Abstract
The identification and characterization of mouse basophils have historically been hampered by the extreme rarity of this cell type. Virtually no photomicrographs of hematologically stained (eg, Wright-Giemsa) examples of mouse basophils exist in the literature. However, 4 recent studies in the past 2 years have used flow cytometry and a defined set of cell-surface markers to identify and subsequently isolate mouse “basophils,” including the publication of stained cytospin preparations of these cells. Surprisingly, a reevaluation of the data from all 4 of the studies revealed several issues of concern that suggest that the cells under study are not necessarily basophils. Nonetheless, we propose that these studies do provide the foundation for a reevaluation of the defining characteristics of a basophil and/or provide support for the provocative conclusion that a new previously overlooked leukocyte subtype has been identified. The purpose of this commentary is to revisit these previously published studies, highlight the relevant issues, and provide a different perspective in the hope of developing a consensus within the research community as to the true identity of the “basophils” described in these studies.
Introduction
The roles of the different granulocyte subtypes in allergy and asthma have been the subject of continued study and debate. Reports from both the clinical literature1 and studies of mouse models2-5 have focused particular attention on basophils as potentially critical cells in the development of inflammatory diseases such as asthma. The ability to identify unambiguously human basophils, and subsequently to isolate and characterize these granulocytes, has uniquely enabled physician-scientists to develop and test hypotheses linking these cells with pathologic changes occurring in patients. Unfortunately, similar advantages have been by and large out of the reach of investigators using mouse models of disease. Unlike humans, mouse basophils are exceptionally rare6 and, until recently, only a single photomicrographic example of these leukocytes was reported in the literature.7 As a consequence, electron microscopic identification has generally been substituted as a criterion for identification of basophils in the mouse (eg, see Dvorak8 ). However, during the past 2 years, 4 studies using mouse models2-5 used flow cytometry and a defined set of cell-surface markers to identify mouse “basophils” and to provide photomicroscopic evidence of their appearance following hematologic staining (eg, using DiffQuik ‘Fisher Scientific, Pittsburgh, PA’). The provocative character and clinical relevance of the conclusions in those studies are significant and warrant careful reexamination of the data presented, particularly in regard to the identification of the leukocytes under study as “basophils.” This is especially true given the interdependence and self-fulfilling character of those reports. That is, the second of the studies published3 cites the first study5 to confirm the identification of the leukocytes under study as “basophils” and the third4 and fourth2 studies, in turn, cite the previous two as support for their identity.
Immunology versus hematology
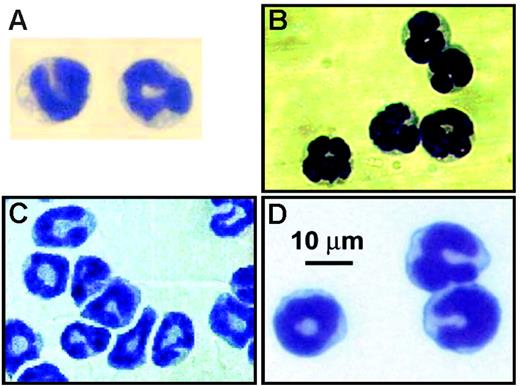
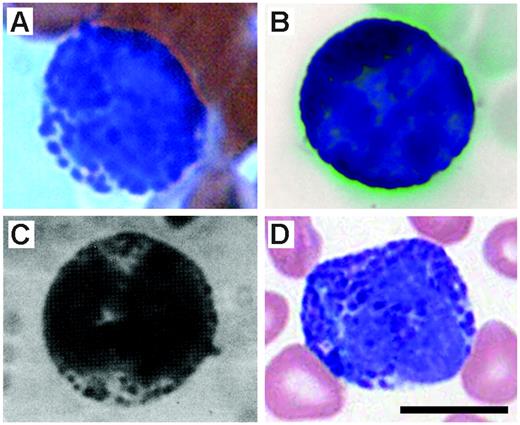
The studies by Voehringer et al,5 Min et al,3 and Gessner et al4 each assessed the cellular source of IL-4 expression in naive mice as well as animals following infection with the intestinal nematode Nippostrongylus brasiliensis using unique GFP “knock-in” mice. In contrast, Mukai et al2 examined the role of “basophils” in the development of IgE-mediated chronic allergic inflammation. Despite the use of different models (ie, parasite-infected animals versus noninfected animals) and strains of mice (BALB/cJ versus C57BL/6J), those studies share 3 important commonalities. (1) Through the use of flow cytometry and antibodies specific for unique cell-surface markers, the investigators describe cells with a surface immunophenotype that they characterized as representing a “basophil” (ie, FSClo/SSClo, Thy-1.2+, CD69+, CD49b(DX-5)+, FcϵRI+, 2B4+, CD11bdull, CD24−, CD19−, CD80−, CD14−, CD23−, Ly49C−, CD122−, CD11c−, Gr-1−, α4- and β7-integrin−, c-kit−, NK1.1−, CD3−, B220−, γδTCR−, αβTCR−). The basis for this definition initially arose from studies of human leukocytes (eg, see Falcone et al9 and Kinet10 ) and has continued to evolve in the mouse (eg, see Lantz et al11 and Seder et al12 ). Ultimately, the definition is fundamentally simplistic: granulocytes with lower than expected scatter characteristics in flow cytometry that are FcϵRI+, CD49b(DX-5)+, and negative for a host of lineage markers that rule out other leukocyte subtypes. (2) The “basophils” in those studies are relatively prevalent leukocytes. In 3 of the studies,2-4 the investigators noted that the identified leukocytes were approximately 1% of nucleated marrow cells, and in the fourth study5 the identified leukocytes comprised more than 2% of the total IL-4–expressing cells accumulating in the lungs of mice following N brasiliensis infection. (3) Each of the studies used cell sorting followed by preparation of cytospins and hematologic staining to confirm the identity of the leukocytes as “basophils.” Figure 1 reproduces the photomicrographs from each of those studies as they appeared in the original publications. The stained cells in each panel display identical morphologies: the nuclei are polymorphic, and the cytoplasmic regions are slightly basophilic and remarkably devoid of any granulation (ie, stained as well as unstained granules). Herein, is the problem: these are neither the morphology nor the staining characteristics of basophils! Panels A and B of Figure 2 contain representative photomicrographs of basophils we have collected from Wright-stained blood films of mice. These cells display the characteristic features associated with basophils: leukocytes similar in size with other blood granulocytes (ie, eosinophils and neutrophils) that, however, contain intensely metachromatic-stained granules that are asymmetrically distributed throughout the cytoplasm and a lobulate nucleus that has comparatively weak staining chromatin.13 Interestingly, the cited studies suggest that the leukocytes that were identified by flow cytometry have the correct hematologic morphology through a series of references that ultimately lead to the single study in the literature that had previously presented a photomicrograph (unfortunately in black and white) of a mouse basophil.7 For example, Mukai et al2 references Seder et al,12 which, in turn, ultimately leads to Urbina et al.7 This singular photomicrograph is reproduced here and is shown in Figure 2C. The hematologic staining character of this leukocyte is remarkably similar to the other representative mouse basophils presented in Figure 2, all of which share the hallmark morphologies that define a basophil. In particular, Urbina et al7 note in their 1981 study, “The mouse basophils differ from those of other species in that they have relatively few, loosely packed granules of unequal size. Still, these granules appear intensely azurophil and characteristically metachromatic in the blood smears.” Surprisingly, the “basophils” identified in the 4 cited studies under discussion (Figure 1) bear little resemblance to any of the representative mouse basophils in Figure 2, including the photograph from Urbina et al.7 The photomicrographs of Figure 2 also include a comparison of mouse basophils with a human basophil from a peripheral blood film (Figure 2D). This comparison clearly shows the commonality of this staining pattern between species and the evolutionary conservation of this leukocyte.
Photomicrographs of leukocytes characterized as basophils in 4 recently published studies. (A) FcϵRI+ IL-4–expressing FSClo/SSClo cells recovered from the lungs of N brasiliensis–infected mice (BALB/c) stained with Wright-Giemsa (from Voehringer et al5(Fig 1B); reprinted with permission from Elsevier. (B) IL-4–expressing DX5 (CD49b)+ non-CD4+ T cells from the liver of N brasiliensis–infected mice (BALB/c) stained with Wright-Giemsa (from Min et al3(Fig 3B); reproduced from The Journal of Experimental Medicine, 2004, 200:507-517, by copyright permission of The Rockefeller University Press). (C) FcϵRI+ IL-4–expressing CCR3− cells recovered from the blood of naive mice (BALB/c) stained with Wright-Giemsa (from Gessner et al4(Fig 3C); copyright 2005, American Association of Immunologists, Inc). (D) FcϵRI+/DX5+ bone marrow cells recovered from normal C57BL/6J mice stained with Giemsa (from Mukai et al2(Fig 5C); copyright 2005, reprinted with permission from Elsevier.)
Photomicrographs of leukocytes characterized as basophils in 4 recently published studies. (A) FcϵRI+ IL-4–expressing FSClo/SSClo cells recovered from the lungs of N brasiliensis–infected mice (BALB/c) stained with Wright-Giemsa (from Voehringer et al5(Fig 1B); reprinted with permission from Elsevier. (B) IL-4–expressing DX5 (CD49b)+ non-CD4+ T cells from the liver of N brasiliensis–infected mice (BALB/c) stained with Wright-Giemsa (from Min et al3(Fig 3B); reproduced from The Journal of Experimental Medicine, 2004, 200:507-517, by copyright permission of The Rockefeller University Press). (C) FcϵRI+ IL-4–expressing CCR3− cells recovered from the blood of naive mice (BALB/c) stained with Wright-Giemsa (from Gessner et al4(Fig 3C); copyright 2005, American Association of Immunologists, Inc). (D) FcϵRI+/DX5+ bone marrow cells recovered from normal C57BL/6J mice stained with Giemsa (from Mukai et al2(Fig 5C); copyright 2005, reprinted with permission from Elsevier.)
Photomicrographs of peripheral blood leukocytes displaying the classical staining and morphology of basophils. (A) A basophil from a Wright-Leishman–stained mouse (strain unknown) peripheral blood film. (B) Wright-Giemsa–stained basophil from a peripheral blood film of a mouse on a C57BL/6J background14 (Fig S2C) (originally published as Supporting On-line Material in Science, 2004;305:1773-1776). (C) The single previous photomicrograph of a mouse basophil appearing in a study by Urbina et al.7(Fig 1) (Reprinted with permission from S. Karger AG, Basel.) (D) A human basophil from a Wright-Giemsa–stained peripheral blood film. Scale bar = 10 μm. The photographs were collected using a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) (Plan-NeoFluar 63×/1.25 mm) and a AxioCam MRc5 digital camera (Carl Zeiss). Adobe Photoshop (San Diego, CA) was used to assemble the figure; however, no enhancements or manipulations of the images were made.
Photomicrographs of peripheral blood leukocytes displaying the classical staining and morphology of basophils. (A) A basophil from a Wright-Leishman–stained mouse (strain unknown) peripheral blood film. (B) Wright-Giemsa–stained basophil from a peripheral blood film of a mouse on a C57BL/6J background14 (Fig S2C) (originally published as Supporting On-line Material in Science, 2004;305:1773-1776). (C) The single previous photomicrograph of a mouse basophil appearing in a study by Urbina et al.7(Fig 1) (Reprinted with permission from S. Karger AG, Basel.) (D) A human basophil from a Wright-Giemsa–stained peripheral blood film. Scale bar = 10 μm. The photographs were collected using a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) (Plan-NeoFluar 63×/1.25 mm) and a AxioCam MRc5 digital camera (Carl Zeiss). Adobe Photoshop (San Diego, CA) was used to assemble the figure; however, no enhancements or manipulations of the images were made.
In addition to lacking the appropriate morphology and staining characteristics, the “basophils” identified in the 4 cited studies are also far too prevalent. Mouse basophils are extraordinarily rare cells (as witnessed by the virtual lack of photo documentation in the literature) such that in our combined 50 years of mouse hematologic studies we have identified only a total of 5 mouse basophils with the correct morphology and staining pattern. Indeed, we recently scanned and typed by light microscopy 10 000 leukocytes from peripheral blood films as well as bone marrow brush smears of BALB/cJ and C57BL/6J mice and were unable to identify a single cell with the appropriate staining characteristics; this despite a projected (with 99.9% confidence) identification of 70 basophils from 10 000 leukocytes if they represented approximately 1% of nucleated cells.
There is no reason to doubt that each of the cited studies have identified a leukocyte with the suggested inventory of cell-surface markers. It is also clear from those studies that this leukocyte is prevalent, representing approximately 1% of marrow mononuclear cells. Furthermore, the hematologic staining characteristics described in these studies are consistent with one another and in each case represent the leukocytes with the described cell-surface marker profile. However, the identification of a leukocyte as a basophil is not dependent on assorted “CD” markers present or absent in flow cytometry. Instead, it is a consequence of a cell's appearance and characteristic morphology following hematologic staining. By these distinguishing hematologic features, the leukocytes identified in the cited studies are not basophils.
What's in a name?
It is possible that the discrepancy between the 1981 study of Urbina et al7 and the cited studies under discussion (ie, regarding the staining morphology characteristic of mouse basophils) occurred over time because succeeding references incrementally misinterpreted statements made by the original investigators. Specifically, the statement by Urbina et al7 “The mouse basophils differ from those of other species.” appears to have evolved to mouse basophils do not look like human basophils. In addition, the statement “they (basophils) have relatively few, loosely packed granules of unequal size” is now interpreted as mouse basophils have no granules. However, and irrespective of the ultimate source of this confusion, the leukocytes identified in the 4 cited studies do not display the classical morphology and staining characteristic of basophils as first described in Urbina et al7 and now confirmed in this perspective (Figure 2).
So, where does this leave us? We suggest that 3 explanations exist for the observations noted in this perspective. First, the leukocytes in the cited studies and those identified here as basophils may represent the same cell at different points of maturation. Although possible, the identification of leukocytes with similar morphology in both bone marrow and peripheral tissues would suggest that the cells in the cited studies do not substantially change as they mature and are therefore different from the basophils shown here. The second possibility is that basophils in rodents include 2 morphologically distinct subtypes: one that is classical in appearance, rare, and has a yet-to-be-defined cell-surface marker profile, versus leukocytes with a unique surface immunophenotype that are less distinct in appearance but nonetheless more prevalent. The lack of such a described dichotomy in other mammalian species, including humans, would suggest that its unique appearance in the mouse is unlikely. However, this issue has certainly not been fully explored, and it is also possible that these 2 apparently different and distinct subtypes are simply morphologic variations of a single class of leukocytes. Moreover, the possible presence of “basophils” in humans with the characteristics noted in the 4 cited studies has not been investigated. The final possibility is that these reports have identified a distinct and previously underappreciated leukocyte. That is, this new cell type shares some characteristics of basophils (eg, the cell-surface marker profile) but is a nongranulated polymorphonuclear leukocyte subtype distinct from the classical metachromatically staining granulated basophil. We suggest that, although provocative, this conclusion has merit and may not easily be dismissed on the basis of available data.
Future directions and possibilities
The resolution of this paradox and the unambiguous identification of the cells in the cited studies are critical to the integration of data from patients with asthma and mouse models of disease. Specifically, is there a need, consensus, or both in the research community to modify the definition of a basophil to include the morphologically distinct leukocytes in the 4 cited studies under study? Alternatively, are these cells a potentially new leukocyte subtype and is this a mouse-specific phenomenon or do these cells also exist in humans? We believe that each of these questions is important for the hematologic as well as immunologic communities. Furthermore, we also believe that future studies are required to resolve these issues or, at a minimum, are needed to develop a consensus. Regardless of the eventual outcome of this dialogue, if past experiences are any indication of future events, these studies will also no doubt create more questions than they answer.
Authorship
Contribution: J.J.L. and M.P.McG. each contributed equally to all 3 facets of this perspective (ie, the manuscript), including the intellectual design of the study, collection of the data presented (cell differential analyses of mouse and human blood films), and writing of this perspective.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James J. Lee, Division of Pulmonary Medicine, S.C. Johnson Medical Research Center, Mayo Clinic Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: jjlee@mayo.edu.
Acknowledgments
We thank the invaluable contributions of all the members of Lee Laboratories who endured what must have seemed like endless discussions of what a basophil is and is not. We also wish to thank Dr Terri Cunliffe-Beamer (Wyeth) for her contribution of a rare example of a Wright-stained mouse basophil (the photomicrograph contained in Figure 2A) as well as Pam Salalac and Dan Otero (Hematology/Pathology Group, Department of Laboratory Medicine, Mayo Clinic Arizona) for their preparation and staining of human blood films. Finally, special thanks go to Marv Ruona whose skills as a medical graphic artist are unparalleled and our administrative staff, Linda Mardel and Margaret (Peg) McGarry, without whom we could not function as an integrated group.
This work was supported by the Mayo Foundation and grants from the National Institutes of Health (grant HL065228) (J.J.L.) and the American Heart Association (grant AHA 045580Z) (J.J.L.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal