The contribution of Btk to innate immune responses of myeloid cells is poorly understood. In this issue of Blood, Sochorova and colleagues demonstrate that deficient Btk function selectively impairs dendritic cell cytokine induction in response to viral single-stranded RNAs that activate Toll-like receptor 8 (TLR-8).
The discovery of pathways that may be deficient in patients with susceptibility to infection is a key impetus to the study of human immunology. In 1952, Colonel Ogden Bruton1 first noted an absence of the γ-globulin peak in the serum of a boy with recurrent infections, thereby describing an index case of X-linked agammaglobulinemia (XLA). Recombinant DNA techniques allowed for the identification of the gene defect in XLA patients leading to impaired function of the cytoplasmic tyrosine kinase named Bruton tyrosine kinase (Btk),2 expressed at all stages of B-cell lineage development. Thus, patients with defects in Btk have impaired B-cell production, have agammaglobulinemia, and present with recurrent infections.
Btk is also expressed by non-B cells, including myeloid cells, but its roles and contributions in this context are less clear. The recent characterization of the Toll-like receptor (TLR) family of transmembrane proteins that contribute to microbial recognition is a major breakthrough in our understanding of innate immunity3,4 and represents an opportunity to better define the roles of Btk in innate responses of myeloid cells. In the report by Sochorova and colleagues in this issue of Blood, the authors studied TLR-mediated responses of monocyte-derived dendritic cells (DCs) from patients with XLA compared with adult controls. Although responses to diverse agonists of TLRs 2 through 5 were normal, TNF-α and IL-6 responses to ssRNAs that activate via TLR-8 were markedly impaired. Treatment of normal DCs with the Btk-selective inhibitor LFM-A13 mimicked the phenotype of XLA cells, demonstrating selective impairment in TLR-8–mediated TNF-α and IL-6 production. A member of a subfamily of TLRs expressed in endosomes, TLR-8 serves to recognize viral single-stranded RNAs, such as those of influenza virus, as well as synthetic immunomodulatory imidazoquinoline compounds that mimic purines.5,6
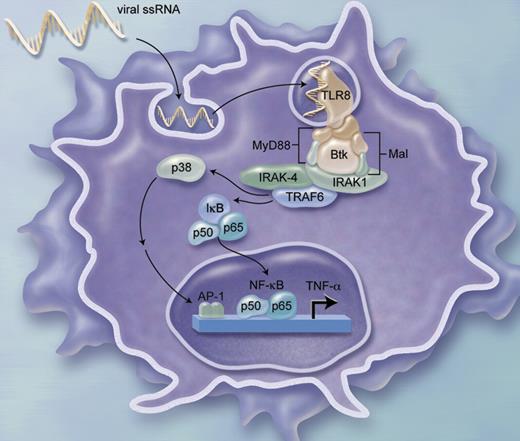
From the standpoint of TLR-mediated cell activation, Sochorova et al have helped us delineate novel and distinct aspects of TLR-8–mediated signaling (see figure). Importantly, Btk has been shown to directly interact with the intracellular domain of TLR-8.7 It has also been recently demonstrated that TLR-8 agonists induce especially robust responses from human monocytes and DCs, including those derived from newborns that generally show impaired responses to many other stimuli.8 Taken as a whole, these studies suggest distinct pathways by which TLR-8 activates cells and position TLR-8 agonists as robust immune adjuvants.
Role of Btk in TLR-8–mediated ssRNA-induced TNF-α production from monocyte-derived DCs. Illustration by A. Y. Chen.
Role of Btk in TLR-8–mediated ssRNA-induced TNF-α production from monocyte-derived DCs. Illustration by A. Y. Chen.
From a clinical perspective, it is notable that patients with XLA have a profound susceptibility to infection with Enteroviruses that contain ssRNAs, including echoviruses, Coxsackie viruses, and polioviruses.9 Thus, the study by Sochorova et al raises the possibility that impaired TLR-8–mediated signaling in DCs of patients with XLA may contribute to their susceptibility to Enteroviruses.
Much remains to be defined with respect to the roles of Btk in TLR signaling, a challenging but worthwhile task that is being pursued in cell culture lines and primary cells. Btk mediates TLR-4–induced phosphorylation of the TLR adaptor molecule Mal in THP-1 cells10 and contributes to TLR-2– and TLR-4–induced TNF-α production in human mononuclear cells.11 Further dissection of these pathways will define the specificity of innate immune pathways, providing further insights into human health and disease.
The author declares no conflicting financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal