Abstract
Blood cell progenitors were scanned for the presence of the coagulation starter protein tissue factor (TF) by immunoelectron microscopy. Thereby, substantial TF expression was observed in the precursor cells of eosinophils. TF levels were lower in basophil precursors and barely detectable in neutrophil progenitors. In peripheral blood immediately processed to avoid activation of the TF gene, mature eosinophils were found to considerably express TF, unique among the granulocyte and monocyte fractions. TF was preferentially located in the specific granules in resting eosinophils. Platelet-activating factor (PAF), and more pronounced, granulocyte-macrophage colony-stimulating factor (GM-CSF) plus PAF, caused translocation of preformed TF to the eosinophil cell membrane. GM-CSF/PAF also increased the TF transcript levels. The activated eosinophils exhibited procoagulant activity that was abrogated by TF inhibition. Targeting the extracellular domain of TF with specific antibodies markedly suppressed the initial phase of the eosinophil passage across the IL-4–activated endothelium. Eosinophil rolling and firm adhesion remained unaffected. This suggests that TF specifically facilitates the early transendothelial migration of the eosinophils. In summary, eosinophils maintain a high TF expression during maturation, providing a main source of preformed TF in blood, which might be relevant for the thrombogenesis promoted by hypereosinophilic conditions.
Introduction
Tissue factor (TF), the crucial starter protein of hemostasis and a major determinant of its pathologic sequelae,1 is basically expressed in the plasma membrane of cells located in the adventitial and medial layers of the vascular wall. TF binds the serine protease factor VIIa with high affinity, thereby enhancing its proteolytic activity by several orders of magnitude, whereby coagulation is initiated. A series of new findings suggests that preformed TF is present in intravascular compartments in humans, at variance with its designation. For a deeper understanding of the start process of intravascular coagulation, it is essential to know where TF is located and how its expression is regulated. Recently, circulating microvesicles (or microparticles) were revealed as source for preexisting TF in blood, which are apparently derived from leukocytes and platelets.2-5 They most likely represent the earlier described TF pool in acellular plasma6-9 because the selective removal of microvesicles strongly decreases the plasma TF contents.3 Microvesicles are rapidly recruited to the site of vascular injury in vivo, where they elicit the coagulation start in a TF-dependent way.10 Moreover, TF has been reported to appear on the cell surface and on microvesicles secreted from activated platelets.11-13 In other work, TF was not detectable on platelets.14 In addition, TF has been proposed to be present in neutrophils (summarized by Nakamura et al15 ), although this has been called into question.16 Notably, the functional competence of TF is not restricted to coagulation. Substantial evidence suggests an eminent role for the protein in angiogenesis,17,18 especially in the induction of tumor capillaries, and in inflammation/sepsis.19 Moreover, TF contributes to cell migration because it supports the reverse migration of monocytes20 and the invasion of metastatic cells into the tissue.21 In the present investigation, we have performed a scan through the bone marrow and the peripheral blood to reveal unidentified sources of preformed TF. Blood processing times were minimized to preclude any induction of TF during the preparation. Thereby, blood eosinophils were found to express TF, which is rapidly exposed by cell-specific stimulation, pointing to a role for the eosinophils in the initiation process of fibrin formation.
Materials and methods
Materials
The PE-labeled monoclonal anti-CD15 antibody was from Biotrend (Cologne, Germany), and the FITC-labeled isotype control antibody as well as Cell-Fix were from Becton Dickinson (Heidelberg, Germany). Isotonic Ficoll was from Pharmacia (Freiburg, Germany). The 2 monoclonal anti–human TF antibodies VD8 and VIC7 used have been characterized previously, whereby their TF specificity has been established.20,22,23 Both antibodies have been evaluated at the Tissue Factor Workshop Panel (Kyoto, 1996; Morrisssey et al23 ). Thereby, their suitability for use in procoagulant assays, enzyme-linked immunosorbent assays (ELISAs), Western blots, flow cytometry, and immunohistochemistry has been confirmed by 5 independent laboratories. Furthermore, the epitope specificity of the anti-TF antibodies has been established,24 showing that VIC7 and VD8 recognize epitopes in the extracellular domain of TF, encoded by exon 5 of the TF gene and in proximity to the N-terminus, respectively. For flow cytometry experiments, VD8 was labeled with FITC. Soluble TF (sTF; 1-214) was kindly provided by V. Magdolen. The chromogenic substrate S2222 and Beriplex P/N 500 were from Chromogenix (Mølndal, Sweden) and Dade-Behring (Marburg, Germany). Recombinant TF was obtained from Ortho Diagnostic Systems (Raritan, NJ). The microbeads conjugated with anti-CD3, anti-CD14, and anti-CD16 antibodies and the positive selection column were from Miltenyi Biotec (Bergisch Gladbach, Germany) and from StemSep (Vancouver, BC, Canada). Hydroxyethyl starch (HES, 6%) was from Fresenius Kabi (Bad Homburg, Germany). GM-CSF (Leucomax150) was provided by Novartis (Basel, Switzerland) and platelet-activating factor (PAF) was purchased from Sigma (Deisenhofen, Germany). Human recombinant IL-4 was from R&D Systems (Minneapolis, MN). Human plasma albumin (HSA) was from Bayer (Toronto, ON, Canada); M199 and Lymphoprep 1077 were from Invitrogen Life Technologies (Carlsbad, CA).
Preparation of eosinophils
Blood from healthy or mildly atopic individuals was drawn into syringes containing sodium citrate (or heparin). The anticoagulated blood was centrifuged at 180g for 15 minutes, and the leukocyte-rich buffy coat was carefully recovered. The buffy coat was mixed with 6% HES and centrifuged at 28g to enable the erythrocyte sedimentation. Alternatively, erythrocyte sedimentation was performed on dextran.25 The granulocyte fractions were separated from the rest of the leukocytes by density sedimentation at 210g for 25 minutes using Ficoll-Paque (density 1.077 g/mL). The fraction containing the peripheral blood mononuclear cells (PBMCs), from which residual erythrocytes were removed by hypotonic lysis on ice, was carefully recovered. The remaining granulocyte pellet was washed in PBS. Thereafter, the granulocytes were incubated with anti-CD16–coupled, and, in some cases (mRNA and cell surface expression levels), additionally with anti-CD3– and anti-CD14–coupled immunomagnetic beads. Then, the eosinophils were isolated by negative selection with the magnetic-activated cell sorting (MACS) cell separation system. The eosinophils were washed once with PBS, and the resulting pellet was taken up in resuspension buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.4 mM NaH2PO4, 1 mM MgCl2, 5 mM glucose, 5 mM HEPES, pH 7.35). The purity of the eosinophil preparations was greater than 94%. Approval for these studies was obtained from the Ethics Committees of the Medical Faculties of the Ludwig-Maximilians-University and the University of Calgary. Informed consent was provided according to the Declaration of Helsinki.
Isolation of platelets and nucleated blood cells
To isolate platelets, venous blood from healthy volunteers drawn into sodium citrate (0.38% final concentration) was centrifuged at 190g for 15 minutes. After supplementation of the upper two thirds of the supernatant (platelet-rich plasma [PRP]) with apyrase (0.2 U/mL) and iloprost (10 ng/mL), the PRP was centrifuged (330g, 10 minutes). The pellet was recovered and resuspended in saline buffer, and the isolated platelets were additionally washed twice. The suspensions of the isolated platelets thus obtained contained less than 0.01% of total leukocytes. Monocytes were isolated by buoyant density centrifugation and subsequent purification with anti-CD14 antibodies, as described previously.3 Subsequently, they were stimulated for 4 hours with LPS (10 ng/mL). For isolation of the peripheral blood lymphocytes (PBLs), the buffy coat was separated on a density gradient using Ficoll, and the peripheral blood mononuclear fraction thus obtained was incubated with anti-CD14–conjugated immunomagnetic beads. The PBLs were subsequently prepared by negative selection using the MACS cell separation system.
Eosinophil accumulation and transmigration
Eosinophil interactions under flow conditions were quantified as described.25 First-passage human umbilical vein endothelial cells (HUVECs) were isolated as previously described 26 and maintained in M199 with 20% human serum. For all experiments, HUVECs were used 2 days after confluence. Briefly, confluent monolayers of endothelial cells were stimulated with 20 ng/mL IL-4. After 24 hours, the incubation buffer was removed, the flow chamber was assembled, and freshly isolated eosinophils (5 × 105/mL) were perfused across endothelial monolayers at a wall shear stress of 2 dyn/cm2. After 4 minutes of perfusion, the inlet line was transferred to Hanks balanced salt solution (HBSS) to prevent the binding of new eosinophils and shear was maintained at 2 dyn/cm2 for an additional 3 minutes. Interacting cells were visualized using either × 200 or × 400 magnification and recorded via a CCD camera for later analysis. Accumulation (rolling plus firmly adherent cells) and transmigration were determined at the specified times by examining the cells in 4 to 6 separate fields, essentially as previously described.26 Briefly, rolling and adherent cells on the surface of the endothelium appear phase-bright, whereas transmigrated cells are flattened and phase-dark. Transmigration is expressed as the number of transmigrated cells divided by the total cells counted. For kinetic experiments, transmigration was assessed every 15 seconds beginning at time 0 and continuing to 5 minutes. Results obtained by inspection of 4 to 6 separate fields were comparable with those gained from inspections of 5 to 10 fields. In some experiments, HUVECs alone or eosinophils plus HUVECs were treated with 10 μg/mL of the anti-TF antibodies VIC7 or VD8, or with sTF (1-214) for 15 minutes prior to perfusion through the flow chamber.
ECP release
Eosinophils were primed with GM-CSF (50 ng/mL; 20 minutes) and subsequently stimulated with PAF (10 μM; 25 minutes) at 37°C. Control eosinophils were kept at 37°C without stimulation. Thereafter, the samples were centrifuged at 1100g for 5 minutes, and the cell-free supernatants were recovered. ECP was detected by fluorescence immunoassay (UniCAP ECP, Pharmacia).
Flow cytometry
Eosinophils (5 × 105) were stimulated at 37°C with PAF (10 μM; 10 minutes) or GM-CSF (50 ng/mL; 20 minutes) and PAF (25 minutes) and immediately thereafter fixed with Cell-Fix according to the manufacturer's instructions. The fixed cells were washed with PBS, blocked with a 50-fold excess of unlabeled IgG for 30 minutes, and washed again. Then, the samples were incubated with 5 μL PE-labeled anti-CD15 and FITC-labeled anti-TF antibody (20 μg/mL) for 30 minutes in the dark. For all samples, an FITC-labeled control antibody (20 μg/mL) was used in parallel. After a further washing step, the eosinophils were resuspended in PBS and analyzed using a Becton Dickinson Coulter EPICS XL-MCL (EXPO2 software).
Electron microscopy of peripheral blood cells
To immediately prevent activation of the blood components, human blood was drawn into anticoagulant plus fixative solution (3% paraformaldehyde, 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2; 50 μL/mL). Following centrifugation, the platelet-poor plasma was removed and the cell pellet was covered with the fixative solution and incubated for 30 minutes (4°C). Then, the upper layer containing the leukocytes (plus platelets) was recovered and cut into small blocks. The washed blocks were suspended in cacodylate buffer (containing 2.3 M sucrose) and plunge-frozen in liquid propane at −180°C (KF80, Reichert-Jung, Vienna, Austria). After dehydration with ethanol under progressive lowering of the temperature, the samples were embedded at −30°C in Lowicryl HM20 (Chemische Werke Lowi, Waldkraiburg, Germany). Ultrathin serial sections of the blood samples fixed on Pioloform-coated Ni-grids were incubated with the primary anti-TF antibody (VD8) for 60 minutes at room temperature. VD8 was previously shown to specifically detect TF in morphologic analyses.23,27,28 After washing, the grids were incubated with a gold-labeled secondary antibody (goat antimouse, 10 nm gold conjugate; Aurion, Wageningen, The Netherlands). The grids were analyzed in series with an EM 109 electron microscope (Carl Zeiss, Oberkochen, Germany). Images were captured by FotoLook software version 2.09.6; for image processing, Adobe Photoshop version 5.01 was used (Adobe Systems, San Jose, CA). In control experiments, the grids were blocked with fetal calf serum (10% in PBS) before incubation with the primary antibody. Thereby, the cellular TF labeling was comparable as without serum. As a further control, the samples were incubated with the gold-labeled secondary antibody alone, whereby no labeling was observed.
Preparation of bone marrow cells for electron microscopy
Human bone marrow obtained from the sternum of healthy volunteers under a protocol approved by the local ethics committee (Medical Faculty of the University of the Saarland) was fixed with the fixative solution (see “Electron microscopy of peripheral blood cells”) for 30 minutes at 4°C. After washing in cacodylate buffer, blocks of the bone marrow cells were resuspended in cacodylate buffer containing 2.3 M sucrose and plunge-frozen in liquid propane at −180°C (KF80, Reichert-Jung), and subsequently treated as described for peripheral blood cells.
TF mRNA analysis
Total RNA was isolated from the different blood cells using TRIzol reagent (Gibco-BRL, Karlsruhe, Germany), and reverse-transcribed into cDNA as follows: 1 μg RNA was mixed with 100 ng oligo(dT)15 and incubated for 5 minutes at 65°C; 1 mM dNTPs, 60 mM KCL, 15 mM Tris-HCl (pH 8.4), 3 mM MgCl2, 0.3% Tween-20, 10 mM β-mercaptoethanol, 10 U RNasin (Promega, Mannheim, Germany), and 100 U Mo-MLV RT (Invitrogen) were added, and the mix was incubated for 55 min at 37°C, followed by an incubation for 5 min at 95°C. Reverse transcription-polymerase chain reaction (RT-PCR) was performed with 20 ng cDNA and Taq DNA polymerase (Promega) on a MJ research machine PTC 200 (BioRad, Hercules, CA). PCR primers for the full-length TF were: 5′-CCTGGAGACAAACCTCGGACAGCC-3′ forward, 5′-CCAGCTCTGCC-CCACTCCTGC-3′ reverse. Real-time PCR was carried out with the LightCycler Instrument (Roche, Mannheim, Germany) using the DNA-binding dye SYBR Green I for detection of PCR products. The expression levels of the target genes were quantified by the ratio of TF mRNA to aldolase mRNA.
TF ELISA
Eosinophil TF contents were assessed by a double-sandwich ELISA, which uses 2 different antibodies, recognizing N-terminal and membrane-proximal epitopes on the extracellular domain of TF.22,23 Briefly, cell pellets were frozen and thawed and were diluted with 0.05 M Tris/HCl, 0.1 M NaCl, 1% Triton X-100, 10 mM EDTA, pH 7.6, and incubated for 1 hour at 37°C. Microtiter plates (Nunc, Roskilde, Denmark) were coated with purified anti-TF antibody VIC7 at 4°C overnight. After blocking with buffer containing 2% calf serum, cell lysates were added. After 3 washing steps, peroxidase-conjugated anti-TF antibody IIID8 was added and incubated for 2 hours. TMB (Kirkegaard and Perry Lab, Gaithersburg, MD) was used as substrate. Absorbance was converted into fmol/mg cell protein by reference to a standard curve using recombinant TF (American Diagnostica, Greenwich, CT).
Procoagulant activity
The isolated eosinophils (resting or activated by GM-CSF [20 minutes] plus PAF [25 minutes]; 5 × 105) were incubated with a coagulation factor concentrate containing factors VII, IX, X, and II (Beriplex P/N 500; ZLB Behring, Marburg, Germany). The chromogenic substrate S2222 was added, and the formation of factor Xa was monitored as previously described.3
Statistics
The determinations were compared by one-way or repeated measurements ANOVA. Mean values are given plus or minus SEM. Differences of P below .05 were considered to be significant.
Results
Detection of stored TF in eosinophil precursors
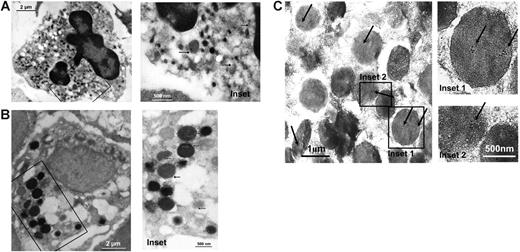
To identify precursors of nucleated blood cells capable of expressing TF, thin sections of human bone marrow preparations were screened for the presence of the protein by immunoelectron microscopy. We focused on the precursor cells of granulocyte subfractions, because TF has already been detected in monocyte progenitor cells.29 In neutrophil metamyelocytes, recognized by their characteristic morphology (Figure 1A), TF was detected at very low levels in intracellular locations, especially in the primary granules (Figure 1A). TF could not be found in neutrophil myelocytes (not shown). Moreover, TF staining was consistently noted for the intracellular granules of basophil myelocytes (Figure 1B). Furthermore, eosinophil myelocytes exhibited noticeable TF expression (Figure 1C), exceeding the one observed for the progenitor cells of the other granulocyte subfractions. The protein was in particular expressed within the coreless granules, specific for the eosinophil precursor cells.
TF storage in eosinophil myelocytes. In thin sections of human bone marrow, TF is found to be expressed in intracellular compartments of leukocyte progenitor cells. The hematopoietic cells were visualized by immunoelectron microscopy, using a monoclonal anti-TF antibody and a secondary antibody coupled with gold particles. (A) Neutrophil metamyelocytes (recognized by their characteristic nuclear morphology and the dense heterochromatin) and (B) basophil myelocytes (identified by the morphology and size of their intracellular granules) contain TF in different types of granules. (C) In the eosinophil myelocytes, high amounts of TF are expressed in the coreless granules (identified by the presence of arginine-rich crystals in intracellular granules). The marked areas in the left-hand pictures are magnified on the right-hand side. Arrows indicate specific TF labeling.
TF storage in eosinophil myelocytes. In thin sections of human bone marrow, TF is found to be expressed in intracellular compartments of leukocyte progenitor cells. The hematopoietic cells were visualized by immunoelectron microscopy, using a monoclonal anti-TF antibody and a secondary antibody coupled with gold particles. (A) Neutrophil metamyelocytes (recognized by their characteristic nuclear morphology and the dense heterochromatin) and (B) basophil myelocytes (identified by the morphology and size of their intracellular granules) contain TF in different types of granules. (C) In the eosinophil myelocytes, high amounts of TF are expressed in the coreless granules (identified by the presence of arginine-rich crystals in intracellular granules). The marked areas in the left-hand pictures are magnified on the right-hand side. Arrows indicate specific TF labeling.
Selective expression of preformed TF in mature eosinophils
In a next step, we examined whether the mature eosinophils were endowed with TF. Because the TF gene transcription might be triggered during the blood cell isolation, causing rapid TF synthesis, we used a preparation procedure minimizing the activation of the blood cells. To this purpose, we added fixative solution directly to the freshly drawn blood, thereby excluding inadvertent transcription and translation of the TF gene. TF was consistently observed in the specific granules equipped with arginine-rich crystals, typical of eosinophils (Figure 2A-C). TF was mostly located within these granules, whereas a smaller part was located in the cytoplasm. Within the granules, TF appeared to be associated with the membrane and with the internal matrix. For comparison, TF expression was evaluated in mature forms of granulocytes and in monocytes. TF staining in mature neutrophils (Figure 2D; concordant with Leon et al13 ), basophils, and resting monocytes was barely detectable (data not shown), suggesting that TF is specifically silenced/removed from these cells during maturation. The amount of TF antigen associated with freshly isolated neutrophils, the quantitatively major CD15+ blood cell, was below the detection limit (not shown). In summary, preformed TF is stored in intracellular compartments of the eosinophils under resting conditions.
Detection of TF expression in peripheral blood eosinophils. Immediately processed whole blood was scanned to identify leukocytic sources of TF. In mature (unstimulated) eosinophils (A), the protein is predominantly stored in the specific granules (arrows in the insets in panels B and C), low amounts being present on membranes of other cell organelles in the cytoplasm. (D) TF is practically absent from mature neutrophils. N indicates nucleus.
Detection of TF expression in peripheral blood eosinophils. Immediately processed whole blood was scanned to identify leukocytic sources of TF. In mature (unstimulated) eosinophils (A), the protein is predominantly stored in the specific granules (arrows in the insets in panels B and C), low amounts being present on membranes of other cell organelles in the cytoplasm. (D) TF is practically absent from mature neutrophils. N indicates nucleus.
Augmented TF exposure in response to eosinophil activation
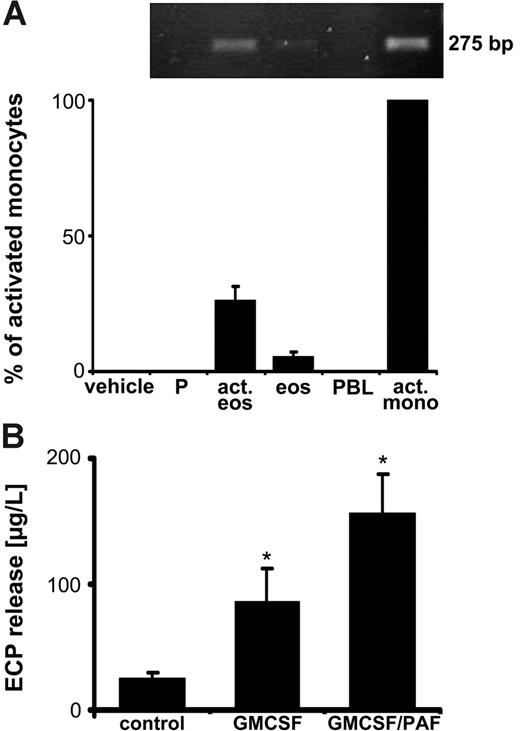
Next, a primer pair specifically detecting the full-length form TF was used to evaluate the presence of TF mRNA in the isolated eosinophils. Small amounts of a transcript of the expected size were indeed amplified, migrating at the same height as the positive control (LPS-stimulated monocytes; Figure 3A). In resting platelets and PBLs, no transcript for the full-length TF was detected. To explore the potential regulation of the eosinophil TF gene by cell-specific stimulation, we primed the leukocytes with GM-CSF, followed by activation with PAF. This resulted in the shape change indicative of eosinophil activation (not shown). Moreover, following the 2-step activation procedure, substantial release of the eosinophil cationic protein (ECP) was observed (Figure 3B), a cell-specific protein stored in high amounts in the specific granules. Although the ECP release was already evident on activation with GM-CSF, it was further enhanced by PAF (Figure 3B). Under the latter activation conditions, the quantity of the TF transcript was elevated (Figure 3A), amounting to 25% of the one found in the LPS-stimulated monocytes. Thus, stimulation of eosinophils augments their TF transcript levels.
Strong eosinophil activation enhances TF mRNA. (A) Full-length TF mRNA levels are increased in response to eosinophil stimulation. Sequential activation of isolated eosinophils (act eos; 5 × 105) with GM-CSF (50 ng/mL; 20 minutes), followed by PAF (10 μM; 25 minutes), increases the mRNA expression for TF (top panel, conventional RT-PCR; bottom panel, real-time PCR). Comparisons with the mRNA levels in LPS-stimulated monocytes (act mono), resting eosinophils (eos), platelets (P), and PBLs. The expression levels were calculated as the ratio of TF mRNA to aldolase mRNA. Means ± SEM, n = 3. (B) GM-CSF/PAF causes ECP release. Degranulation of specific granules by GM-CSF (50 ng/mL; 20 minutes), and by GM-CSF plus PAF (10 μM; 25 minutes), as verified by ECP secretion. Means ± SEM, n = 4. *P < .05 (versus control).
Strong eosinophil activation enhances TF mRNA. (A) Full-length TF mRNA levels are increased in response to eosinophil stimulation. Sequential activation of isolated eosinophils (act eos; 5 × 105) with GM-CSF (50 ng/mL; 20 minutes), followed by PAF (10 μM; 25 minutes), increases the mRNA expression for TF (top panel, conventional RT-PCR; bottom panel, real-time PCR). Comparisons with the mRNA levels in LPS-stimulated monocytes (act mono), resting eosinophils (eos), platelets (P), and PBLs. The expression levels were calculated as the ratio of TF mRNA to aldolase mRNA. Means ± SEM, n = 3. (B) GM-CSF/PAF causes ECP release. Degranulation of specific granules by GM-CSF (50 ng/mL; 20 minutes), and by GM-CSF plus PAF (10 μM; 25 minutes), as verified by ECP secretion. Means ± SEM, n = 4. *P < .05 (versus control).
In parallel, we analyzed whether the eosinophil release reaction, which involves fusion of the membrane of the specific granules with the plasma membrane, would result in the presentation of TF on the cell surface. Under resting conditions, low cell surface expression of TF was noted (Figure 4A), as determined by flow cytometry. However, after a 10-minute incubation with PAF, the TF exposure was clearly enhanced (Figure 4A). This was further increased after stimulation with GM-CSF/PAF for 45 minutes. In the presence of the control antibody, no surface labeling was observed, indicating the TF specificity of the signal. Inhibiting translation by cycloheximide slightly lowered the TF surface presentation in cells stimulated with GM-CSF/PAF (Figure 4A). Suppression of transcription with actinomycin D barely affected the TF exposure. Next, we analyzed the TF protein contents of the eosinophils by a double-sandwich ELISA that selectively recognizes the full-length form of TF. The TF contents of the freshly isolated eosinophils amounted to 4.4 ± 1.4 fmol/mg cell protein (means ± SEM of 5 different donors), thus confirming the presence of preformed TF. Activation with GM-CSF/PAF tended to increase the TF contents to 6.4 ± 1.5 fmol/mg cell protein. The latter value corresponds to about 1200 total TF molecules/cell. Together, the findings show that the surface TF originates mainly from presynthesized TF.
Surface TF exposure in response to eosinophil stimulation. (A) TF exposure elicited by GM-CSF/PAF. Left-hand panel: TF presentation on the surface of eosinophils was determined by flow cytometry using an FITC-labeled anti-TF antibody (▪). In parallel, the FITC-labeled control antibody was used (□). Activation of the eosinophils for the indicated time periods was performed by PAF alone (10 μM) or by GM-CSF (50 ng/mL) plus PAF. Means ± SEM, n = 4. *P < .05 (versus control). Right-hand panel: Contribution of de novo synthesized protein to TF exposure. Isolated eosinophils of 3 different donors were preincubated with cycloheximide or actinomycin D (both at 10 μg/mL; 30 minutes) and then stimulated for 45 minutes with GM-CSF/PAF. No statistically significant differences were seen between the mean values. (B) TF exposure in degranulating eosinophils as detected by immunoelectron microscopy. (C) Procoagulant activity of isolated eosinophils. Eosinophils activated by GM-CSF/PAF were lysed by freezing and thawing as described previously.30 In parallel, lysed monocytes previously activated with LPS were analyzed. The anti-TF antibody and isotype control IgG were present at 10 μg/mL. No cells indicates presence of coagulation factor concentrate alone. Means ± SEM, n = 4. *P < .05 (versus control of respective cell).
Surface TF exposure in response to eosinophil stimulation. (A) TF exposure elicited by GM-CSF/PAF. Left-hand panel: TF presentation on the surface of eosinophils was determined by flow cytometry using an FITC-labeled anti-TF antibody (▪). In parallel, the FITC-labeled control antibody was used (□). Activation of the eosinophils for the indicated time periods was performed by PAF alone (10 μM) or by GM-CSF (50 ng/mL) plus PAF. Means ± SEM, n = 4. *P < .05 (versus control). Right-hand panel: Contribution of de novo synthesized protein to TF exposure. Isolated eosinophils of 3 different donors were preincubated with cycloheximide or actinomycin D (both at 10 μg/mL; 30 minutes) and then stimulated for 45 minutes with GM-CSF/PAF. No statistically significant differences were seen between the mean values. (B) TF exposure in degranulating eosinophils as detected by immunoelectron microscopy. (C) Procoagulant activity of isolated eosinophils. Eosinophils activated by GM-CSF/PAF were lysed by freezing and thawing as described previously.30 In parallel, lysed monocytes previously activated with LPS were analyzed. The anti-TF antibody and isotype control IgG were present at 10 μg/mL. No cells indicates presence of coagulation factor concentrate alone. Means ± SEM, n = 4. *P < .05 (versus control of respective cell).
Cell surface presentation of the protein in response to eosinophil activation and degranulation was confirmed by immunoelectron microscopy (Figure 4B). Then, the procoagulant activity of the eosinophils was measured. The ability of the lysed cells to activate factor X was nearly completely blocked by the anti-TF antibody (Figure 4C). The isotype-matched control antibody did not affect the factor Xa formation, indicating the TF dependence of the procoagulant activity. The procoagulant activity of the activated eosinophils amounted to one third of the one of LPS-stimulated monocytes (Figure 4C), the latter being almost entirely prevented by the anti-TF antibody. Thus, degranulation of eosinophils causes translocation of intracellular TF to the eosinophil surface.
TF inhibition obstructs early transmigration of eosinophils across the endothelium
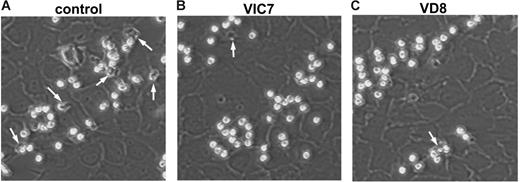
The participation of eosinophils in parasite elimination and adaptive immunity requires their emigration into the tissue. Moreover, TF has been implicated in the transendothelial passage of monocytes.20 We therefore examined the role of TF on eosinophil binding to endothelial cells under physiologic flow conditions. Endothelial cells were challenged for 24 hours with IL-4 to induce the expression of VCAM-1 and P-selectin. The stimulated endothelial cells, which themselves expressed TF (as indicated by Western blots; data not shown), were placed into a parallel plate flow chamber, and freshly isolated eosinophils were perfused across the monolayer for 4 minutes at 2 dyn/cm2. This led to accumulation and transmigration of the blood cells (Figure 5A). Virtually all eosinophils were firmly adherent at this time point. Pretreatment of endothelial cells and eosinophils with the anti-TF antibodies VIC7 and VD8 had no significant effect on the accumulation of the eosinophils (Figure 5B-C), or on the percentage of cells that were firmly adherent (data not shown). Quantification of the results supported the observation that the accumulation of eosinophils under flow conditions was not altered by the anti-TF antibodies (Figure 6A).
Differential effects of anti-TF antibodies on eosinophil accumulation and transmigration under flow conditions. Endothelial cells treated for 24 hours with 20 ng/mL IL-4 (20 ng/mL) were assembled into the flow chamber, and freshly isolated eosinophils (5 × 105/mL) were perfused over the monolayer at 2 dyn/cm2 for 4 minutes. In some experiments, the endothelial cells and eosinophils were pretreated for 15 minutes with VIC7 or VD8 (10 μg/mL). Representative images of eosinophils bound to IL-4–treated endothelial cells in (A) control, (B) VIC7, and (C) VD8 are shown at ×200 magnification. Transmigrated cells are indicated with white arrows. Data are representative of at least 3 independent experiments.
Differential effects of anti-TF antibodies on eosinophil accumulation and transmigration under flow conditions. Endothelial cells treated for 24 hours with 20 ng/mL IL-4 (20 ng/mL) were assembled into the flow chamber, and freshly isolated eosinophils (5 × 105/mL) were perfused over the monolayer at 2 dyn/cm2 for 4 minutes. In some experiments, the endothelial cells and eosinophils were pretreated for 15 minutes with VIC7 or VD8 (10 μg/mL). Representative images of eosinophils bound to IL-4–treated endothelial cells in (A) control, (B) VIC7, and (C) VD8 are shown at ×200 magnification. Transmigrated cells are indicated with white arrows. Data are representative of at least 3 independent experiments.
Anti-TF antibodies suppress early eosinophil transmigration under flow conditions. Endothelial cells were treated with 20 ng/mL IL-4 for 24 hours. Stimulated endothelial cells were assembled into the flow chamber and freshly isolated eosinophils (5 × 105/mL) were perfused over the monolayer at 2 dyn/cm2. The endothelial cells and eosinophils were pretreated for 15 minutes with buffer alone, 10 μg/mL VIC7 or VD8. (A) Total accumulated cells were determined between 4 and 5 minutes as described in “Materials and methods.” Over 90% of the accumulating cells were firmly adherent. No statistically significant differences were observed between the mean values. (B) Transmigration was assessed from multiple fields between 3 and 4 minutes or (C) between 6 and 7 minutes. (D) Transmigration kinetics as assessed every 15 seconds between 2 and 5 minutes. In some cases, the endothelial cells were selectively precoated with VD8 (VD8(EC)). Data represent means ± SEM of at least 3 experiments. *P < .05 (versus control).
Anti-TF antibodies suppress early eosinophil transmigration under flow conditions. Endothelial cells were treated with 20 ng/mL IL-4 for 24 hours. Stimulated endothelial cells were assembled into the flow chamber and freshly isolated eosinophils (5 × 105/mL) were perfused over the monolayer at 2 dyn/cm2. The endothelial cells and eosinophils were pretreated for 15 minutes with buffer alone, 10 μg/mL VIC7 or VD8. (A) Total accumulated cells were determined between 4 and 5 minutes as described in “Materials and methods.” Over 90% of the accumulating cells were firmly adherent. No statistically significant differences were observed between the mean values. (B) Transmigration was assessed from multiple fields between 3 and 4 minutes or (C) between 6 and 7 minutes. (D) Transmigration kinetics as assessed every 15 seconds between 2 and 5 minutes. In some cases, the endothelial cells were selectively precoated with VD8 (VD8(EC)). Data represent means ± SEM of at least 3 experiments. *P < .05 (versus control).
Once bound to IL-4–stimulated endothelial cells, eosinophils rapidly transmigrate. We found that treatment with anti-TF antibodies blocked eosinophil transmigration. Quantification of transmigration during the initial 3- to 4-minute interval showed that VIC7 and VD8 profoundly decreased the transendothelial passage of the eosinophils (Figure 6B). Under the same conditions, an isotype control antibody did not alter the transmigration (not shown). When later time points were analyzed (between 6 and 7 minutes), the anti-TF antibodies still had a significant influence on transmigration; however, the degree of inhibition was less pronounced (Figure 6C). During the same time period and under identical experimental conditions, sTF lowered the eosinophil transmigration to the same extent as the anti-TF antibodies (by 26.8%; mean value of 2 experiments). We next examined the kinetics of transmigration in the presence of the anti-TF antibodies. In these experiments, the transendothelial passage was assessed every 15 seconds between 1 and 5 minutes. Control eosinophils began to migrate at 3 minutes, with transmigration reaching 14% to 23% between 3 and 5 minutes (Figure 6D). After selective precoating of the endothelial cells with VD8, the early translocation of the eosinophils was unaffected (Figure 6D). In contrast, when VD8 (and VIC7) were added to the whole system, the transmigration between 3 and 5 minutes was strongly diminished (Figure 6D), suggesting that the antibodies specifically target the eosinophil TF. Together, the data indicate that TF regulates the passage of eosinophils across the endothelial layer, its role being more pronounced during initial transmigration.
Discussion
We document that blood eosinophils store the coagulation initiator TF, which is mainly embodied within their specific granules. Activation by PAF results in rapid TF exposure on the eosinophil cell membrane, which is enforced when the cells are first primed with GM-CSF, a stimulus accompanied by augmented expression of TF transcripts. Within the bone marrow, TF is contained in appreciable quantities in the coreless granules of the eosinophil myelocytes. However, TF is also detected in basophil myelocytes, and previous work suggests its expression in monocyte precursor cells.29 Rapid processing of the peripheral blood before the fixation for the immunoelectron microscopy analyses ensured that no mature protein was generated via induction of TF synthesis, a major concern during the preparation of blood-associated leukocytes.
When scanning the thus prepared blood for TF-positive components, the mature (resting) monocytes were found to exhibit very low expression levels of the protein, in particular no TF being detectable on the cell surface. By consequence, TF must be inactivated or removed during the development from the monoblast/promonocyte precursor stage to the mature cell type. Similarly, the genesis of blood basophils is apparently accompanied by a loss of TF. Moreover, in mature neutrophils and their progenitor cells, TF was barely detectable. The plasma membrane proteome of the eosinophils considerably overlaps with the one of the neutrophils, as exemplified by the presence of PSGL-1 and CD15. Our findings are of consequence for the ongoing debate on the association of TF with neutrophils,15,16 since eosinophils can be included in neutrophil preparations. Thus, future studies on the presence of TF in granulocyte fractions will have to carefully differentiate between the diverse cell types. Cells are capable of shedding substantial amounts of TF via microvesicles,2,5 which could account for the decrease in TF expression in the course of cell maturation. Vesicular loss of cell membrane proteins during blood cell development in vivo has indeed been previously proposed for erythroid complement receptor 1 and decay accelerating factor.31 Alternatively, TF gene transcription could be suppressed during the leukocyte development via differentiation-specific transcription factors, as known for several genes.32
Eosinophils thus selectively maintain a high TF expression during the maturation process. Consequently, part of the protein stored in mature eosinophils is likely to represent presynthesized TF derived from eosinophil precursor cells. Another portion might originate from uptake of TF, such as via microparticle transfer,33 a mechanism proposed to be of relevance for the acquisition of TF by granulocytes in mice in vivo.34 The contribution of de novo synthesized TF to TF exposure appeared to be low. Thus, TF exposed in eosinophils stimulated with GM-CSF/PAF is almost entirely derived from preformed protein.
Our observations indicate TF as one of the critical mediators of the initial eosinophil migration across the activated endothelium. We find that 2 different anti-TF antibodies virtually abrogate the early phase of the eosinophil passage through monolayers of IL-4–stimulated endothelial cells. In this model system, eosinophils tether and roll on P-selectin and VCAM-1 expressed on the endothelium, the interactions being mediated by eosinophil-based PSGL-1 and α4β1. Once bound, endothelial eotaxin-3 rapidly activates eosinophils, resulting in shape change and subsequent transmigration. The anti-TF antibodies could neutralize the migratory capacity of the leukocytes by targeting TF present on the eosinophil surface. We indeed observe that the surface expression of TF is rapidly augmented by eosinophil stimulation. Alternatively (or additionally), the antibodies might interfere with the endothelial TF, which could interact with potential targets on eosinophils, including α4β1, based on the ability of TF to modulate the cell migration supported by β1 integrins.35 Nonetheless, the rolling and tethering of the eosinophils are apparently not controlled by TF, because these parameters were unaffected by TF inhibition. Future studies will dissect the contributing roles of the eosinophil or endothelial cell TF during eosinophil transmigration.
The ability of the resting eosinophils to store TF and to rapidly translocate it to the cell membrane during activation suggests a pronounced functional relevance for the protein in this leukocyte subtype. In vivo, the eosinophil TF might acquire its full functionality following the leukocyte emigration from the bloodstream. Within the tissue, it could foster fibrin formation, which protects against hemorrhage caused by parasite infection,36 a major chemotactic stimulus for eosinophils. Furthermore, given the major participation of the eosinophils in the pathogenesis of allergic diseases, the eosinophil-based TF could be implicated in the hyperresponsiveness characteristic of asthma, which is facilitated by coagulation components, such as thrombin.37,38 Moreover, it is anticipated that the eosinophil TF might contribute to the multiple prothrombotic manifestations of the hypereosinophilic syndrome, which include cardiac thrombosis,39 deep vein thrombosis,40 thrombotic microangiopathies,41 and others. This is supported by the presence of activated eosinophils in the vicinity of thrombi typical of the hypereosinophilic syndrome,39 which could directly foster thrombogenesis via cell surface presentation of TF.
Authorship
Contribution: C.M., S.L.C., D.M., K.B., S.A., and P.L. performed the research. E.M. performed the research and analyzed part of the results. K.D.P. designed, performed, and analyzed the research. B.E. designed and analyzed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernd Engelmann, Institut für Klinische Chemie, Ludwig-Maximilians-Universität, Marchioninistr. 15, 81377 München, Germany; e-mail: bernd.engelmann@med.uni-muenchen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (B.E.). C.M., D.M., and K.B. have been participants of the graduate programs Molekulare Medizin (Medical Faculty, Ludwig-Maximilians-Universität) and DFG-Graduiertenkolleg Vaskuläre Biologie in der Medizin. We thank Matthias Kotzsch and Matthias Kramer for the ELISA measurements and the determinations of the ECP release. We are also grateful to Thomas Luther for helpful suggestions.
The current address for K.B. is Molecular Cardiovascular Research, University Hospital, Aachen, Germany.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal