Abstract
Aspirin is effective in the prevention of cardiovascular events in high-risk patients. The primary established effect of aspirin on hemostasis is to impair platelet aggregation via inhibition of platelet thromboxane A2 synthesis, thus reducing thrombus formation on the surface of the damaged arterial wall. Growing evidence also indicates that aspirin exerts additional antithrombotic effects, which appear to some extent unrelated to platelet thromboxane A2 production. Aspirin can reduce thrombin generation with the subsequent attenuation of thrombin-mediated coagulant reactions such as factor XIII activation. Aspirin also acetylates lysine residues in fibrinogen resulting in increased fibrin clot permeability and enhanced clot lysis as well as directly promoting fibrinolysis with high-dose aspirin. The variable effectiveness of aspirin in terms of clinical outcomes and laboratory findings, which has been termed aspirin resistance, may be related to these additional antithrombotic effects that are altered when associated with common genetic polymorphisms such as the Leu33Pro β3-integrin or Val34Leu factor XIII mutations. However, the clinical relevance of these observations is still unclear. Elucidation of the actual impacts of aspirin other than antiaggregation effects could be important in view of the widespread use of this drug in the prevention of thrombotic manifestations of atherosclerosis.
Introduction
Acetylsalicylic acid, or aspirin, was synthesized by Hoffmann in 1898. Initially, this simple chemical compound was used as an antipyretic and anti-inflammatory agent. In the late 1960s aspirin emerged as a potent agent that prolongs the bleeding time and inhibits platelet aggregation.1 During the last 30 years, many studies have reinforced aspirin's position in the armamentarium of physicians as an antithrombotic wonder drug that has saved the lives of millions of patients with cardiovascular disease.2 Compelling evidence indicates that therapy with aspirin results in a 25% reduced risk of nonfatal myocardial infarction, nonfatal stroke, or vascular death in high-risk patients, regardless of sex, age, the presence of arterial hypertension, or diabetes.3
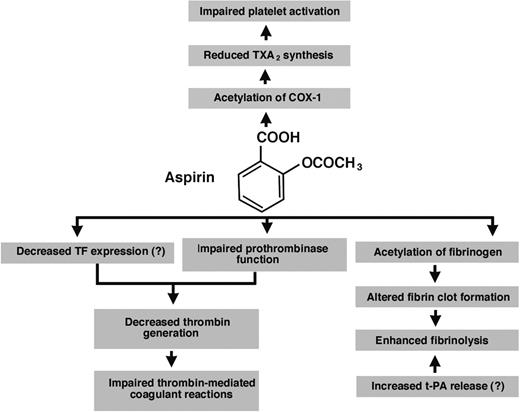
In this article we review a number of the reported effects of aspirin on 3 basic elements of hemostasis: platelet activation and aggregation, the formation of the fibrin network, and the fibrinolytic process (Figure 1). A role of these effects in the phenomenon of aspirin resistance will also be discussed.
Antithrombotic effects of aspirin (acetylsalicylic acid) reported in the literature. The major well-documented antithrombotic action of aspirin is to acetylate cyclooxygenase-1 (COX-1) in platelets, leading to the inhibition of thromboxane A2 (TXA2) synthesis. Decreased production of this lipid platelet agonist impairs platelet aggregation. Additional effects of aspirin may include reduced thrombin formation and changes in the fibrin structure such as increased clot permeability. The former effect is probably due to impaired platelet function or possibly decreased tissue factor (TF) expression, whereas the latter effect is induced by acetylation of fibrinogen and can result in faster fibrin clot lysis. Another profibrinolytic effect of aspirin might be increased tissue-type plasminogen activator (t-PA) release from endothelial cells.
Antithrombotic effects of aspirin (acetylsalicylic acid) reported in the literature. The major well-documented antithrombotic action of aspirin is to acetylate cyclooxygenase-1 (COX-1) in platelets, leading to the inhibition of thromboxane A2 (TXA2) synthesis. Decreased production of this lipid platelet agonist impairs platelet aggregation. Additional effects of aspirin may include reduced thrombin formation and changes in the fibrin structure such as increased clot permeability. The former effect is probably due to impaired platelet function or possibly decreased tissue factor (TF) expression, whereas the latter effect is induced by acetylation of fibrinogen and can result in faster fibrin clot lysis. Another profibrinolytic effect of aspirin might be increased tissue-type plasminogen activator (t-PA) release from endothelial cells.
Platelet activation and aggregation
COX-dependent actions of aspirin
The conversion of arachidonic acid to various eicosanoids is regulated by the enzyme cyclooxygenase (COX) of which there are 2 isoforms: COX-1 and COX-2.4 The COX-1 isoform, present in all tissues, represents the constitutive form of the enzyme, whereas the COX-2 isoform is expressed in inflammatory states in response to oxygen reactive species, endotoxins, cytokines, or growth factors.5 COX-2 can be found in human atherosclerotic plaques6 and also in small amounts in newly formed platelets.7
The aspirin-sensitive pathway in platelets initiates the release of arachidonic acid from membrane phospholipids. Aspirin inhibits the COX activity of prostaglandin (PG) G/H synthase (PGHS), by acetylating a single serine residue at position 529 in COX-1.8,9 This enzyme is also referred to as PGHS-1. X-ray crystallographic analysis has revealed that the aspirin-induced inhibition of platelet COX-1 consists of blocking the access of arachidonic acid through a narrow hydrophobic channel to the catalytic site in the core of the enzyme molecule.10
In human platelets, the product of COX-1 is PGH2, which is the direct precursor of PGD2, PGE2, PGF2α, prostacyclin, and thromboxane A2 (TXA2). TXA2 is the main product that is formed, by the specific isomerase called TXA2 synthase, and it acts as a platelet agonist, vasoconstrictor, and vascular smooth muscle cell mitogen.9,11 It has been demonstrated that 95% suppression of platelet COX-1 activity inhibits TXA2-dependent platelet aggregation and this effect is achieved with low-dose aspirin.12 Aspirin-treated platelets can still aggregate in response to potent agonists such as collagen or thrombin, provided at the site of vascular injury.5,9 However, aspirin reduces the potency of agonists released from activated platelets without any direct effect on β3-integrin activation13 and thus impairs platelet thrombus formation on collagen surfaces.14 The prostanoid, prostacyclin, which is generated largely through COX-2 in healthy individuals,2 counteracts these effects by preventing platelet aggregation and stimulating vasodilatation. In contrast to anucleate platelets, nucleated cells can resynthesize their enzymes, thus prostacyclin production is recovered after a few hours.9,15 Moreover, the systemic vasculature is partly protected by presystemic metabolism of aspirin to salicylate by hepatic esterases.2,16
Aspirin is 170 times less potent as an inhibitor of the COX-2 enzyme by acetylating Ser5169 and, therefore, much higher doses of aspirin are required to suppress COX-2 activity and consequently produce anti-inflammatory effects. It is known that selective COX-2 inhibition suppresses prostacyclin and can promote arterial thrombosis.17 However, aspirin at therapeutic doses has most likely a negligible effect on potential COX-2-dependent modulation of hemostasis in vivo. The synthesis of PGE2, the main product of platelet COX-2, is also inhibited. Importantly, acetylated COX-2 has 15-lipooxygenase activity and produces 15(R) hydroxyeicosatetraenoic acid (HETE).2 The anti-inflammatory properties of aspirin also involve the inhibition of inflammation-mediated endothelial dysfunction18 and the reduction of platelet release of interleukin 7.19
A third COX isoenzyme is derived as a splice variant of the COX-1 gene with persistence of the first intron. Whether this isoform is involved in aspirin efficacy is unresolved.20
After a single dose of aspirin, COX-1 activity is restored at the rate of 10% daily if platelet turnover is normal.2 Consequently, a dosage of one 30 to 100 mg tablet per day is sufficient to block platelet TXA2 synthesis in most individuals even if aspirin is able to acetylate COX-1 in megakaryocytes.21
In atherosclerotic vascular disease, especially in unstable coronary syndromes, TXA2 synthesis is only partly suppressed by aspirin. This is evidenced by relatively large amounts of thromboxane metabolites in urine despite inhibition of platelet TXA2 production.22 Agents used to achieve greater suppression of TXA2 activity such as blockers of the TXA2/PGH2 receptor or TXA2 synthase have been shown to provide no further benefit beyond what is seen during aspirin therapy.9
In many studies, aspirin use in cardiovascular disease appears surprisingly effective given the fact that the principal role ascribed to this agent is the impairment of platelet aggregation by inhibiting the formation of only one (and not the most potent) of several platelet agonists, operative in vivo. On the other hand, growing evidence indicates that aspirin may produce additional antithrombotic effects that are not attributable to the blocked COX-1 or COX-2.
COX-independent actions of aspirin
Thrombin generation.
When the vascular endothelium is damaged, blood factor (F) VIIa comes in contact with exposed/expressed tissue factor (TF) and forms the vitamin K-dependent extrinsic tenase complex, which activates FX and FIX.23 FXa (pM concentrations) on a membrane activates a small amount of prothrombin to thrombin, which further amplifies thrombin production by activating platelets, the pro-cofactors FV and FVIII, and the protransglutaminase FXIII, which is cross-linked by FXIIIa and cleaves fibrinogen, releasing fibrinopeptide (FP) A and B, to form fibrin. The activated platelets supply the surface for the vigorous generation of thrombin through the kinetically more efficient intrinsic tenase (FIXa-FVIIIa, Ca2+, membrane) complex and the prothrombinase (FXa, FVa, Ca2+, membrane) complex. The formation of α-thrombin by prothrombinase, in purified systems, occurs via cleavage at Arg320 yielding meizothrombin, which is subsequently cleaved at Arg271 and processed by autolytic cleavage at Arg284. The resulting serine protease α-thrombin is composed of an A chain (residues 285-320) and a B chain (residues 321-579). In vitro, α-thrombin is associated (Kd = 10 nM) with prothrombin fragment 2 or the precursor fragment 1.2 or both. Thrombin generation is attenuated and terminated by a collection of stoichiometric and enzymatic inhibitors (mainly antithrombin III, tissue factor pathway inhibitor [TFPI], and the protein C pathway). Clinical assays for the coagulation markers, thrombin-antithrombin (TAT) complex, prothrombin fragment 1 + 2 (F1 + 2), and FPA, have not revealed an aspirin mediated anticoagulant effect.24,25 No significant changes in coagulation markers following aspirin use have been found in plasma from peripheral venous blood in most but not all studies.26
With the introduction of a model of microvascular injury, blood collected from standardized bleeding time skin incisions on a forearm, the complex hemostatic events occurring following vascular wall damage could be evaluated qualitatively and quantitatively. Weiss and Lages27 demonstrated that thrombin formation proceeds via the TF-initiated coagulation pathway. This process is accompanied by rapid and massive platelet activation most probably stimulated by exposed collagen.28 The effects of aspirin on hemostasis in coagulation using the microvascular injury model were described by Kyrle et al.24 They reported that aspirin at a dose of 30 mg/d given for 1 week decreased thrombin formation in healthy volunteers. Further reports provided evidence that at the site of microvascular injury, aspirin at a dose of both 75 mg/d and 300 mg/d decreased the concentrations of thrombin markers to a similar level.24,29,30 This reduction was observed after a single dose of 500 mg aspirin,31 following a 7-day administration30 or 90 days of therapy.26 The thrombin-lowering action of aspirin was detected in both healthy young individuals24,29,31 and patients at increased risk of coronary artery disease.25,32
Thrombin regulation and the effect of various doses of aspirin.
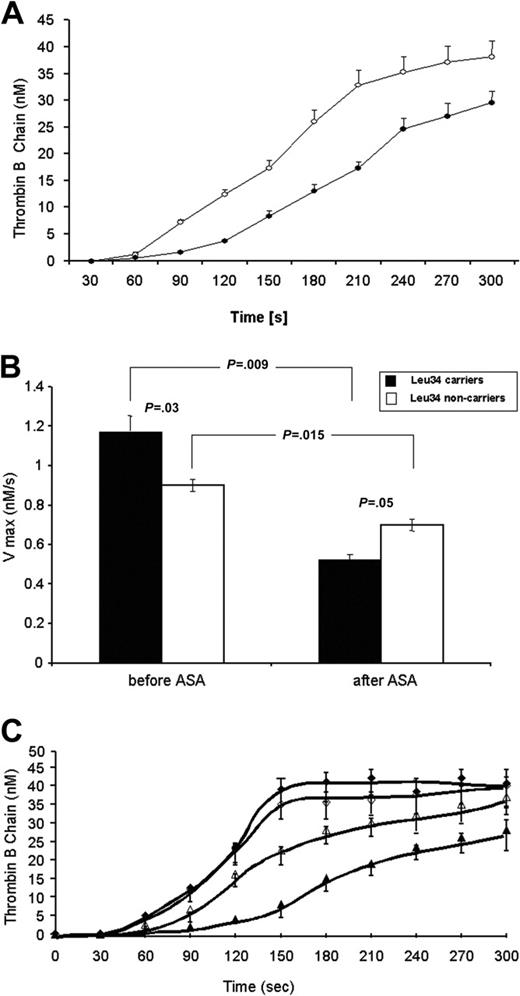
To extend observations regarding aspirin-induced alterations in blood clotting at the site of microvascular injury, we evaluated the coagulant reactions that regulate thrombin formation or are catalyzed by this protease such as activation of FV and FXIII and the inactivation of activated FV (FVa) by activated protein C (APC).30 Consistent with reduced maximum rates of formation of F1 + 2 and TAT (by about 30% and 33%), a 7-day administration of aspirin (75 mg/d) resulted in a significantly lower velocity of prothrombin consumption (by 29%), thrombin generation (by 27.2%; Figure 2A), and prothrombinase formation (by 29%). The posttreatment rates of appearance of both chains of FVa were reduced by 25% (heavy chain) and 29.6% (light chain), respectively. The peak levels of the light and heavy FVa chains were also decreased in blood collected at the site of vascular injury following aspirin ingestion. Conversely, FVa inactivation by APC remained either unaltered or was slightly enhanced. Aspirin also delayed FXIII activation by thrombin and decreased the maximum rate of FXIII cleavage.30
The effect of aspirin of select populations using a microvascular injury model. (A) Quantitative analysis of the generation of a-thrombin B-chain (circles) in bleeding time blood from 12 individuals (mean ± SEM).30 This product, estimated by densitometry and comparison to standards, is presented as a function of time (seconds). Concentration of thrombin B-chain before (○) and after 7-day aspirin (ASA, 75 mg/d) treatment (•). (B) The maximal velocities of activated factor XIII A subunit (FXIIIA) removal from bleeding time blood (mean ± SEM) in the Leu34-negative subjects (n = 23) and Leu34 carriers (n = 14) both prior to and following 7-day aspirin (ASA, 70 mg/d) treatment.30 (C) Quantitative analysis of thrombin B-chain generation showing concentrations of thrombin B-chain in 12 PlA1A1 subjects (triangles) and in 12 PlA2 carriers (diamonds) before (open symbols) and after 7-day aspirin (70 mg/d) treatment (closed symbols).34 Values are plotted as mean ± SEM.
The effect of aspirin of select populations using a microvascular injury model. (A) Quantitative analysis of the generation of a-thrombin B-chain (circles) in bleeding time blood from 12 individuals (mean ± SEM).30 This product, estimated by densitometry and comparison to standards, is presented as a function of time (seconds). Concentration of thrombin B-chain before (○) and after 7-day aspirin (ASA, 75 mg/d) treatment (•). (B) The maximal velocities of activated factor XIII A subunit (FXIIIA) removal from bleeding time blood (mean ± SEM) in the Leu34-negative subjects (n = 23) and Leu34 carriers (n = 14) both prior to and following 7-day aspirin (ASA, 70 mg/d) treatment.30 (C) Quantitative analysis of thrombin B-chain generation showing concentrations of thrombin B-chain in 12 PlA1A1 subjects (triangles) and in 12 PlA2 carriers (diamonds) before (open symbols) and after 7-day aspirin (70 mg/d) treatment (closed symbols).34 Values are plotted as mean ± SEM.
Studies probing direct effects of high-dose aspirin on the plasma and blood coagulation systems in vitro have provided mixed results. The dampening of thrombin formation by aspirin has been reported in citrated plasma35 and in whole blood taken after administration of 500 mg aspirin and then left to clot in vitro.36 The calibrated automated thrombin generation system, which displays thrombin levels by measuring the consumption of a fluorogenic substrate, has also reported a decrease in thrombin formation by 25% to 40% following a single dose of aspirin.37 Moreover, 300 mg aspirin daily given for 1 month has been reported to inhibit thrombin formation at shear rates, similar to those encountered in the arteries.38 In contrast, Butenas et al39 reported no significant effect of 650 mg aspirin taken orally on TAT formation measured in a whole blood system in which non-anticoagulated blood was mixed with relipidated TF in the presence of corn trypsin inhibitor that blocks activated FXII. Given the interindividual variability of the thrombin profiles,39 the 3 healthy volunteers tested in this study potentially formed too small a group size to detect subtle changes that could be induced by aspirin. Available data suggest that aspirin's effect on thrombin production may be partially related to depressed platelet reactivity, but also associated with the properties of the damaged tissues exposed on injury. This and the absence of shear potentially limits the chance of observation of a major aspirin influence in static systems.
Initiation of coagulation and the effect of aspirin.
Because in vivo coagulation is triggered by the exposure of membrane-bound TF present in the subendothelium, an explanation for the very significant aspirin-induced down-regulation of thrombin generation following microvascular injury might be by decreased TF expression or increased TFPI secretion. The prolongation of the lag phase of thrombin generation, which is observed in the blood flowing from a microvascular injury during aspirin use,30 is largely determined by the activity of the TF-FVIIa complex and its inhibition by TFPI.40 Of note are reports showing that aspirin may inhibit TF synthesis in human monocytes41 and reduced TF expression in human atherosclerotic plaques.42 These effects may might be mediated by the inhibition of IκB kinase-β.41 Further, it is not known to what extent aspirin-induced suppression of COX-1 activity with the subsequent inhibition of TXA2 synthesis may influence thrombin formation at the platelet with shear surface. At present the suggestion that impaired platelet function by aspirin translates into lower thrombin production cannot be excluded.
Cholesterol and aspirin.
Elevated levels of cholesterol appear to diminish the influence of aspirin on thrombin formation at the site of hemostatic plug formation.43 Aspirin at a dose of 300 mg daily has been reported to impair thrombin generation in patients with a total cholesterol less than 240 mg/dL and low-density lipoprotein (LDL) cholesterol less than 155 mg/dL.43 There is a positive correlation between total cholesterol or LDL cholesterol and the total amount of thrombin generated following aspirin administration. Moreover, aspirin does not prolong the bleeding time in subjects with a total cholesterol greater than 240 mg/dL.43 Aspirin at a dose of 75 mg/d has been reported to reduce thrombin generation only in patients whose total cholesterol level is below 200 mg/dL. Low-dose aspirin thus failed to impair thrombin formation at the site of microvascular injury in subjects with total cholesterol levels between 200 and 250 mg/dL.32 Importantly, simvastatin added to long-term aspirin use resulted in a further decrease in thrombin generation.32 This finding not only provides additional evidence for antithrombotic effects of statins but also suggests that combined aspirin-statin therapy may function in a synergistic fashion in lowering thrombin generation in vivo.
Aspirin and the fibrin network
Influence of polymorphisms
Thrombin is essential to the formation of the fibrin network through the activation of platelet receptors, fibrinogen to fibrin conversion and the stability of the clot through the activation of FXIII. The stability of this network may be altered by various genetic polymorphisms and potentially directly and indirectly influenced by aspirin.
FXIII.
FXIII, a heterotetamer composed of 2 A chains and 2 B chains, functions as a transglutaminase that can form cross-linked amide bonds between specific glutamine and lysine residues on polypeptide chains. Activation of the zymogen by thrombin and calcium occurs in the NH2-terminus of the A chains at Arg37-Gly38 and releases a 36-amino acid activation peptide from each of the A chains. Catalytic activity is expressed only after the A-chain dimer is dissociated from the B chain through a calcium-dependent process after thrombin proteolysis. Fibrin is the main physiologic substrate for FXIIIa and stabilizes fibrin clots through formation of covalent bonds between the ϵ-amino group of specific lysines and the γ-carboxylamide group of glutamines within fibrin fibrils.44 It has been suggested that a Val34Leu polymorphism in the A chain of FXIII may be associated with a lower risk of myocardial infarction despite the fact that this allelic variant results in a higher rate of FXIII activation by thrombin.45 This polymorphism, found in 25% of the population, is 3 amino acids proximal to the thrombin cleavage site at Arg37-Gly38. Due to its close proximity to the thrombin cleavage site, it has been postulated that this mutation modulates FXIII activation. We demonstrated using the model of microvascular injury that inhibition of FXIII activation following a 7-day administration of 75 mg/d aspirin is more pronounced in the Leu34-positive healthy men as compared to individuals with the Val34Val genotype (Figure 2B).33 Because FXIII activation is enhanced about 100-fold by fibrinogen and fibrin,44 incorporation of acetyl groups by aspirin into fibrin might interfere with FXIII activation and activated FXIII function following administration of this drug. These findings suggest a pharmacogenetic association of aspirin's antithrombotic effects and the presence of the FXIII Leu34 allele.
A higher rate of FXIII activation in the presence of the Leu34 allele suggests earlier fibrin network cross-linking catalyzed by activated FXIII and increased resistance to lysis.46 We have studied fibrin clot properties in healthy subjects with all 3 allelic variants of the FXIII Val34Leu mutation both prior to and following ingestion of 300 mg aspirin. Four hours after aspirin intake, clot permeability increased in association with the Val34Leu polymorphism and this effect was significantly greater in subjects possessing the Leu34 allele.47 In a turbidimetric lysis test using tissue plasminogen activator, clots formed from plasma obtained from subjects receiving aspirin were more prone to fibrinolysis in carriers of the Leu34 allele than in Leu34-negative individuals. These experiments suggest that in carriers of the Leu34 allele, aspirin alters fibrin cross-linking (likely via lysine acetylation48 ) to a greater extent than in subjects with the Val34Val genotype. It might be speculated that a novel mechanism of variability in sensitiveness to aspirin in terms of its antithrombotic effects can be related to FXIII activity or function. FXIIIa has multiple substrates including fibrin(ogen), fibronectin, α2-plasmin inhibitor, collagen, vitronectin, von Willebrand factor, actin, myosin, factor V, and thrombospondin.
Platelet glycoproteins.
An additional modulator of aspirin-related changes in thrombin formation may be a common polymorphism of the β3-integrin gene, termed PlA1A2. A single nucleotide substitution at position 1565 in exon 2 of the gene encoding β3-integrins results in the substitution of leucine to proline at position 33 of the protein. The PlA2 allele, present in approximately 20% of whites, has been reported to increase the risk of coronary thrombosis,49 although the available data are inconsistent.50 The same is true for platelet reactivity in the presence of the PlA2 allele.51–53 While studying platelet response to aspirin, Cooke et al54 reported impaired aggregability of platelets obtained from subjects carrying the PlA2 allele. In contrast, Undas et al55 reported that carriers of the Pro33 allele showed a tendency to greater thrombin formation. In contrast to subjects homozygous for the Leu33 allele, 75 mg/d aspirin did not affect this reaction. Analyses of coagulant reactions at the site of microvascular injury have shown that in subjects homozygous for the Leu33 allele, there were significant reductions in the velocity of thrombin formation (by ∼30%), FVa generation (by ∼30%), fibrinogen cleavage (by ∼40%), and also thrombin-mediated FXIII activation (by ∼20%)34 (Figure 2C). At the end of bleeding, FPA and FPB levels were significantly lower following aspirin administration for 7 days, but only in men with the PlA1A1 genotype.34 Moreover, at 4 hours after ingestion of 300 mg aspirin, the bleeding time, an indirect marker of aspirin-induced alterations in hemostasis, became significantly less prolonged in healthy young men positive for the PlA2 allele as compared to individuals homozygous for the PlA1 allele.56 The mechanism(s) of any aspirin-mediated effects on β3-integrin activity is as yet unknown. Domingez-Jimenez et al57 has suggested that nonsteroidal anti-inflammatory drugs alter β3-integrin activation in a COX-independent manner by interfering with intracellular signaling pathways. Aspirin has also been reported to acetylate GPIIb and GPIIIa molecules,58 which are crucial for platelet aggregation at high shear stress. However, it remains to be established how this effect might modulate thrombin generation in subjects receiving aspirin.
Plasma protein acetylation
Mechanisms by which aspirin depresses thrombin generation remain largely hypothetical. Acetylation of prothrombin in vivo has been suggested31 ; however, there are no fundamental data to support this conjecture. It is also possible that fibrinogen/fibrin acetylated by aspirin is less efficient in enhancing thrombin formation during blood clotting. Aspirin has been reported to acetylate antithrombin59 and this mechanism, if present in vivo, may contribute to the decrease in plasma levels of thrombin markers observed after aspirin use.
One of the potential mechanisms independent of the activity of COX-1 might be related to factors responsible for fibrin clot architecture and network.60 Acetylation of fibrinogen has been demonstrated in vitro at relatively high pH61 and in vivo following high-dose treatment with aspirin (650 mg every 12 hours).48 Mass spectrometry used to evaluate fragments of fibrinogen preincubated with aspirin showed that some fragments have higher molecular weights consistent with incorporation of the mass of an acetyl group (∼42 Da).62 Furthermore, acetylation of lysines proceeded at different velocities depending on their locations in the fibrinogen molecule.62 It has also been speculated that in vivo acetylation of fibrinogen changes its structure, which might result in altered properties of the fibrin clot formed from the modified fibrinogen. Indeed, several studies have shown that aspirin alters fibrin gel porosity. Aspirin has also been reported to increase plasma clot permeability.63,64 In healthy individuals, low-dose aspirin increases clot porosity and fiber mass-to-length ratio by 65%.64 Aspirin withdrawal has been associated with a return to baseline permeability as early as after 7 days.64 In a purified system, He et al65 demonstrated that aspirin increases fibrin fiber thickness and enhances fibrinolysis. In healthy individuals taking 650 mg aspirin twice daily, the extent of acetylation in the fibrinogen molecule correlated inversely with clot lysis time in vitro.48 However, it remains to be established which ϵ-amino groups of lysines in the fibrinogen molecules can be acetylated in human blood at conventional aspirin doses.
Fibrinolysis
Aspirin has been reported to induce changes in tissue plasminogen activator (t-PA) activity.66 Aspirin (650 mg given twice daily) has no effect on t-PA expression but decreases t-PA activity induced by venous occlusion primarily by inhibiting the release of t-PA.67 This effect appears to be associated with inhibition of prostacyclin production by aspirin.67 Some investigators found in various experimental systems that aspirin increased occlusion-induced increments in t-PA levels68 or had no effect.69 In a population of patients with stable angina, long-term aspirin treatment was inversely correlated with t-PA antigen concentration.70 It has also been suggested that aspirin may stimulate plasmin activity.71 Taken together, a role of aspirin at conventional doses in the modulation of fibrinolytic activity is unclear.
Other possible effects
The acetylation of cell-membrane proteins has been suggested to alter membrane fluidity that is known to modulate cell function. Aspirin has been reported to increase rigidity of cell membranes in vitro72 and in subjects taking aspirin at therapeutic doses.73,74 This effect did not depend on the inhibition of COX-1 by aspirin.74 It has been hypothesized that alterations in platelet lipid-protein matrix that render membrane proteins less accessible for acetylation by aspirin.43 Very high doses of aspirin (> 1.5 g/d) have been reported to act as a vitamin K antagonist.75 However; it is unlikely that this effect contributes to antithrombotic actions of aspirin given at therapeutic doses.
Another potential mechanism may be related to the stoichiometric inhibitor, TFPI, which modulates TF-dependent coagulation in vivo by binding the FXa-TF-FVIIa product complex (extrinsic pathway). TFPI is the principal regulator of the initiation phase of thrombin generation. Inhibition of TFPI has been found to result in arterial thrombus formation, previously abolished by administration of high-dose aspirin in a rabbit model.76 Since 10% of TFPI is released from platelets and TFPI, like other proteins, may be acetylated by aspirin, it is conceivable that aspirin might affect the TFPI function; however, this concept has not been reported.
Aspirin resistance
The term “aspirin resistance” has been applied to describe a clinical situation in which a subgroup of patients taking therapeutic doses of aspirin experience thrombotic vascular events.77–79 This so-called clinical aspirin resistance is based on the somewhat naive premise that atherothrombotic events can be controlled by using a single pharmaceutical agent. From the biochemical/cell biologic perspective, aspirin resistance may be defined as the failure of aspirin to produce an expected effect such as inhibition of platelet aggregation, suppression of TXA2 production, or prolongation of the bleeding time.77–79 Such a biochemical aspirin resistance in patients with stroke or coronary artery disease (CAD), assessed using an ex vivo platelet function test, have been estimated to occur in 5% to 45% of users.78 The lack of a commonly accepted definition of aspirin resistance largely accounts for marked differences in the frequency reported by various investigators.
Growing evidence indicates that aspirin resistance might have important practical implications. It has been reported that urinary levels of 11-dehydrothromboxane B2 in the highest quartile of patients is associated with a greater risk for a composite end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death, compared to subjects in the lowest quartile of urinary 11-dehydrothromboxane TXB2 levels.22 A prospective study by Gum et al80 demonstrated that reduced responsiveness to aspirin is associated with an increased risk of cardiovascular events in patients with stable angina after a mean follow-up of 2.1 years. A similar tendency among aspirin-resistant survivors of acute myocardial infarction was observed following a 4-year follow-up.81 Moreover, as compared to aspirin-sensitive patients, the aspirin nonresponders had also a 2.9-fold increase in the risk of creatine kinase-MB elevation observed following percutaneous coronary interventions in patients with stable CAD.82 Among patients who had a history of stroke, aspirin resistance has been reported to be associated with a 10-fold increase in the risk of recurrent vascular events.83 Increased incidence of recurrent cerebral ischemic attacks in patients resistant on aspirin has been confirmed by Grundmann et al.84 However, the current evidence for clinical relevance of aspirin resistance is not firm and comes from studies limited by drawbacks such as study design (series of cases or case-control studies) and a small number of patients or cardiovascular events during a follow-up.
A number of mechanisms underlying such a complex phenomenon as aspirin resistance is continuously increasing (Table 1)A substantial body of evidence suggests that nonresponsiveness to aspirin results from an interplay of several genetic and environmental factors. However, contribution of these factors most likely varies from one subject to another and changes over time. Most investigators believe that some of mechanisms accounting for aspirin resistance are modifiable.
Potential mechanisms of aspirin resistance
| Reduced bioavailability of aspirin |
| Low compliance in aspirin-treated subjects85 |
| Underdosing or poor absorption, especially in case of the use of enteric-coated aspirin, or increased potential for aspirin hydrolysis by esterases during proton pump inhibitor administration79 |
| Simultaneous administration of other nonsteroidal anti-inflammatory drugs, especially ibuprofen, that interfere with aspirin-mediated COX-1 inhibition in platelets86–90 |
| Comorbid conditions, cigarette smoking,89 and hypercholesterolemia43 |
| Transient increase of platelet COX-1/COX-2 expression in new platelets: platelet turnover is accelerated in response to stress, eg, following coronary artery bypass grafting (CABG)90,91 |
| Alternative pathways of platelet activation |
| Augmented COX-2 expression in monocytes/macrophages leading to TXA2 synthesis92 |
| Increased sensitivity to collagen or adenosine diphosphate78,79 |
| Isoprostane formation, resulting from nonenzymatic lipid peroxidation, that may amplify the response to platelet agonists, eg, in diabetics, smokers, and hyperlipidemic patients79 |
| Expression of a new COX-2 isoform (COX-2a), demonstrated in patients after CABG93 |
| Common genetic polymorphisms affecting |
| COX-1 (eg, 50T), COX-2 (−765C), TXA2 synthase, or other enzymes involved in arachidonate metabolism94,95 ; the first 2 variants appear associated with changes in TXA2 production96 and recently, COX-1 haplotype has been reported to be related to platelet aggregation in the presence of aspirin97 |
| Platelet glycoproteins such as the β3 integrin (Pro33Leu), glycoprotein Ia/IIa (C807T), or Ib/V/IX34,94 |
| Proteins involved in blood coagulation, eg, FXIII (Val34Leu)33 |
| An ADP receptor P2Y198 |
| Tachyphylaxis99,100 |
| Other factors |
| Interference of aspirin- and nitric oxide-mediated antiplatelet effects |
| Elevated norepinephrine levels, especially following physical stress101 |
| Reduced bioavailability of aspirin |
| Low compliance in aspirin-treated subjects85 |
| Underdosing or poor absorption, especially in case of the use of enteric-coated aspirin, or increased potential for aspirin hydrolysis by esterases during proton pump inhibitor administration79 |
| Simultaneous administration of other nonsteroidal anti-inflammatory drugs, especially ibuprofen, that interfere with aspirin-mediated COX-1 inhibition in platelets86–90 |
| Comorbid conditions, cigarette smoking,89 and hypercholesterolemia43 |
| Transient increase of platelet COX-1/COX-2 expression in new platelets: platelet turnover is accelerated in response to stress, eg, following coronary artery bypass grafting (CABG)90,91 |
| Alternative pathways of platelet activation |
| Augmented COX-2 expression in monocytes/macrophages leading to TXA2 synthesis92 |
| Increased sensitivity to collagen or adenosine diphosphate78,79 |
| Isoprostane formation, resulting from nonenzymatic lipid peroxidation, that may amplify the response to platelet agonists, eg, in diabetics, smokers, and hyperlipidemic patients79 |
| Expression of a new COX-2 isoform (COX-2a), demonstrated in patients after CABG93 |
| Common genetic polymorphisms affecting |
| COX-1 (eg, 50T), COX-2 (−765C), TXA2 synthase, or other enzymes involved in arachidonate metabolism94,95 ; the first 2 variants appear associated with changes in TXA2 production96 and recently, COX-1 haplotype has been reported to be related to platelet aggregation in the presence of aspirin97 |
| Platelet glycoproteins such as the β3 integrin (Pro33Leu), glycoprotein Ia/IIa (C807T), or Ib/V/IX34,94 |
| Proteins involved in blood coagulation, eg, FXIII (Val34Leu)33 |
| An ADP receptor P2Y198 |
| Tachyphylaxis99,100 |
| Other factors |
| Interference of aspirin- and nitric oxide-mediated antiplatelet effects |
| Elevated norepinephrine levels, especially following physical stress101 |
With regard to other than antiaggregatory effects of aspirin, it would be of interest to demonstrate that thrombin formation in vivo and its modulation by aspirin is associated to aspirin resistance. Our preliminary findings have shown that, indeed, in patients at risk of CAD whose platelets aggregated in the presence of arachidonic acid despite treatment with 300 mg/d aspirin, thrombin generation, measured in the model of microvascular injury, was increased as compared to similar subjects with inhibited platelet aggregability (A.U., unpublished data, January 2006). The concept of undiminished or increased thrombin formation in subjects with “aspirin resistance” warrants further investigation.
Conclusions
Beyond any reasonable doubt, a major effect of aspirin on hemostasis is its COX-dependent inhibition of platelet function. However, a growing body of evidence indicates that aspirin may also affect blood coagulation at several levels, thus reducing thrombin generation with the subsequent inhibition of thrombin-mediated coagulant reactions. Another plausible effect of aspirin could by acetylation of fibrinogen that results in increased fibrin clot permeability and enhanced clot lysis under various experimental conditions. The variable effectiveness of aspirin, termed “aspirin resistance,” is most likely associated with insufficient inhibition of thromboxane synthesis or the presence of thromboxane-independent platelets stimulators. Decreased responsiveness to aspirin could also be related to these additional antithrombotic properties that are to some extent determined by environmental or genetic factors. The clinical relevance of these observations needs to be established. Given the widespread use of aspirin in the prevention of thrombotic manifestations of atherosclerosis, elucidation of the actual impact of other than strictly antiplatelet effects of this agent are of importance in clinical practice. A position paper of the Working Group of the International Society on Thrombosis and Haemostasis states that “the correct treatment, if any, of aspirin's ‘resistance’ is unknown.”102 However, future regimens of antithrombotic therapy might be tailored to the individual patient following identification of patients who may require other antiplatelet agents or administration of another antithrombotic drug.
Authorship
Conflict-of-interest disclosure: The authors declare that no financial conflicts of interest exist.
Correspondence: Kenneth G. Mann, University of Vermont, Biochemistry, 208 S Park Dr, Ste 2, Rm T227C, Colchester, VT 05446; e-mail: kenneth.mann@uvm.edu.
Acknowledgment
This work was supported by a Program Project Grant No. HL 46703 (Project 1) from the National Institutes of Health (K.G.M.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal