF-κB and STAT3, which in turn leads to the down-regulation of antiapoptotic gene products and increased apoptosis.
Materials and methods
Reagents and antibodies
Resveratrol with purity greater than 98% was purchased from Alexis Biochemicals (San Diego, CA). A 20-mM stock solution of resveratrol (molecular weight, 228.2) was prepared in ethanol and further diluted in cell culture medium to make working concentrations. Maximum final concentration of ethanol was less than 0.1% and was used as a control. Penicillin, streptomycin, RPMI 1640 medium, and fetal calf serum (FCS) were obtained from GIBCO (Grand Island, NY). Glycine, phorbol myristate acetate, lipopolysaccharide (LPS), ceramide, bovine serum albumin, and mounting medium DPX were obtained from Sigma-Aldrich Chemicals (St Louis, MO).
Rabbit polyclonal antibodies to p50, p65, AKT, STAT3, and STAT5 and mouse antibodies against phospho-STAT3 and phospho-STAT5 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against cyclin D1, cIAP2, Bcl-2, Bcl-xL, A1, and TRAF2 were also obtained from Santa Cruz Biotechnology. Anti-IKKα and anti-IKKβ antibodies were kindly provided by Imgenex (San Diego, CA). Anti-XIAP antibody was obtained from BD Biosciences (San Jose, CA); poly (ADP) ribose polymerase (PARP) antibody was from PharMingen (San Diego CA); phospho-IκBα (Ser32) antibody was from New England BioLabs (Beverly, MA); phospho-specific anti-AKT (Ser 473), phospho-specific anti-p65 (serine 536), and cleaved caspase-3 were from Cell Signaling (Beverly, MA); and goat anti–rabbit Alexa 594 was from Molecular Probes (Eugene, OR). Hoechst 33342, dexamethasone, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Chemicals, and γ32 P-ATP was from ICN Pharmaceuticals (Costa Mesa, CA). Protein blocking solution (ref no. X0909), Dako Cytomation LSAB+System-HRP kit (ref no. K0690), DAB chromogen (DAKO ref. no. K3466), and hematoxylin (DAKO, no. S3309) were obtained from Dako Cytomation (Glostrup, Denmark). Bortezomib (PS-341) was obtained from Millennium (Cambridge, MA). Thalidomide was obtained from Tocris Cookson (St Louis, MO).
Cell lines and culture conditions
The human MM cell lines U266 (ATCC TIB-196) and RPMI 8226 (ATCC CCL-155), both plasmacytomas of B-cell origin, were obtained from the American Type Culture Collection (Manassas, VA). U266 is known to produce IL-6 and monoclonal antibodies11,20 ; RPMI 8226 produces only Ig L chains, with no evidence of H-chain or IL-6 production. RPMI-8226–Dox-6 (a doxorubicin-resistant clone) and RPMI-8226–LR-5 (a melphalan-resistant clone) were kindly provided by Dr William S. Dalton (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL). MM.1 (also called MM.1S) cells were established from a peripheral blood sample of a patient with IgA myeloma; these cells secrete L chains, do not express the Epstein-Barr virus genome, and do express the leukocyte antigen DR, plasma cell Ag-1, T9, and T10 antigens.21 MM.1R, a dexamethasone-resistant variant of MM.1 cells,22 was kindly provided by Dr Steven T. Rosen of Northwestern University Medical School (Chicago, IL). U266, RPMI-8226, MM.1, and MM.1R cells, Dox-6 and LR-5 variants were cultured in RPMI 1640 medium containing 1 × antibiotic-antimycotic with 10% FCS. Cells were periodically tested by Hoechst staining and by custom polymerase chain reaction (PCR) for mycoplasma contamination.
Clinical samples
Marrow samples were obtained from patients with MM who gave informed consent and underwent treatment at the University of Texas M.D. Anderson Cancer Center (Houston). Approval was obtained from the University of Texas M.D. Anderson Cancer Center institutional review board for these studies. The CD138+ cells were separated as described previously.7 Table 2 describes the analysis of samples from these patients for various assays.
MTT assay
The antiproliferative effects of resveratrol on drug-sensitive and drug-resistant cells and patient samples were determined by the MTT dye uptake method as described.23
Electrophoretic mobility shift assay for NF-κB
NF-κB activation was analyzed by electrophoretic mobility gel shift assay (EMSA) as described.24
Immunocytochemistry for NF-κB p65 and STAT3 localization
Both MM cell lines as well as CD138+ samples from patients with MM were examined for NF-κB and for STAT3 by immunocytochemistry method essentially as described.7,8 One hundred cells were counted for each patient, and the sample was graded on the basis of a 4-point scale: − indicates no nuclear-positive cells (0%); +, 10% to 20% cells with nuclear positivity; ++, greater than 50% with nuclear positivity; +++, greater than 80% cells with nuclear positivity. Images were captured with a Labophot-2 microscope (Nikon, Tokyo, Japan) equipped with a CFWN 10×/1.5 oil objective. Cells were mounted in mounting media (Sigma-Aldrich). Photographs were taken with a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and processed with MetaMorph version 4.6.5 software (Universal Imaging, Downington, PA).
IKK assay
The effect of resveratrol on IKK activation was examined by immune complex kinase assay as previously described.25
Western blotting
Resveratrol-treated cells were examined by Western blot analysis for phospho-IκBα levels in the cytoplasm; phospho-p65 in the nucleus; and for STAT3, STAT5, phospho-STAT3, phospho-STAT5, phospho-AKT, Bax, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1 and TRAF2, procaspase-3, cleaved caspase-3, and PARP proteins in the whole-cell extracts as described.26 The whole-cell extracts were prepared by lysing resveratrol-treated cells in lysis buffer (20 mM Tris [pH 7.4], 250 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mM phenylmethylsulphonylfluoride, and 4 mM sodium orthovanadate). After electrophoresis, the proteins were electrotransferred to a nitrocellulose membrane, blocked with 5% nonfat milk, and probed with various antibodies overnight at 4°C. The blots were then washed, exposed to horseradish peroxidase–conjugated secondary antibodies for 1 hour, and finally examined by an enhanced chemiluminescence (ECL) reagent (Amersham, Piscataway, NJ).
Flow cytometry for cell-cycle distribution
To determine the effect of resveratrol on the cell cycle, U266 and MM1.S cells were first synchronized by serum starvation and then exposed to resveratrol for indicated time intervals. Thereafter, cells were washed, fixed with 70% ethanol, and incubated for 30 minutes at 37°C with 0.1% RNAse A in PBS. Cells were then washed again, resuspended, and stained in PBS containing 25 μg/mL propidium iodide (PI) for 30 minutes at room temperature. Cell distribution across the cell cycle was analyzed with a FACS Calibur (Becton Dickinson, Bedford, MA).
Live/dead assay
Apoptosis of cells was also determined by live/dead assay (Molecular Probes, Eugene, OR) that measures intracellular esterase activity and plasma membrane integrity as described.27
Annexin V assay
One of the early indicators of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected by using the binding properties of annexin V. This assay was performed as indicated.27
Statistical analysis
Statistical analysis was performed by Student unpaired t test. Probability (P) values below .05 were considered statistically significant.
Results
The goal of this study was to determine whether resveratrol can sensitize drug-resistant multiple myeloma cells through the regulation of NF-κB and STAT3 activation. To determine this, several MM cell lines and CD138+ cells from patients with MM were used.
Resveratrol suppresses the proliferation of drug-resistant MM cell lines and potentiates the apoptotic effect of bortezomib and thalidomide
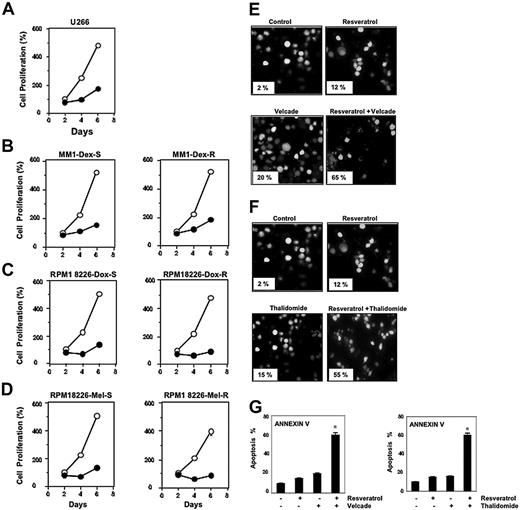
Resveratrol, at a concentration of 50 μM, suppressed the proliferation of all MM cell types tested, including U266, MM.1R cells (resistant to dexamethasone), RPMI 8226–Dox6 cells (resistant to doxorubicin), and RPMI 8226–LR5 cells (resistant to melphalan) (Figure 1A-D).
Resveratrol suppresses the proliferation of drug-resistant MM cell lines and potentiates the apoptotic effect of bortezomib and thalidomide. (A-D) U266 cells (5 × 103/100 μL; A), dexamethasone-sensitive (B, left) and dexamethasone-resistant (B, right) MM.1 cells (20 × 103/100 μL), doxorubicin-sensitive (C, left) and doxorubicin-resistant (C, right) RPMI 8266 cells (20 × 103/100 μL), and melphalan-sensitive (D, left) and melphalan-resistant (D, right) RPMI 8266 cells were plated in triplicate, treated with 50 μM resveratrol, and then subjected to MTT assay on days 2, 4, or 6 to analyze proliferation of cells. ○ represents control and • represents resveratrol-treated cells. Each point on line is an average of triplicate value. Resveratrol induced inhibition of cell growth at days 2 and 4 was statistically significant (P < .05). (E-F) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol and 20 nM bortezomib (E) or 10 μg/mL thalidomide (F) alone or in combination for 24 hours at 37°C. Cells were stained with a live/dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” Percentage of apoptosis is indicated in the inset. (G) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol, 20 nM bortezomib (left), or 10 μg/mL thalidomide (right) alone or in combination for 24 hours at 37°C. Cells were incubated with anti–annexin V antibody conjugated with FITC and then analyzed with a flow cytometer for early apoptotic effects. The results shown are representative of 3 independent experiments. *Values significantly (P < .05) different than control as well as single agent. Error bars represent SD of triplicate values.
Resveratrol suppresses the proliferation of drug-resistant MM cell lines and potentiates the apoptotic effect of bortezomib and thalidomide. (A-D) U266 cells (5 × 103/100 μL; A), dexamethasone-sensitive (B, left) and dexamethasone-resistant (B, right) MM.1 cells (20 × 103/100 μL), doxorubicin-sensitive (C, left) and doxorubicin-resistant (C, right) RPMI 8266 cells (20 × 103/100 μL), and melphalan-sensitive (D, left) and melphalan-resistant (D, right) RPMI 8266 cells were plated in triplicate, treated with 50 μM resveratrol, and then subjected to MTT assay on days 2, 4, or 6 to analyze proliferation of cells. ○ represents control and • represents resveratrol-treated cells. Each point on line is an average of triplicate value. Resveratrol induced inhibition of cell growth at days 2 and 4 was statistically significant (P < .05). (E-F) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol and 20 nM bortezomib (E) or 10 μg/mL thalidomide (F) alone or in combination for 24 hours at 37°C. Cells were stained with a live/dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” Percentage of apoptosis is indicated in the inset. (G) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol, 20 nM bortezomib (left), or 10 μg/mL thalidomide (right) alone or in combination for 24 hours at 37°C. Cells were incubated with anti–annexin V antibody conjugated with FITC and then analyzed with a flow cytometer for early apoptotic effects. The results shown are representative of 3 independent experiments. *Values significantly (P < .05) different than control as well as single agent. Error bars represent SD of triplicate values.
Bortezomib, an inhibitor of proteasome, and thalidomide, an inhibitor of TNF expression, have been approved for the treatment of patients with MM. Whether resveratrol can potentiate the effect of these drugs was examined. For this, U266 cells were treated with resveratrol together with either bortezomib or thalidomide, and then examined for apoptosis. As shown in Figure 1E-F, resveratrol potentiated the effect of both bortezomib and thalidomide. Whether the potentiation by resveratrol was dose dependent was examined. For this cells were treated with different concentrations of resveratrol together with different concentrations of either bortezomib or thalidomide. As can be seen in Table 1, potentiation can be seen at both 25 and 30 μM resveratrol. To further confirm the potentiation effect of resveratrol, we used annexin V staining, which detects an early stage of apoptosis. These results also indicated enhancement of apoptotic effects of bortezomib (Figure 1G, left) and thalidomide (Figure 1G, right) by resveratrol.
U266 cells treated with resveratrol, bortezomib, and thalidomide alone or in combination
| Resveratrol . | Resveratrol . | Bortezomib, % apoptosis . | Thalidomide, % apoptosis . | ||||
|---|---|---|---|---|---|---|---|
| 10 nM . | 20 nM . | 30 nM . | 5 μg/mL . | 10 μg/mL . | 20 μg/mL . | ||
| 0 μM | 1 ± 0.57 | 5 ± 0.5 | 20 ± 1.63 | 45 ± 2.45 | 5 ± 0.82 | 15 ± 3.32 | 35 ± 2.45 |
| 15 μM | 6 ± 0.82 | 12 ± 0.96 | 25 ± 1.15 | 45 ± 8.54 | 10 ± 1.63 | 20 ± 2.99 | 35 ± 6.45 |
| 25 μM | 12 ± 2.06 | 25 ± 3.56 | 65 ± 4.08 | 92 ± 5.06 | 30 ± 1.15 | 55 ± 4.08 | 85 ± 4.08 |
| 30 μM | 40 ± 2.98 | 60 ± 6.45 | 98 ± 1.15 | 100 ± 0.5 | 45 ± 4.08 | 90 ± 3.09 | 100 ± 0.5 |
| Resveratrol . | Resveratrol . | Bortezomib, % apoptosis . | Thalidomide, % apoptosis . | ||||
|---|---|---|---|---|---|---|---|
| 10 nM . | 20 nM . | 30 nM . | 5 μg/mL . | 10 μg/mL . | 20 μg/mL . | ||
| 0 μM | 1 ± 0.57 | 5 ± 0.5 | 20 ± 1.63 | 45 ± 2.45 | 5 ± 0.82 | 15 ± 3.32 | 35 ± 2.45 |
| 15 μM | 6 ± 0.82 | 12 ± 0.96 | 25 ± 1.15 | 45 ± 8.54 | 10 ± 1.63 | 20 ± 2.99 | 35 ± 6.45 |
| 25 μM | 12 ± 2.06 | 25 ± 3.56 | 65 ± 4.08 | 92 ± 5.06 | 30 ± 1.15 | 55 ± 4.08 | 85 ± 4.08 |
| 30 μM | 40 ± 2.98 | 60 ± 6.45 | 98 ± 1.15 | 100 ± 0.5 | 45 ± 4.08 | 90 ± 3.09 | 100 ± 0.5 |
U266 cells (1 × 106/mL) were treated with indicated concentrations of resveratrol, bortezomib, and thalidomide alone or in combination for 24 hours at 37°C. Cells were stained with a live/dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope as described in “Materials and methods.” The results shown are the percentage of apoptosis and are representative of 3 independent experiments. Standard deviations between the triplicates are indicated.
Resveratrol causes accumulation of MM cells in sub-G1 phase, increases release of Bax protein accumulation, and activates caspase-3
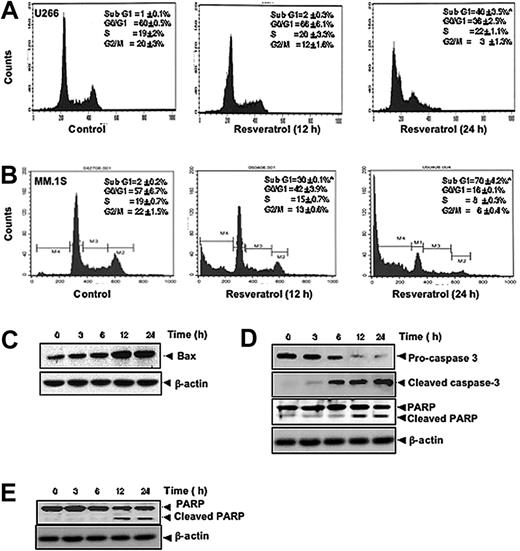
To further confirm that resveratrol inhibits proliferation of MM cells through induction of cell-cycle arrest, we analyzed cell-cycle distribution after PI staining. We found that resveratrol caused statistically significant accumulation of cell population in the sub-G1 phase after the treatment of U266 (Figure 2A) and MM1.S cells (Figure 2B) with resveratrol for 12 hours and 24 hours. Increased accumulation of proapoptotic protein Bax was also seen in a time-dependent manner on treatment of U-266 cells with resveratrol (Figure 2C). Cleavage of procaspase-3 to caspase-3 and caspase-3–mediated cleavage of PARP are other characteristic feature of apoptosis that were induced by resveratrol in U266 (Figure 2D) and in MM1.S cells (Figure 2E). Thus, these results suggest that resveratrol induces apoptosis through the activation of caspases.
Resveratrol causes accumulation of MM cells in sub-G1 phase, increases release of Bax protein accumulation, and activates caspase-3. (A) U266 cells (2 × 106/mL) were synchronized by incubation overnight in the absence of serum and then treated with 50 μM resveratrol for 0, 12, or 24 hours, after which the cells were washed, fixed, stained with PI, and analyzed for DNA content by flow cytometry. Results typical of 3 independent experiments are shown. *P < .05. (B) MM1.S cells (2 × 106/mL) were synchronized by incubation overnight in the absence of serum and then treated with 50 μM resveratrol for 0, 12, or 24 hours, after which the cells were washed, fixed, stained with PI, and analyzed for DNA content by flow cytometry. Results typical of 3 independent experiments are shown. *P < .05. (C) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to Western blot using antibody against Bax. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. (D) U266 cells were treated as described in panel C, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibodies against indicated proteins. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. (E) MM1.S cells were treated as described in panel C, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibody against PARP. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of 3 independent experiments.
Resveratrol causes accumulation of MM cells in sub-G1 phase, increases release of Bax protein accumulation, and activates caspase-3. (A) U266 cells (2 × 106/mL) were synchronized by incubation overnight in the absence of serum and then treated with 50 μM resveratrol for 0, 12, or 24 hours, after which the cells were washed, fixed, stained with PI, and analyzed for DNA content by flow cytometry. Results typical of 3 independent experiments are shown. *P < .05. (B) MM1.S cells (2 × 106/mL) were synchronized by incubation overnight in the absence of serum and then treated with 50 μM resveratrol for 0, 12, or 24 hours, after which the cells were washed, fixed, stained with PI, and analyzed for DNA content by flow cytometry. Results typical of 3 independent experiments are shown. *P < .05. (C) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to Western blot using antibody against Bax. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. (D) U266 cells were treated as described in panel C, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibodies against indicated proteins. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. (E) MM1.S cells were treated as described in panel C, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibody against PARP. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of 3 independent experiments.
Resveratrol suppresses AKT activation and inhibits the expression of antiapoptotic proteins in MM cells
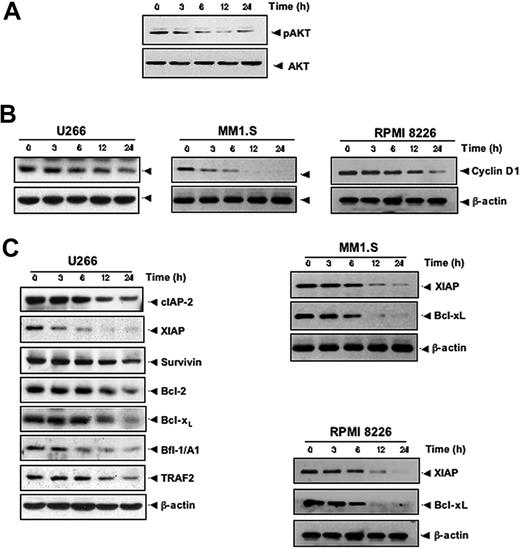
Activation of AKT also plays a major role in cell survival.28 Whether resveratrol modulates the activation of AKT in MM cells was therefore investigated. AKT was found to be constitutively active in U266 cells, and resveratrol was found to suppress constitutively phosphorylated AKT levels in a time-dependent manner (Figure 3A), indicating that these reduced AKT levels may contribute toward increasing the apoptosis of MM cells. Whether the expression of genes implicated in tumor-cell proliferation (cyclin D1) and survival (cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2) are regulated by resveratrol was investigated. We found that resveratrol down-regulated the constitutive expression of cyclin D1 in 3 different MM cell lines, including U266 (Figure 3B, left), MM1.S (Figure 3B, middle), and RPMI 8826 (Figure 3B, right) cells. Also resveratrol treatment decreased the levels of antiapoptotic gene products cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2 proteins in U266 (Figure 3C, left), XIAP and Bcl-xL (Figure 3C middle panel) in MM1.S, and (Figure 3C, right) in RPMI 8826 cells.
Resveratrol suppresses AKT activation and inhibits the expression of antiapoptotic proteins in MM cells. (A) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blotting with a phospho-AKT antibody. The same blot were stripped and reprobed with AKT antibody to show equal protein loading. (B) U266 cells (left)/MM1.S (middle)/RPMI 8826 (right) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot analysis using cyclin D1 antibody. The same blot was stripped and reprobed with β-actin antibody to verify equal protein loading. (C) U266/MM1.S/RPMI 8826 cells were treated as described in panel A, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibodies against indicated proteins. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of 3 independent experiments.
Resveratrol suppresses AKT activation and inhibits the expression of antiapoptotic proteins in MM cells. (A) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blotting with a phospho-AKT antibody. The same blot were stripped and reprobed with AKT antibody to show equal protein loading. (B) U266 cells (left)/MM1.S (middle)/RPMI 8826 (right) were treated with 50 μM resveratrol for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot analysis using cyclin D1 antibody. The same blot was stripped and reprobed with β-actin antibody to verify equal protein loading. (C) U266/MM1.S/RPMI 8826 cells were treated as described in panel A, and whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot using antibodies against indicated proteins. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown are representative of 3 independent experiments.
Resveratrol inhibits constitutively active NF-κB in MM cells
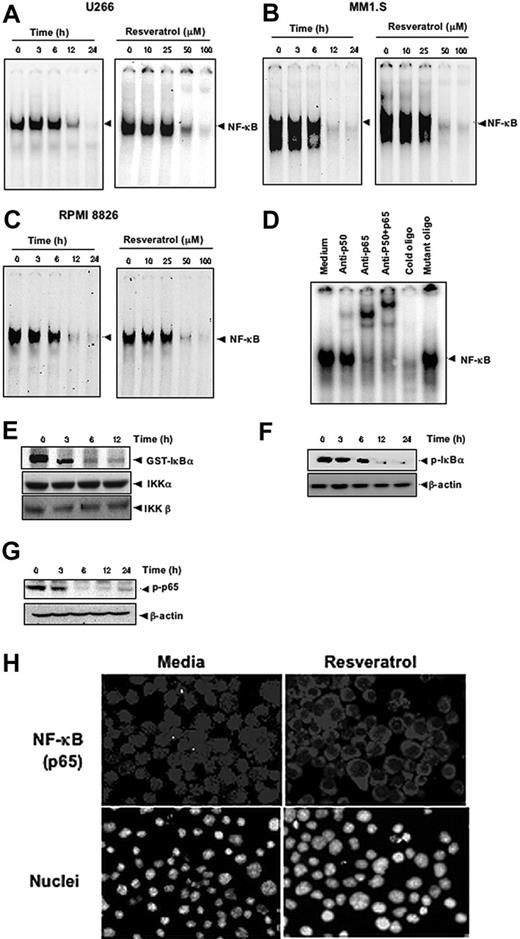
Because the expression of cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2 is regulated by NF-κB,29 whether suppression of expression of these gene products by resveratrol is through the down-regulation of NF-κB was examined. By using EMSA, We found that treatment of MM cells with resveratrol suppressed the constitutive active NF-κB in a dose- and time-dependent manner in U266 (Figure 4A), in MM1.S (Figure 4B), and in RPMI 8826 (Figure 4C) cells.
Resveratrol inhibits constitutively active NF-κB in MM cells. Resveratrol suppressed NF-κB in a time-dependent and a dose-dependent manner (A) in U266, (B) in MM1.S, and (C) RPMI 8826 cells. (A, left) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (A, right) U266 cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (B, left) MM1.S cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (B, right) MM1.S cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (C, left) RPMI 8826 cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (C, right), RPMI 8826 cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (D) NF-κB DNA binding was specific, and the activated complex consisted of p50 and p65 subunits. The results shown are representative of 3 independent experiments. (E) To measure IKK activity, U266 cells (4 × 106/mL) were incubated with 50 μM resveratrol for the indicated times, after which whole-cell lysates were prepared and immunoprecipitated with an antibody against IKK-α and analyzed with an immunocomplex kinase assay. (Bottom) IKK protein levels were assessed by fractionating whole-cell extracts on SDS-PAGE and examining them with Western blot using anti-IKKα and anti-IKKβ antibodies. (F-G) To assess phosphorylation of IκBα (F) and p65 (G), U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for indicated times and subjected to cytoplasmic fractionation. Then, 30-μg extracts were resolved on 7.5% SDS-PAGE gel and electrotransferred onto nitrocellulose membranes. The cytoplasmic fraction was probed for phospho-IκBα (F), and the nuclear fraction was probed for phospho-p65 (G). The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (H) Resveratrol induced translocation of activated nuclear p65 from the nucleus to the cytoplasm. U266 cells (2 × 106/mL) were incubated with medium (left) or with 50 μM resveratrol (right) for 24 hours and then analyzed for the intracellular distribution of p65 by immunocytochemistry (original magnification ×200). The results shown are representative of 3 independent experiments.
Resveratrol inhibits constitutively active NF-κB in MM cells. Resveratrol suppressed NF-κB in a time-dependent and a dose-dependent manner (A) in U266, (B) in MM1.S, and (C) RPMI 8826 cells. (A, left) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (A, right) U266 cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (B, left) MM1.S cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (B, right) MM1.S cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (C, left) RPMI 8826 cells (2 × 106/mL) were treated with 50 μM resveratrol for 0, 3, 6, 12, and 24 hours and then tested for NF-κB by EMSA. (C, right), RPMI 8826 cells (2 × 106/mL) were treated with the indicated concentrations of resveratrol for 24 hours and then tested for NF-κB by EMSA. (D) NF-κB DNA binding was specific, and the activated complex consisted of p50 and p65 subunits. The results shown are representative of 3 independent experiments. (E) To measure IKK activity, U266 cells (4 × 106/mL) were incubated with 50 μM resveratrol for the indicated times, after which whole-cell lysates were prepared and immunoprecipitated with an antibody against IKK-α and analyzed with an immunocomplex kinase assay. (Bottom) IKK protein levels were assessed by fractionating whole-cell extracts on SDS-PAGE and examining them with Western blot using anti-IKKα and anti-IKKβ antibodies. (F-G) To assess phosphorylation of IκBα (F) and p65 (G), U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for indicated times and subjected to cytoplasmic fractionation. Then, 30-μg extracts were resolved on 7.5% SDS-PAGE gel and electrotransferred onto nitrocellulose membranes. The cytoplasmic fraction was probed for phospho-IκBα (F), and the nuclear fraction was probed for phospho-p65 (G). The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (H) Resveratrol induced translocation of activated nuclear p65 from the nucleus to the cytoplasm. U266 cells (2 × 106/mL) were incubated with medium (left) or with 50 μM resveratrol (right) for 24 hours and then analyzed for the intracellular distribution of p65 by immunocytochemistry (original magnification ×200). The results shown are representative of 3 independent experiments.
To show that the inhibited band visualized by EMSA in U266 cells was indeed NF-κB, we incubated the nuclear extracts from these cells with antibodies against p50 or p65 subunits and performed the EMSA. Antibodies to either subunit of NF-κB shifted the band to one of higher molecular weight (Figure 4D), suggesting that the constitutively activated complex consisted of p50 and p65 subunits. A 100-fold excess of cold oligo nearly eradicated the band, but incubation with the mutated oligo failed to compete, further confirming the specificity of NF-κB binding.
To determine whether the inhibition of NF-κB by resveratrol resulted from the inhibition of IKK, U266 cells were exposed to resveratrol for various times, after which cells were lysed, and IKK complexes were extracted by immunoprecipitating with IKK-α antibody and subjected to an immunocomplex kinase assay with GST-IκBα used as a substrate. Resveratrol inhibited IKK activity without affecting the levels of IKK-α and IKK-β proteins (Figure 4E). Resveratrol also suppressed the phosphorylation of both IκBα (Figure 4F) and of p65 (Figure 4G). Whether resveratrol suppresses the p65 nuclear localization was examined by immunocytochemistry after 24-hour incubation with 50 μM resveratrol. The results show that resveratrol inhibited the appearance of p65 in the nucleus (Figure 4H).
Resveratrol inhibits constitutively active and IL-6–inducible STAT3 activation in MM cells
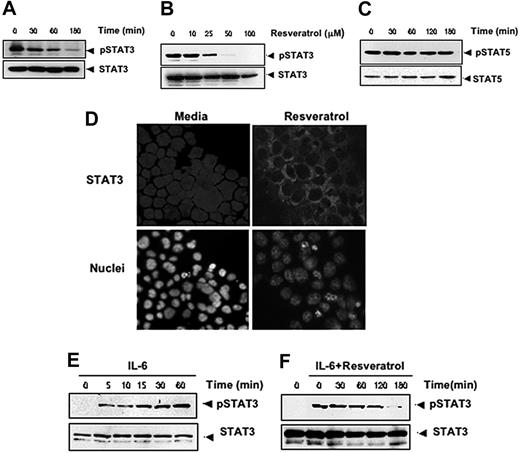
Because the expression of cyclin D1 and Bcl-xL is also regulated by STAT3,30,31 whether resveratrol also modulates STAT3 activation was investigated. For this, we exposed U266 cells with various doses of resveratrol and for various times and assessed the levels of phosphorylated STAT3 by Western blotting. STAT3 was found to be constitutively active in these cells, and resveratrol down-regulated phospho-STAT3 levels in a time- and dose-dependent manner (Figure 5A-B). This treatment did not change the expression of phosphorylated STAT5 levels (Figure 5C), indicating that the effect of resveratrol is specific to STAT3.
Resveratrol inhibits constitutively active and IL-6–inducible STAT3 activation in MM cells. Resveratrol suppressed phospho-STAT3 levels in a time-dependent (A) and dose-dependent (B) manner. (A) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times; after which whole-cell extracts were prepared; and 30-μg portions of those extracts were resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for phospho-STAT3. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (B) U266 cells (2 × 106/mL) were treated with the indicated concentration of resveratrol for 3 hours, after which Western blotting was performed as described for panel A. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (C) Resveratrol had no effect on STAT5 or phospho-STAT5 protein levels. U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times. Whole-cell extracts were prepared, fractionated on SDS-PAGE, and examined by Western blot using antibodies against phospho-STAT5 and STAT5. (D) Resveratrol causes inhibition of translocation of STAT3 to the nucleus. U266 cells (1 × 105/mL) were incubated with or without 50 μM resveratrol for 3 hours and then analyzed for the intracellular distribution of STAT3 by immunocytochemistry (original magnification ×200). (E-F) Resveratrol down-regulates IL-6–induced phospho-STAT3. (E) RPMI 8226 cells (2 × 106/mL) were treated with IL-6 (10 ng/mL) for the indicated times, whole-cell extracts were prepared, and phospho-STAT3 was detected by Western blot as described in “Materials and methods.” The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. Arrowheads indicate pSTAT3 and STAT3, respectively. (F) RPMI 8226 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times and then stimulated with IL-6 (10 ng/mL) for 15 minutes. Whole-cell extracts were then prepared and analyzed for phospho-STAT3 by Western blotting. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. The results shown are representative of 3 independent experiments.
Resveratrol inhibits constitutively active and IL-6–inducible STAT3 activation in MM cells. Resveratrol suppressed phospho-STAT3 levels in a time-dependent (A) and dose-dependent (B) manner. (A) U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times; after which whole-cell extracts were prepared; and 30-μg portions of those extracts were resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for phospho-STAT3. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (B) U266 cells (2 × 106/mL) were treated with the indicated concentration of resveratrol for 3 hours, after which Western blotting was performed as described for panel A. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (C) Resveratrol had no effect on STAT5 or phospho-STAT5 protein levels. U266 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times. Whole-cell extracts were prepared, fractionated on SDS-PAGE, and examined by Western blot using antibodies against phospho-STAT5 and STAT5. (D) Resveratrol causes inhibition of translocation of STAT3 to the nucleus. U266 cells (1 × 105/mL) were incubated with or without 50 μM resveratrol for 3 hours and then analyzed for the intracellular distribution of STAT3 by immunocytochemistry (original magnification ×200). (E-F) Resveratrol down-regulates IL-6–induced phospho-STAT3. (E) RPMI 8226 cells (2 × 106/mL) were treated with IL-6 (10 ng/mL) for the indicated times, whole-cell extracts were prepared, and phospho-STAT3 was detected by Western blot as described in “Materials and methods.” The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. Arrowheads indicate pSTAT3 and STAT3, respectively. (F) RPMI 8226 cells (2 × 106/mL) were treated with 50 μM resveratrol for the indicated times and then stimulated with IL-6 (10 ng/mL) for 15 minutes. Whole-cell extracts were then prepared and analyzed for phospho-STAT3 by Western blotting. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. The results shown are representative of 3 independent experiments.
Phosphorylation of STAT3 induces its dimerization and translocation from the cytoplasm into the nucleus.32 To confirm that resveratrol suppresses nuclear translocation of STAT3, we stained resveratrol-treated and untreated cells with anti-STAT3 antibody and found that exposure to resveratrol substantially inhibited the translocation of STAT3 from the cytoplasm to the nucleus (Figure 5D).
Because IL-6 is known to activate STAT3, we tested whether resveratrol would affect STAT3 activation induced by IL-6. We confirmed that IL-6 induced phospho-STAT3 in RPMI 8266 cells as early as 5 minutes after exposure and that this induction increased further over time (Figure 5E). We also found that treatment with resveratrol led to suppression of IL-6–induced phosphorylation of STAT3, an effect that also increased over time (Figure 5F). These results suggest that resveratrol down-regulates both constitutive and inducible STAT3 activation.
Resveratrol potentiates the effect of bortezomib and thalidomide on NF-κB and STAT3 activation in MM cells
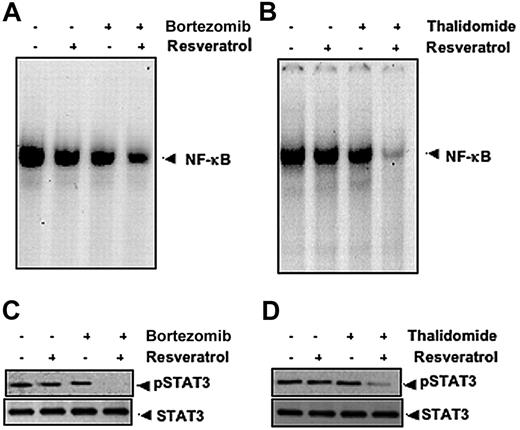
Our results indicate that resveratrol can potentiate the apoptotic effects of bortezomib and thalidomide. Whether this correlates with down-regulation of NF-κB and STAT3 activation was examined. To investigate this, U266 cells were exposed to suboptimal doses of resveratrol, bortezomib, and thalidomide and then examined for NF-κB and STAT3. As shown in Figure 6, resveratrol potentiated the effect of bortezomib and thalidomide on NF-κB (Figure 6A-B) and on STAT3 (Figure 6C-D).
Resveratrol potentiates the effect of bortezomib and thalidomide on NF-κB and STAT3 activation in MM cells. (A) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 nM bortezomib alone or in combination for 24 hours at 37°C and then tested for NF-κB by EMSA. (B) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 μg/mL thalidomide alone or in combination for 24 hours at 37°C and then tested for NF-κB by EMSA. The results shown are representative of 3 independent experiments. (C) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 nM bortezomib alone or in combination for 3 hours at 37°C. Whole-cell extracts were prepared, and 30-μg portions of those extracts were resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for antibody against phospho-STAT3. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (D) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 μg/mL thalidomide alone or in combination for 3 hours at 37°C after which Western blotting was performed as described for panel C. The results shown are representative of 3 independent experiments.
Resveratrol potentiates the effect of bortezomib and thalidomide on NF-κB and STAT3 activation in MM cells. (A) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 nM bortezomib alone or in combination for 24 hours at 37°C and then tested for NF-κB by EMSA. (B) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 μg/mL thalidomide alone or in combination for 24 hours at 37°C and then tested for NF-κB by EMSA. The results shown are representative of 3 independent experiments. (C) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 nM bortezomib alone or in combination for 3 hours at 37°C. Whole-cell extracts were prepared, and 30-μg portions of those extracts were resolved on 7.5% SDS-PAGE gel, electrotransferred onto nitrocellulose membranes, and probed for antibody against phospho-STAT3. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. (D) U266 cells (1 × 106/mL) were treated with 25 μM resveratrol or 10 μg/mL thalidomide alone or in combination for 3 hours at 37°C after which Western blotting was performed as described for panel C. The results shown are representative of 3 independent experiments.
Resveratrol inhibits the proliferation of CD138+ cells from patients with MM and down-regulates NF-κB and STAT3 activation
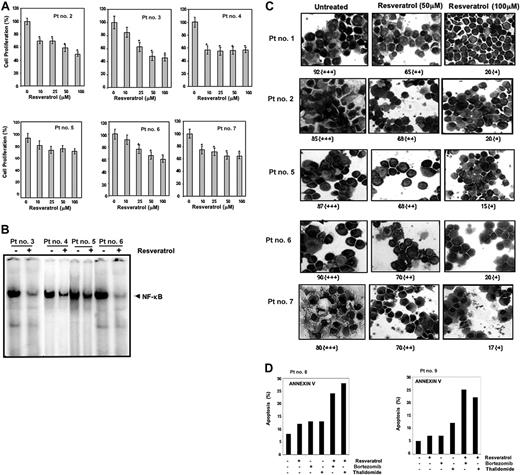
Until now all the studies were carried out with MM cell lines. We also investigated the effect of resveratrol on proliferation of CD138+ cells from 6 patients with MM as described in Table 2. Cells were exposed to different concentrations of resveratrol and then examined for cell proliferation by the MTT method. As shown in Figure 7A, exposure of CD138+ cells from all 6 patients with MM to resveratrol decreased cell proliferation in a dose-dependent manner, except patient no. 4 whereby maximum inhibition was observed at 10 μM resveratrol concentration and not beyond.
Analysis of CD138+ samples derived from multiple myeloma patients for proliferation, apoptosis, STAT3, and NF-κB
| Patient no. . | MTT . | STAT3 . | NF-κB . | Annexin . |
|---|---|---|---|---|
| 1 | ND | + | ND | ND |
| 2 | + | + | ND | ND |
| 3 | + | ND | + | ND |
| 4 | + | ND | + | ND |
| 5 | + | + | + | ND |
| 6 | + | + | + | ND |
| 7 | + | + | ND | ND |
| 8 | ND | ND | ND | + |
| 9 | ND | ND | ND | + |
| Patient no. . | MTT . | STAT3 . | NF-κB . | Annexin . |
|---|---|---|---|---|
| 1 | ND | + | ND | ND |
| 2 | + | + | ND | ND |
| 3 | + | ND | + | ND |
| 4 | + | ND | + | ND |
| 5 | + | + | + | ND |
| 6 | + | + | + | ND |
| 7 | + | + | ND | ND |
| 8 | ND | ND | ND | + |
| 9 | ND | ND | ND | + |
Proliferation was measured by the MTT method, and apoptosis was measured by the annexin method.
ND indicates not done.
Resveratrol inhibits the proliferation of CD138+ cells from patients with MM and down-regulates NF-κB and STAT3 activation. (A) Enriched CD138+ cells (2 × 105/0.1 mL) from bone marrow aspirates of patients with multiple myeloma were cultured in the absence or presence of indicated concentrations of resveratrol for 24 hours, and cell proliferation was measured by MTT assay as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. *P < .05. (B) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of patients with MM as indicated were cultured in the absence or presence of resveratrol (50 μM) for 12 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Materials and methods.” (C) STAT3 activation status was determined by fixing the untreated and 12-hour resveratrol-treated (50 μM, 100 μM) enriched CD138+ cells (2 × 106 cells) on slides by cytospin followed by immunocytochemistry for STAT3 as described in “Materials and methods.” Patient's numbers are indicated beside each panel. Original magnification, × 200. One hundred cells were counted for each patient. Grading was as follows: + indicates less than 10% cells with nuclear positivity; ++, 10% to 50% cells with nuclear positivity; +++, greater than 51% cells with nuclear positivity. The ++ and + indicate significantly different nuclear (P < .05) positivity than untreated cells. (D) CD138+ cells (2 × 104 cells) from patients no. 8 and no. 9 were treated with 25 μM resveratrol, 20 nM bortezomib, 10 μg/mL thalidomide either alone or in combination for 24 hours at 37°C. Cells were incubated with anti–annexin V antibody conjugated with FITC and then analyzed by a flow cytometer for early apoptotic effects.
Resveratrol inhibits the proliferation of CD138+ cells from patients with MM and down-regulates NF-κB and STAT3 activation. (A) Enriched CD138+ cells (2 × 105/0.1 mL) from bone marrow aspirates of patients with multiple myeloma were cultured in the absence or presence of indicated concentrations of resveratrol for 24 hours, and cell proliferation was measured by MTT assay as described in “Materials and methods.” Values represent the mean ± SD of triplicate cultures. *P < .05. (B) Enriched CD138+ cells (2 × 106 cells) from bone marrow aspirates of patients with MM as indicated were cultured in the absence or presence of resveratrol (50 μM) for 12 hours and then tested for NF-κB activity in the nuclei by electrophoretic mobility shift assay as described in “Materials and methods.” (C) STAT3 activation status was determined by fixing the untreated and 12-hour resveratrol-treated (50 μM, 100 μM) enriched CD138+ cells (2 × 106 cells) on slides by cytospin followed by immunocytochemistry for STAT3 as described in “Materials and methods.” Patient's numbers are indicated beside each panel. Original magnification, × 200. One hundred cells were counted for each patient. Grading was as follows: + indicates less than 10% cells with nuclear positivity; ++, 10% to 50% cells with nuclear positivity; +++, greater than 51% cells with nuclear positivity. The ++ and + indicate significantly different nuclear (P < .05) positivity than untreated cells. (D) CD138+ cells (2 × 104 cells) from patients no. 8 and no. 9 were treated with 25 μM resveratrol, 20 nM bortezomib, 10 μg/mL thalidomide either alone or in combination for 24 hours at 37°C. Cells were incubated with anti–annexin V antibody conjugated with FITC and then analyzed by a flow cytometer for early apoptotic effects.
Whether resveratrol-induced inhibition of proliferation of CD138+ cells from patients with MM leads to the down-regulation of NF-κB and STAT3 activation was investigated. We found that resveratrol was able to inhibit constitutively active NF-κB (Figure 7B) and nuclear translocation of STAT3 (Figure 7C) in CD138+ cells from patients with MM in a statistically significant manner (P < .05).
Resveratrol potentiates the apoptotic effect of bortezomib and thalidomide in CD138+ cells from patients with MM
Whether resveratrol potentiates the apoptotic effect of bortezomib and thalidomide in CD138+ cells from patients with MM was also investigated. Using annexin V staining (which detects an early stage of apoptosis), we found that resveratrol enhances the apoptotic effect of bortezomib and thalidomide in CD138+ cells derived from patients with MM no. 8 and no. 9 (Figure 7D).
Discussion
The aim of this study was to determine whether resveratrol could suppress the proliferation of multiple myeloma (MM) cells by interfering with NF-κB and STAT3 pathways. Resveratrol inhibited the proliferation of human multiple myeloma cell lines regardless of whether they were sensitive or resistant to the conventional chemotherapeutic agents. It also potentiated the apoptotic effects of bortezomib and thalidomide. Resveratrol induced sub-G1 accumulation and increased Bax, leading to caspase-3 activation. This correlated with down-regulation of various proliferative and antiapoptotic gene products, including cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2. These effects of resveratrol are mediated through suppression of constitutively active NF-κB through inhibition of IκBα kinase. Resveratrol also inhibited both the constitutive and the interleukin 6–induced activation of STAT3. We found that resveratrol inhibited the survival of CD138+ plasma cells from patients with MM and potentiated the apoptotic effect of bortezomib and thalidomide, and this correlated with suppression of constitutive activation of both NF-κB and STAT3.
In agreement with previous reports,4,5,33 we found that all MM cell lines expressed constitutively activated NF-κB and that resveratrol suppressed the activation. Although resveratrol has been shown to inhibit inducible NF-κB activation in cell lines of various origins,18 whether resveratrol can also inhibit constitutively activated NF-κB in MM cell lines has not been previously reported. Resveratrol inhibits NF-κB through suppression of constitutively active IKK, which is needed for NF-κB activation. We found that inhibition of IKK by resveratrol led to inhibition of phosphorylation of both IκBα and p65; we also found that resveratrol suppressed constitutively active AKT. Both AKT and IKK have been shown to phosphorylate p65.34–36 AKT has been shown to provide survival signals and inhibit apoptosis.28,37
In addition to NF-κB, we also found for the first time that resveratrol could suppress both constitutive and inducible STAT3 activation in multiple myeloma cells and that these effects were specific to STAT3, as resveratrol had no effect on STAT5 phosphorylation. STAT3 phosphorylation plays a critical role in transformation and proliferation of tumor cells. All Src-transformed cell lines have persistently activated STAT3, and dominant-negative STAT3 blocks transformation.38,39 Dominant-negative STAT3 has also been shown to induce apoptosis in cells with constitutively active STAT3.6 Other forms of cancer, including head and neck cancers,40 hepatocellular carcinoma,41 lymphomas, and leukemia,42 also have constitutively active STAT3. The suppression of constitutive active STAT3 in MM cells by resveratrol raises the possibility that this novel STAT3 inhibitor might also inhibit the constitutively activated STAT3 in other types of cancer cells.
We also found that resveratrol suppressed several genes that are regulated by NF-κB and STAT3, including the proliferative (cyclin D1) and antiapoptotic gene products (cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF233 ). Constitutively active STAT3 can contribute to oncogenesis by protecting cancer cells from apoptosis; this implies that suppression of STAT3 activation by agents such as resveratrol could facilitate apoptosis. Constitutively active STAT3 has been implicated in the induction of resistance to apoptosis,6 possibly through the expression of Bcl-2 and cyclin D1.43,44 Expression of Bcl-xL is known to be regulated by both STAT345 and NF-κB46 and to be overexpressed in MM cells.9 Bcl-xL can block cell death induced by a variety of chemotherapeutic agents,47 and expression of Bcl-xL has been correlated with chemoresistance in patients with MM.9 Our results confirm that resveratrol down-regulated the expression of Bcl-2 and Bcl-xL in MM cells, which may be responsible for the decline in viability of those cells.
To our knowledge this is the first report of the ability of resveratrol to overcome chemotherapy-induced resistance in MM cells. Resveratrol-induced cell death in MM.1R cells (resistant to dexamethasone), RPMI 8226–Dox6-R cells (resistant to doxorubicin), and RPMI 8226–LR5-R cells (resistant to melphalan) was comparable to that in their drug-sensitive counterparts. Resveratrol has been reported to enhance paclitaxel-induced cell death in RPMI 8226 cells,48 but the mechanism by which this takes place is unknown. Because paclitaxel activates NF-κB,49 it is possible that resveratrol sensitizes cells to paclitaxel by down-regulating NF-κB.
Recently, a proteasome inhibitor (PS341, also called bortezomib) and a TNF inhibitor (thalidomide) were approved for the treatment of multiple myeloma.50,51 Both of these inhibitors also suppress NF-κB activation,52,53 but both have severe side effects. We also found for the first time that resveratrol potentiates the apoptotic effect of bortezomib and thalidomide in CD 138+ plasma cells from patients with MM. However, the variation in sensitivity of different patient samples to resveratrol or to the combination of drugs could be due to the regulation of apoptosis by multiple mechanisms in cells from patients; and that these mechanisms may vary from patient to patient.
Resveratrol has been shown to be well tolerated in animal studies, with little toxicity.14,18,54 As high as 3000 mg resveratrol/kg body weight per day for 4 weeks was found to have no side effects in rats.55 We contend that the apparent pharmacologic safety of resveratrol and its ability to down-regulate the expression of several genes involved in cell survival and chemoresistance18 provides a sufficient rationale for testing resveratrol in patients with MM.
MM that has relapsed after conventional-dose therapy or stem cell transplantation is typically treated with high-dose corticosteroids, thalidomide, or bortezomib. However, disease in significant proportions of patients does not respond to these agents. Moreover, prolonged exposure leads to the development of resistance and toxicity, and progression-free and overall survival times are short. Collectively, safety information from preclinical studies and the ability of resveratrol to suppress NF-κB and STAT-3 activation, inhibit IL-6 signaling, down-regulate the expression of cyclin D1 and Bcl-xL, inhibit cell proliferation, and suppress the ability to potentiate the effect of bortezomib and thalidomide and to overcome drug resistance provide a sound basis for conducting clinical trials with resveratrol, alone or in combination with other agents, to enhance treatment efficacy, reduce toxicity, and overcome chemoresistance of relapsed or refractory MM.
Authorship
Contribution: A.B. and G.S. performed research and analyzed the data; Y.T., U.G., A.S.N., S.S. performed research; C.B.-R. and S.V.-R. contributed patient samples and analyzed the data; B.B.A. supervised and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.B. and G.S. contributed equally to this study.
Correspondence: Bharat B. Aggarwal, Cytokine Research Laboratory, Department of Experimental Therapeutics, Unit 143, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: aggarwal@mdanderson.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments. B.B.A. is a Ransom Horne Jr Professor of Cancer Research.
This work was supported by a grant from the Clayton Foundation for Research and from the Multiple Myeloma Research Foundation (B.B.A.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal