Abstract
A key feature of Plasmodium falciparum, the parasite causing the most severe form of malaria in humans, is its ability to export parasite molecules onto the surface of the erythrocyte. The major virulence factor and variant surface protein PfEMP1 (P falciparum erythrocyte membrane protein 1) acts as a ligand to adhere to endothelial receptors avoiding splenic clearance. Because the erythrocyte is devoid of protein transport machinery, the parasite provides infrastructure for trafficking across membranes it traverses. In this study, we show that the P falciparum skeleton-binding protein 1 (PfSBP1) is required for transport of PfEMP1 to the P falciparum–infected erythrocyte surface. We present evidence that PfSBP1 functions at the parasitophorous vacuole membrane to load PfEMP1 into Maurer clefts during formation of these structures. Furthermore, the major reactivity of antibodies from malaria-exposed multigravid women is directed toward PfEMP1 because this is abolished in the absence of PfSBP1.
Introduction
Plasmodium falciparum is the causative agent of the most severe form of malaria in humans, resulting in severe morbidity and mortality with more than 2 million deaths each year.1 On invasion into erythrocytes P falciparum modifies the host cell by export of parasite-encoded proteins across the parasitophorous vacuole membrane (PVM) into the erythrocyte cytosol, and some of these are incorporated beneath and transported onto the host membrane.2,3 The parasite proteins on the erythrocyte surface are exposed to the host immune system and play a role in pathogenesis of infection.4
Transport of proteins to the erythrocyte cytoskeleton and surface is a multistep process involving trafficking across the parasite membrane, PVM, and erythrocyte cytosol.5,6 Here, they associate with membranous structures known as Maurer clefts before reaching their destination. The surface of the infected erythrocyte becomes punctuated with electron-dense elevations called knobs.7,8 These structures provide a platform for variant P falciparum erythrocyte membrane protein-1 (PfEMP1).9-11 PfEMP1 mediates adhesion by binding to host receptors such as CD36 and chondroitin sulfate A (CSA).12,13 Other proteins, such as knob-associated histidine-rich protein (KAHRP) and P falciparum erythrocyte membrane protein 3 (PfEMP3), are exported through the PVM using a hydrophobic signal sequence and a pentameric PEXEL (Plasmodium export element) at the N-terminus, required for translocation across the PVM.2,3,14
PfEMP1 contains a PEXEL-like sequence at the N-terminus, although a second sequence has been suggested in trafficking of this protein to the erythrocyte surface.2,3 This protein does not have a hydrophobic signal sequence and enters the endoplasmic reticulum (ER) by a hydrophobic transmembrane segment toward the C-terminus of the protein.15 The PEXEL-like sequence and transmembrane region may be sufficient for trafficking of PfEMP1 to the surface of the parasite-infected erythrocyte; however, this has not been confirmed.2,15
Interestingly, there are some proteins exported to the parasite-infected erythrocyte that do not contain a PEXEL, and it is not clear how they are translocated. One of these molecules, the 48-kDa P falciparum skeleton-binding protein 1 (PfSBP1) is a type 2 integral membrane protein localized to Maurer clefts.16 The absence of a signal sequence and inhibition of export with brefeldin A suggests it is transported by the ER-Golgi and inserted into Maurer clefts membrane. PfSBP1 has been suggested to anchor Maurer clefts to the erythrocyte cytoskeleton16 or to prevent early rupture of the erythrocyte membrane by interacting with host proteins.17 In this work, we address the role of PfSBP1 and show that it is required for export of PfEMP1 into the erythrocyte cytosol to Maurer clefts.
Materials and methods
Plasmid constructs, parasite strains, culture conditions, and transfection
The pHHT-TK-ΔSBP1 plasmid was derived from pHHT-TK18 by insertion of a 5′ (923 bp [base pair]) and 3′ (818 bp) segment of PfSBP1 with oligonucleotide primers aw549/aw500 and aw200/201, respectively. For complementation, full-length PfSBP1 was amplified using the primer pair aw779/780 and then cloned into pCC-4 using XhoI/XmaI (Maier and Cowman, unpublished observations, June 2006).
The PfEMP1 plasmid was assembled using Multisite recombination as described.19 Entry vectors PfHSP86 5′-pENTR4/1,19 Pf5B1+ N-term TOPO pENTR,20 Pf5B1 TM-ATS C-term pENTR2/3,20 and the destination vector pHBlR-3/419 were used. The resulting BHPfEMP1-YFP is identical to the PfEMP1-YFP as published,20 with the exception that hDHFR has been replaced by blasticidin-S deaminase and selected with 3 μg/mL blasticidin (Figure 6C).
P falciparum asexual stages were maintained in human 0+ erythrocytes. CS2 is a clone of the It isolate.13 It adheres to CSA and hyaluronic acid13,21 in vitro and is recognized by antibodies among pregnant women exposed to placental malaria.22 Prior to transfection CS2 was reselected for adhesion to bovine trachea CSA (Sigma, St Louis, MO). Transfection with 80 μg purified plasmid DNA (Qiagen, Hilden, Germany) and selection for stable transfectants were carried out as described.18
Oligonucleotides and DNA analysis
The following oligonucleotides were used: Aw549, 5′-atcccgcgGTATGTATGTATGTATGTATGCATGTATG-3′; Aw550, 5′-gatactagtCGGTAGTTGCAAGTGCCTCTGCTGC-3′; Aw200, 5′-atcgaattcCAATCCACAACCAAATCCACAAC-3′; Aw201, 5′-gatcctaggTATATGTGTACATTGTTAAATTC-3′; Aw779, 5′-atcctcgagtttttATGTGTAGCGCAGCACGAGCATTTG; Aw780, 5′-gatcccgggTTAGGTTTCTCTAGCAAC-3′. Underlined are restriction sites introduced for cloning.
Genomic DNA was prepared with the DNeasy Tissue Kit (Qiagen) and subjected to Southern Blot analysis using standard protocols.
SDS-PAGE (polyacrylamide gel electrophoresis)and immunoblot analysis
Synchronized trophozoite cultures were saponin lysed, and the pellet was washed 3 times in PBS and taken up in SDS sample buffer (Invitrogen). Proteins were separated on 3% to 8% Tris-Acetate or 10% Bis-Tris gels (Invitrogen, Carlsbad, CA). Western blotting to nitrocellulose (0.45 μm; Schleicher and Schuell, Dassel, Germany) was performed according to standard protocols. Antibodies used were mouse anti-SBP1 (1:2000), rabbit anti-HSP70 (1:4000),23 rabbit anti-ATS (1:1000), and mouse monoclonal anti-ATS 1B/98-6H1-1 (1:200). Both anti-ATS antibodies were preabsorbed on erythrocyte ghosts. Horseradish peroxidase–coupled sheep anti–rabbit Ig or anti–mouse Ig (1:1000; Chemicon, Temecula, CA) were used as secondary antibodies.
CSA binding assay and trypsin cleavage assay
Binding assays were performed using P falciparum–infected erythrocytes at 3% parasitemia and 4% hematocrit.24 For trypsin cleavage, parasites were synchronized by sorbitol, grown to trophozoite stage, and harvested by magnetic cell sorting (CS columns; Miltenyi Biotec, Bergisch Gladbach, Germany) then treated with either TPCK-treated trypsin (Sigma) (1 mg/mL in PBS) or incubated in PBS for 1 hour at 37°C. After incubation soybean trypsin inhibitor (5 mg/mL in PBS; Sigma) was added followed by incubation at room temperature for 15 minutes. Cell pellets were extracted with Triton X-100 (1%) and subsequently with sodium dodecylsulfate (SDS; 2%) as described.25
Fractionation of infected erythrocytes
For saponin lysis, 2 × 107 infected erythrocytes (enriched by Plasmagel floatation26 ) were incubated in a final concentration of 0.09% saponin (Kodak, Rochester, NY) for 10 minutes on ice then centrifuged at 4000g for 5 minutes. The supernatant was resuspended in SDS sample buffer (Invitrogen), and the pellet was washed twice in PBS before resuspending in SDS sample buffer.
The Triton-X 100/SDS extraction was performed as described for the trypsin cleavage assay, without the cells being subjected to a prior trypsin treatment. For the permeabilization of infected erythrocytes with Streptolysin-O (SLO), the hemolytic activity of SLO was determined, and 2 × 107 erythrocytes were incubated with 4 or 2 hemolytic units in PRMI-1640 medium as described.27 Samples were analyzed by Western blot.
Electron microscopy and immunofluorescence microscopy
For scanning electron and transmission electron microscopy, parasite-infected erythrocytes were tightly synchronized and processed by standard methods.28 For immunofluoresence analysis, acetone/methanol (90%/10%)–fixed smears of asynchronous parasites of CS2ΔSBP1-, CS2-infected erythrocytes and transfectants were probed with preabsorbed rabbit anti-ATS (1:200), mouse anti-KAHRP (His; 1:100), mouse anti-SBP1 (1:500), mouse anti-MAHRP (1:500),29 and rabbit anti-GFP (1:200) and were consequently incubated with secondary antibodies Alexa Fluor 488–conjugated anti–rabbit IgG (Molecular Probes, Eugene, OR) and Alexa Fluor 594–conjugated anti–mouse IgG (Molecular Probes). Cells were viewed on a Zeiss (Göttingen, Germany) Axioskop 2 microscope equipped with a PCO SensiCam (12 bit) camera and Axiovision 3 software. Captured images were processed using Photoshop (Adobe, San Diego, CA) and ImageJ software (available from http://rsb.info.nih.gov/ij).
Antibodies to the surface of P falciparum–infected erythrocytes
Serum samples were tested for specific IgG to the surface of trophozoite-infected erythrocytes at 3% to 4% parasitemia, 0.2% hematocrit, using flow cytometry.22 Cells were sequentially incubated with serum diluted 1/20, rabbit anti–human IgG (Fc-specific, 1:100; Dako, Glostrup, Denmark), and Alexa Fluor 488–conjugated anti–rabbit Ig (1:1000; Molecular Probes), with ethidium bromide 10 μg/mL. Incubations were 30 minutes each, performed at room temperature. Samples were analyzed using a fluorescence-activated cell sorting (FACS) Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and Flowjo software (TreeStar, San Carlos, CA). Fluorescence in channel FL1 was used as a measure of IgG binding, and the geometric mean fluorescence of uninfected erythrocytes was deducted from the geometric mean fluorescence of infected erythrocytes. All samples were tested in duplicate.
Serum samples
Sera were collected from malaria-exposed pregnant residents of the Madang Province, Papua, New Guinea (PNG), presenting for routine antenatal care at the Modilon Hospital, Madang. This population experiences year-round transmission of P falciparum. Sera from 5 Australian residents were included as controls. Written informed consent was given by all donors, and ethical clearance was obtained from the Medical Research Advisory Committee, Department of Health, PNG, and the Walter and Eliza Hall Institute Ethics Committee.
Results
Disruption of the PfSBP1 gene in P falciparum–infected erythrocytes
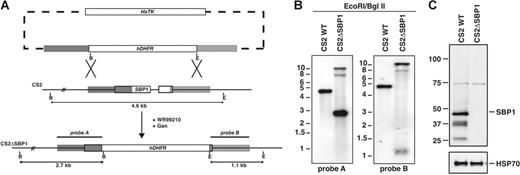
To determine the function of PfSBP1 in P falciparum, we constructed a plasmid to integrate into the gene (PFE0065w) by double crossover recombination (Figure 1A).18 The pHHT-TKΔSBP1 plasmid was transfected into P falciparum CS2-infected erythrocytes and parasites obtained with the hDHFR cassette integrated by homologous recombination resulting in disruption of PfSBP1 (Figure 1A). This was confirmed by Southern blot hybridization (Figure 1B).
Disruption of PfSBP1 and expression of PfSBP1 protein in P falciparum. (A) Schematic representation of parental (CS2) and disrupted PfSBP1 loci (CS2ΔSBP1). The WR99210 resistance gene hDHFR was inserted into the PfSBP1 locus by double crossover recombination resulting in the CS2ΔSBP1 parasite line. Restriction sites used for Southern blot analysis and the predicted fragment sizes are shown (B, BglII; E, EcoRI) (B) Southern blot analysis of the PfSBP1 locus in parental CS2 and transgenic CS2ΔSBP1 cell lines. Genomic DNA was digested with EcoRI/BglII and probed with the 5′ (probe A) and 3′ (probe B) targeting sequence. Predicted sizes for the 5′ probe were wild-type, 4.6 kb (kilobase); disrupted loci, 2.7 kb; and plasmid, 6.9 kb; predicted sizes for the 3′ probe were wild-type, 4.6 kb; disrupted loci, 1.1 kb; and plasmid 6.9 kb. (C) Western blot of saponin pellets of trophozoite-infected erythrocytes of CS2 parent and CS2ΔSBP1 probed with anti-SBP1 antibodies. Equal loading of parasite material was confirmed with anti-PfHSP70 antibodies in the bottom panel.
Disruption of PfSBP1 and expression of PfSBP1 protein in P falciparum. (A) Schematic representation of parental (CS2) and disrupted PfSBP1 loci (CS2ΔSBP1). The WR99210 resistance gene hDHFR was inserted into the PfSBP1 locus by double crossover recombination resulting in the CS2ΔSBP1 parasite line. Restriction sites used for Southern blot analysis and the predicted fragment sizes are shown (B, BglII; E, EcoRI) (B) Southern blot analysis of the PfSBP1 locus in parental CS2 and transgenic CS2ΔSBP1 cell lines. Genomic DNA was digested with EcoRI/BglII and probed with the 5′ (probe A) and 3′ (probe B) targeting sequence. Predicted sizes for the 5′ probe were wild-type, 4.6 kb (kilobase); disrupted loci, 2.7 kb; and plasmid, 6.9 kb; predicted sizes for the 3′ probe were wild-type, 4.6 kb; disrupted loci, 1.1 kb; and plasmid 6.9 kb. (C) Western blot of saponin pellets of trophozoite-infected erythrocytes of CS2 parent and CS2ΔSBP1 probed with anti-SBP1 antibodies. Equal loading of parasite material was confirmed with anti-PfHSP70 antibodies in the bottom panel.
CS2ΔSBP1 transgenic parasites do not express PfSBP1
To confirm that gene disruption had resulted in loss of PfSBP1 expression, we performed immunoblots with anti-PfSBP1 (Figure 1C). A band of approximately 48 kDa was detected in CS2 as well as bands of 37 and 26 kDa most likely representing degradation or processing products. In contrast, no specific band corresponding to PfSBP1 was detected in CS2ΔSBP1. The band at 74 kDa, which is present in both lanes, corresponds to a cross-reactive protein species (see also Figure 2). These results have shown that PfSBP1 can be disrupted in P falciparum. Immunofluorescence experiments with anti-PfSBP1 antibodies confirmed the absence of Maurer clefts labeling in CS2ΔSBP1; in contrast, PfSBP1 was localized to Maurer clefts in CS2 as expected (Figure 2A). The low level fluorescence in CS2ΔSBP1 parasites results from reactivity of the antibody with a cross-reactive protein (Figure 2A).
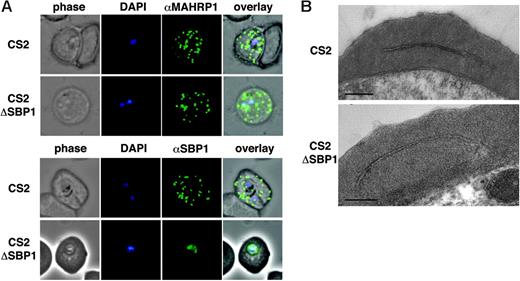
Structure and trafficking to Maurer clefts. (A) Localization of the resident Maurer clefts markers PfSBP1 and PfMAHRP1 in parental CS2 (first row of each panel) and CS2ΔSBP1-infected cells (second row of each panel). PfMAHRP1 was correctly trafficked to Maurer clefts in both cell lines, whereas PfSBP1 showed only cross-reactivity with another protein within the parasite CS2ΔSBP1 without localization on Maurer clefts as observed in the parental CS2 cells. (B) Ultrastructural analysis of CS2- (top) and CS2ΔSBP1-infected cells (bottom) shows typical Maurer clefts in both cell lines. Bars represent 0.5 μm.
Structure and trafficking to Maurer clefts. (A) Localization of the resident Maurer clefts markers PfSBP1 and PfMAHRP1 in parental CS2 (first row of each panel) and CS2ΔSBP1-infected cells (second row of each panel). PfMAHRP1 was correctly trafficked to Maurer clefts in both cell lines, whereas PfSBP1 showed only cross-reactivity with another protein within the parasite CS2ΔSBP1 without localization on Maurer clefts as observed in the parental CS2 cells. (B) Ultrastructural analysis of CS2- (top) and CS2ΔSBP1-infected cells (bottom) shows typical Maurer clefts in both cell lines. Bars represent 0.5 μm.
Maurer clefts develop normally in the absence of PfSBP1
To determine whether trafficking of other proteins had been affected in the absence of PfSBP1 and whether Maurer clefts showed a typical morphology, we used both immunofluorescence with antibodies to the Maurer clefts resident proteins PfSBP119 and PfMAHRP130 and electron microscopy. In both parental and CS2ΔSBP1 transgenic parasite-infected erythrocytes anti-MAHRP1 antibodies showed the same punctate pattern as anti-PfSBP1 antibodies in CS2 (Figure 2A-B). Similar results were obtained for PfREX1, a protein in Maurer clefts (not shown). Transmission electron microscopy could not detect any major alteration of Maurer cleft in CS2ΔSBP1 compared with CS2 (Figure 2B). Therefore, PfSBP1 expression is not required for development of Maurer clefts or trafficking of resident proteins PfMAHRP1 and PfREX1.
PfSBP1 mutants display knobs on the surface of the parasite-infected erythrocyte
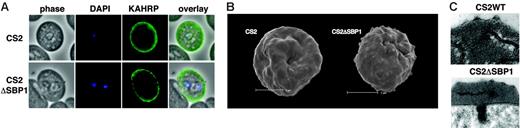
KAHRP is the major component of the knob structure, and this protein is exported through the PVM into the erythrocyte cytosol where it binds to Maurer clefts before assembly underneath the erythrocyte membrane.14 To determine whether loss of PfSBP1 function in CS2ΔSBP1 affected trafficking of KAHRP and assembly into knobs, we used immunofluorescence with anti-KAHRP antibodies and scanning electron microscopy (Figure 3A). Both CS2 and CS2ΔSBP1 showed rim fluorescence, suggesting KAHRP was trafficked to the underside of the host cell membrane (Figure 3 A). In addition, CS2ΔSBP1-infected erythrocytes showed typical knobs on the surface of the host cell compared with CS2 (Figure 3B). Hence, PfSBP1 is not required for trafficking of KAHRP to the erythrocyte cytosol and assembly of knobs.
KAHRP is trafficked normally to the erythrocyte membrane and assembled into knob structures. (A) Immunofluorescence assay to determine the localization of KAHRP in parental CS2 (top) and CS2ΔSBP1-infected erythrocytes (bottom). Cells were reacted with anti-KAHRP antibodies and identical localization patterns for both parasite lines were observed. (B) Scanning electron microscopy revealed typical knob structures in CS2ΔSBP1 parasite-infected erythrocytes compared with parental CS2. The panel shows a representative trophozoite-infected erythrocyte of each cell line. The bar represents 2 μm. (C) Ultrastructural analysis of CS2- and CS2ΔSBP1-infected cells of knob structures (arrow).
KAHRP is trafficked normally to the erythrocyte membrane and assembled into knob structures. (A) Immunofluorescence assay to determine the localization of KAHRP in parental CS2 (top) and CS2ΔSBP1-infected erythrocytes (bottom). Cells were reacted with anti-KAHRP antibodies and identical localization patterns for both parasite lines were observed. (B) Scanning electron microscopy revealed typical knob structures in CS2ΔSBP1 parasite-infected erythrocytes compared with parental CS2. The panel shows a representative trophozoite-infected erythrocyte of each cell line. The bar represents 2 μm. (C) Ultrastructural analysis of CS2- and CS2ΔSBP1-infected cells of knob structures (arrow).
CS2ΔSBP1-infected erythrocytes do not cytoadhere to CSA
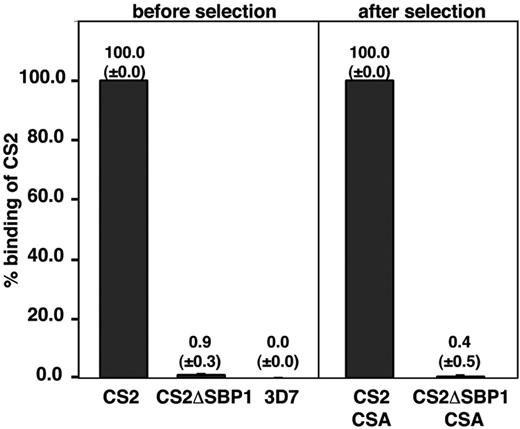
Knobs are platforms on the infected erythrocyte to elevate the major variant surface antigen PfEMP1. CS2 expresses the var2csa gene22 encoding a PfEMP1 protein conferring cytoadherence of parasite-infected erythrocytes to the host cell receptor CSA.31 The adherence phenotype of CSA is stable, suggesting a slow switch to other var genes and adherence phenotypes. We compared adherence properties of CS2ΔSBP1 with CS2-infected erythrocytes to determine whether loss of PfSBP1 had affected the ability of cells to adhere to CSA. The parental CS2-infected erythrocytes bound efficiently to CSA (Figure 4); however, the CS2ΔSBP1 transgenic line showed no binding. 3D7-infected erythrocytes, a parasite line that binds predominantly to CD36, also showed little binding to CSA as expected (Figure 4). To exclude the possibility that CS2ΔSBP1 had switched expression of PfEMP1 to one not binding CSA, we selected both for adherence to CSA. Selection did not increase binding of CS2ΔSBP1 to CSA, and the same relative binding was observed as before selection (Figure 4, right). These results suggested that lack of PfSBP1 had resulted in either changes in PfEMP1 expression or its function on the P falciparum–infected erythrocyte surface.
Adhesion ofP falciparum–infected erythrocytes to CSA. CS2 parent and CS2ΔSBP1-infected erythrocytes were compared before and after panning and subsequent growth for adherence to CSA. Binding of 3D7-parasitized erythrocytes was included as a negative control. Data represents average ± standard deviation (n = 3). Numbers are expressed as the percentage of CS2 parental binding (bound erythrocyte/mm2). The mean number of bound infected erythrocytes per squared millimeter for CS2 parental binding was 1240 above bovine serum albumin control.
Adhesion ofP falciparum–infected erythrocytes to CSA. CS2 parent and CS2ΔSBP1-infected erythrocytes were compared before and after panning and subsequent growth for adherence to CSA. Binding of 3D7-parasitized erythrocytes was included as a negative control. Data represents average ± standard deviation (n = 3). Numbers are expressed as the percentage of CS2 parental binding (bound erythrocyte/mm2). The mean number of bound infected erythrocytes per squared millimeter for CS2 parental binding was 1240 above bovine serum albumin control.
PfEMP1 is not trafficked to the surface of CS2ΔSBP1-infected erythrocytes
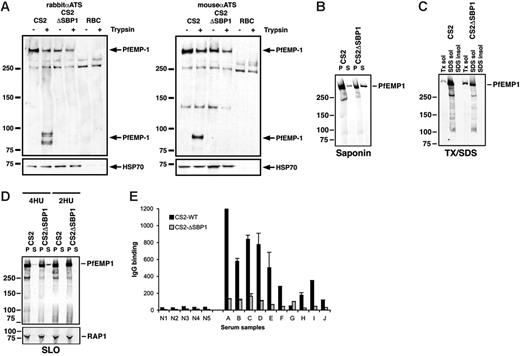
To examine whether the inability of CS2ΔSBP1 to adhere to CSA was due to the absence or altered levels of PfEMP1 on the surface of parasite-infected erythrocytes, we used an assay allowing detection of the pool of protein exposed on the surface by virtue of its sensitivity to trypsin cleavage.32 Cultures were treated with or without trypsin, and PfEMP1 protein was detected using antibody to the conserved acidic terminal segment (ATS), a region located on the cytosolic face of the infected erythrocyte (Figure 5A). The anti-ATS antibody detected an approximately 300-kDa protein, representing the full-length protein in CS2. It also cross-reacts with a blood cell component (most likely spectrin) as shown by comparison with uninfected erythrocytes (Figure 5A). In the parental CS2 only full-length PfEMP1 (surface exposed and nonexposed pools) was observed in the absence of trypsin (Figure 5A, −trypsin), whereas after trypsin cleavage, products of 83 kDa could be detected (Figure 5A, +trypsin). These represent the protected intracellular PfEMP1 domain (transmembrane region and ATS) after cleavage of the surface-exposed PfEMP1. The 300-kDa band after trypsin treatment corresponds to the cytoplasmic pool of PfEMP1. In contrast, CS2ΔSBP1-infected erythrocytes showed no protein truncations after trypsin cleavage, suggesting there was no PfEMP1 on the surface (Figure 5A). This was confirmed using a mouse anti-ATS antibody. The absence of PfEMP1 was consistent with inability of these cells to bind to CSA.
PfEMP1 is not exposed on the surface of erythrocytes infected with CS2ΔSBP1. (A) Intact erythrocytes infected with parental CS2, CS2ΔSBP1, and uninfected erythrocytes were treated with (+) or without (−) trypsin, and extracts were analyzed by Western blotting with rabbit (left) or mouse (right) anti-ATS antibody. Surface-exposed PfEMP1 is cleaved by trypsin, resulting in bands at 83 kDa. The blot was reprobed with anti-HSP70 antibodies to confirm equal loading. (B) PfEMP1 is more abundant in CS2ΔSBP1 saponin supernatant. Saponin pellets and supernatants of trophozoite-infected erythrocytes were analyzed by Western blotting with anti-ATS antibody. (C) Triton X-100–soluble PfEMP1 is more abundant in CS2ΔSBP1. Triton X-100, SDS soluble, and insoluble fractions of trophozoite-infected erythrocytes were analyzed by Western blotting with anti-ATS antibody. (D) More soluble PfEMP1 can be detected in SLO-treated CS2ΔSBP1-infected parasites. Four hemolytic units (HUs) of SLO were used to permeabilize the plasma membrane of infected erythrocytes. The soluble content of the erythrocyte cytosol was then analyzed for the presence of PfEMP1 with anti-ATS antibody. As a comparison the same experiment was done at a noneffective SLO concentration (2 HU). The rhoptry-associated protein RAP1 was used as a loading control. (E) Comparison of reactivity of sera from nonexposed (N1-N5) versus exposed persons (A-J) from Papua, New Guinea, toward erythrocytes infected with parental CS2 and with CS2ΔSBP1 is consistent with the lack of PfEMP1 on the host cell in the absence of PfSBP1 function. IgG binding to the surface of infected erythrocytes among sera from malaria-exposed multigravid women residing in PNG was significantly higher for CS2 than for CS2ΔSBP1 (P < .004, Wilcoxon signed rank sum test). There was little IgG reactivity among sera from Australian residents not exposed to malaria. IgG binding was measured by flow cytometry, and values (arbitrary units) represent the mean + range of samples tested in duplicate.
PfEMP1 is not exposed on the surface of erythrocytes infected with CS2ΔSBP1. (A) Intact erythrocytes infected with parental CS2, CS2ΔSBP1, and uninfected erythrocytes were treated with (+) or without (−) trypsin, and extracts were analyzed by Western blotting with rabbit (left) or mouse (right) anti-ATS antibody. Surface-exposed PfEMP1 is cleaved by trypsin, resulting in bands at 83 kDa. The blot was reprobed with anti-HSP70 antibodies to confirm equal loading. (B) PfEMP1 is more abundant in CS2ΔSBP1 saponin supernatant. Saponin pellets and supernatants of trophozoite-infected erythrocytes were analyzed by Western blotting with anti-ATS antibody. (C) Triton X-100–soluble PfEMP1 is more abundant in CS2ΔSBP1. Triton X-100, SDS soluble, and insoluble fractions of trophozoite-infected erythrocytes were analyzed by Western blotting with anti-ATS antibody. (D) More soluble PfEMP1 can be detected in SLO-treated CS2ΔSBP1-infected parasites. Four hemolytic units (HUs) of SLO were used to permeabilize the plasma membrane of infected erythrocytes. The soluble content of the erythrocyte cytosol was then analyzed for the presence of PfEMP1 with anti-ATS antibody. As a comparison the same experiment was done at a noneffective SLO concentration (2 HU). The rhoptry-associated protein RAP1 was used as a loading control. (E) Comparison of reactivity of sera from nonexposed (N1-N5) versus exposed persons (A-J) from Papua, New Guinea, toward erythrocytes infected with parental CS2 and with CS2ΔSBP1 is consistent with the lack of PfEMP1 on the host cell in the absence of PfSBP1 function. IgG binding to the surface of infected erythrocytes among sera from malaria-exposed multigravid women residing in PNG was significantly higher for CS2 than for CS2ΔSBP1 (P < .004, Wilcoxon signed rank sum test). There was little IgG reactivity among sera from Australian residents not exposed to malaria. IgG binding was measured by flow cytometry, and values (arbitrary units) represent the mean + range of samples tested in duplicate.
It has been shown previously that PfEMP1 has different solubility characteristics during trafficking to the erythrocyte membrane as a consequence of its association with different compartments.33 To compare the solubility of PfEMP1 in parent and CS2ΔSBP1, we used saponin lysis of infected erythrocytes. The pellet fraction includes proteins associated with the parasite and parasitophorous vacuolar and erythrocyte membranes (Figure 5B). PfEMP1 was detected in the pellet of CS2, which mainly represents the membrane-associated pool of PfEMP1. In the absence of PfSBP1 the CS2ΔSBP1-infected erythrocytes showed significantly less PfEMP1 in the saponin pellet consistent with the absence of membrane-associated PfEMP1 in and on the erythrocyte surface. In contrast, in CS2ΔSBP1 more PfEMP1 was found in the supernatant relative to the amount detectable in the pellet. To see whether this correlates with the membrane association of PfEMP1, fractionation was performed (Figure 5C). The Triton X-100–soluble fraction of the parental CS2 trophozoite-infected cells contained only a small amount of PfEMP1; as previously reported10 the majority of PfEMP1 is found in the SDS-soluble fraction. However, the amount of PfEMP1 found in the Triton X-100–soluble fraction of the CS2ΔSBP1-infected erythrocytes was significantly increased, arguing for a larger pool of tightly membrane-associated PfEMP1 in CS2 than in CS2ΔSBP1-infected erythrocytes.
To determine the nature of the membrane association, we permeabilized erythrocytes infected with CS2 and CS2ΔSBP1 parasites with streptolysin O (SLO). SLO permeabilizes the erythrocyte membrane and leaves membranes of parasitic origin intact (eg, parasite membrane, parasitophorous vacuole, or Maurer clefts) (Figure 5D). A small but observable increase of soluble PfEMP1 is found in the supernatant of SLO-permeabilized CS2ΔSBP1-infected erythrocytes relative to the CS2-infected cells. These results suggest that a small quantity of PfEMP1 in CS2ΔSBP1 is present in the erythrocyte in a soluble state that cannot associate with the membranes of parasite-induced compartments or the erythrocyte membrane because of the lack of PfSBP1 function.
To further establish the finding that there was no detectable PfEMP1 present on CS2ΔSBP1-infected erythrocytes, we determined the level of antibody reactivity to the host cell surface using sera from multigravid women resident in a malaria-endemic area of Papua, New Guinea. Multigravid women are generally exposed to P falciparum parasites that present novel antigenic phenotypes that adhere to the receptor CSA in the placenta.34 These sera should react strongly with the surface of CS2-infected erythrocytes because they express the var2csa-encoded PfEMP1, a known ligand for CSA. Levels of IgG binding among the sera of multigravid women were significantly higher for CS2 parent compared with CS2ΔSBP1 (mean ± SEM fluorescence, 483 ± 116 versus 80.7 ± 15.2, respectively; P < .004, Wilcoxon signed-rank sum test) (Figure 5C). IgG binding was higher to CS2- than CS2ΔSBP1-infected erythrocytes for 9 of 10 PNG samples tested (fold difference, 0.5-9.2). There was little binding of IgG among samples from nonexposed donors (mean fluorescence, 27.1 ± 2 for CS2-wt; 7.4 ± 1.4 for CS2ΔSBP1), and IgG binding of PNG sera was significantly higher than IgG binding of nonexposed sera for both CS2- and CS2ΔSBP1-infected erythrocytes (P < .001; Mann-Whitney U test). Interestingly, the response of these persons against the CS2ΔSBP1-infected erythrocytes was almost as low as that of the nonexposed persons (Figure 5C). Sera from multigravid women also had antibodies to non-CSA binding parasite variants as expected for adults exposed to year-round malaria (data not shown). This is consistent with the lack of PfEMP1 on the surface of the CS2ΔSBP1-infected erythrocytes and demonstrates the major reactivity of antibodies from multigravid malaria-infected persons is directed toward a protein that requires PfSBP1 for appropriate erythrocyte surface localization.
PfEMP1 loading into Maurer clefts is impaired in CS2ΔSBP1-infected erythrocytes
Because the transport of PfEMP1 onto the surface of the CS2ΔSBP1-parasitized erythrocytes was dramatically altered, we were interested in the localization of PfEMP1 in these cells compared with CS2. PfEMP1 is translocated from the parasite across the parasitophorous vacuole by an unknown mechanism. It then associates with Maurer clefts before a proportion is transferred onto the erythrocyte surface at approximately 16 to 18 hours after invasion.35 In immunofluorescence assays the CS2 parental parasitized erythrocytes showed a typical pattern of PfEMP1 localization with anti-ATS antibodies. Protein was detected within the parasite but a significant proportion localized to punctate structures within the erythrocyte cytosol (Figure 6A, top). These structures were identified as Maurer clefts because PfEMP1 colocalized with the Maurer cleft resident protein MAHRP1 (Figure 6B, top, and Video S1, which is available on the Blood website; see the Supplemental Videos link at the top of the online article). In contrast, the CS2ΔSBP1 showed PfEMP1 localization associated with the parasite and a high concentration around the periphery of the parasite and parasitophorous vacuole, but there was little or no protein detectable in the erythrocyte (Figure 6A, bottom). Colocalization experiments with anti-MAHRP1 antibodies confirmed that no PfEMP1 was associated with Maurer clefts in the transgenic cell line (Figure 6B, bottom; Video S2).
PfEMP1 cannot be loaded onto Maurer clefts in CS2ΔSBP1 transgenic cells. (A) Immunofluorescence study to assess trafficking of PfEMP1 in erythrocytes infected with parental CS2 and CS2ΔSBP1. Parental cells probed with anti-ATS and anti-SBP1 antibodies showed typical PfEMP1 localization in Maurer clefts in the erythrocyte (first row of panel), whereas PfEMP1 was accumulated in the parasite surrounding parasitophorous vacuole in CS2ΔSBP1-infected cells (second row of panel). Arrowheads show colocalization. DAPI was used to stain the nuclear DNA in all panels. (B) Colocalization with anti-ATS and anti-MAHRP antibodies to Maurer clefts is shown in parental CS2-infected erythrocytes (first row of panel, arrowheads), whereas in the CS2ΔSBP1-infected cells there is no overlap of the 2 proteins in Maurer clefts. (C) Schematic representation of the plasmid to express a truncated PfEMP1 fused to YFP. The PfEMP1-YFP gene is flanked by an hsp86 promoter and a PbDT 3′ terminator. Blasticidin-S deaminase is used as a selection cassette. (D) Expression of PfEMP1-YFP in CS2 wild-type– and CS2ΔSBP1-infected erythrocytes are shown by anti-GFP labeling. In erythrocytes infected with parental CS2 (first row, top panel), the PfEMP1-YFP fusion is localized in the parasite, but the majority of the protein is transported into the erythrocyte cytosol and shows a characteristic Maurer clefts staining. In the CS2ΔSBP1-infected erythrocytes (first row, bottom panel), however, the fusion protein can only be detected within the confines of the parasitophorous vacuole. Both cell lines were also probed with anti-ATS antibody to check for possible trafficking defects caused by the YFP chimera (bottom panels).
PfEMP1 cannot be loaded onto Maurer clefts in CS2ΔSBP1 transgenic cells. (A) Immunofluorescence study to assess trafficking of PfEMP1 in erythrocytes infected with parental CS2 and CS2ΔSBP1. Parental cells probed with anti-ATS and anti-SBP1 antibodies showed typical PfEMP1 localization in Maurer clefts in the erythrocyte (first row of panel), whereas PfEMP1 was accumulated in the parasite surrounding parasitophorous vacuole in CS2ΔSBP1-infected cells (second row of panel). Arrowheads show colocalization. DAPI was used to stain the nuclear DNA in all panels. (B) Colocalization with anti-ATS and anti-MAHRP antibodies to Maurer clefts is shown in parental CS2-infected erythrocytes (first row of panel, arrowheads), whereas in the CS2ΔSBP1-infected cells there is no overlap of the 2 proteins in Maurer clefts. (C) Schematic representation of the plasmid to express a truncated PfEMP1 fused to YFP. The PfEMP1-YFP gene is flanked by an hsp86 promoter and a PbDT 3′ terminator. Blasticidin-S deaminase is used as a selection cassette. (D) Expression of PfEMP1-YFP in CS2 wild-type– and CS2ΔSBP1-infected erythrocytes are shown by anti-GFP labeling. In erythrocytes infected with parental CS2 (first row, top panel), the PfEMP1-YFP fusion is localized in the parasite, but the majority of the protein is transported into the erythrocyte cytosol and shows a characteristic Maurer clefts staining. In the CS2ΔSBP1-infected erythrocytes (first row, bottom panel), however, the fusion protein can only be detected within the confines of the parasitophorous vacuole. Both cell lines were also probed with anti-ATS antibody to check for possible trafficking defects caused by the YFP chimera (bottom panels).
To further investigate the impaired transport of PfEMP1 in CS2ΔSBP1-infected erythrocytes, we transfected both parental and PfSBP1-disrupted parasite lines with a plasmid expressing a truncated form of PfEMP1 (Figure 6C).2 This truncated form consists of the N-terminal portion of the var gene PFL1960w fused to PfEMP1 transmembrane domain and acidic terminal sequence (ATS) and YFP. As expected the protein product of this fusion gene was exported into Maurer clefts in wild-type cells (Marti et al2 ; Figure 6C). However, in erythrocytes infected with CS2ΔSBP1 parasites the PfEMP1-YFP fusion gene could barely be detected in the erythrocyte cytosol, the vast majority being retained within the confines of the parasitophorous vacuole. This strongly suggests that PfSBP1 function is required for localization of PfEMP1 to Maurer clefts.
PfEMP1 trafficking to Maurer clefts can be restored in SBP1 complemented cells
To explore this further, we transfected the CS2ΔSBP1-transgenic parasite line with the plasmid pCC-4/SBP1, where the PfSBP1 gene was inserted between the PfHSP86 promoter and the Plasmodium berghei DT terminator. Cells containing this plasmid were selected by the addition of blasticidin-S, because the plasmid contains blasticidin-S deaminase as a selectable marker. Samples were taken from a stable population at different time points, saponin-lysed, and analyzed on Western blot probed with anti-SBP1 antibodies. A constant expression was detected throughout the different life cycle stages consistent with expression from the PfHSP86 promoter (Figure 7A). In contrast, CS2 showed almost no expression in ring stages and the highest relative expression in trophozoite stages as previously reported.18 An immunofluorescence assay on erythrocytes infected with the CS2ΔSBP1 showed that in complemented cells expressing PfSBP1 from the plasmid, PfEMP1 is transported efficiently beyond the parasitophorous vacuole membrane into the erythrocytes and colocalizes in Maurer clefts with PfSBP1 (Figure 7B). This shows that the PfEMP1 transport deficiency in the CS2ΔSBP1 cells can be complemented by PfSBP1 expressed from an episomally maintained plasmid and confirms its requirement for transport of PfEMP1 to the erythrocyte surface. Furthermore, it demonstrates that only the PfSBP1 gene was targeted by the knockout experiment, and this gene alone is responsible for incorrect trafficking of PfEMP1.
CS2ΔSBP1-complemented transgenic cells export PfEMP1 beyond the parasitophorous vacuole. (A) Western blot showing expression of SBP1 in different stages of CS2WT and CS2ΔSBP1-complemented cells. Saponin pellets of erythrocytes infected with ring- (R), trophozoite- (T), and schizont-stage (S) CS2WT and CS2ΔSBP1-complemented parasites were loaded to detect the level of expression of SBP1. For each cell line, samples were taken from the same culture dish at different time points. The blot was stripped and reprobed with HSP70 to show the amount of parasite material loaded. The cross-reactive band at 80 kDa is shown for the same purpose. Saponin pellet of CS2ΔSBP1 trophozoite-infected erythrocytes was loaded as a control. (B) Immunofluorescence assay to assess trafficking of PfEMP1 in erythrocytes infected with CS2ΔSBP1-complemented parasites. Cells were probed with anti-ATS antibody showing typical PfEMP1 localization with protein detectable within the parasite and in prominent punctate structures in the erythrocyte. These structures were shown to colocalize with the Maurer clefts protein SBP1. The amount and timing of SBP1 expression is different in the CS2ΔSBP1-complemented cells because of the episomal expression under a different promoter. DAPI was used to stain the nuclear DNA.
CS2ΔSBP1-complemented transgenic cells export PfEMP1 beyond the parasitophorous vacuole. (A) Western blot showing expression of SBP1 in different stages of CS2WT and CS2ΔSBP1-complemented cells. Saponin pellets of erythrocytes infected with ring- (R), trophozoite- (T), and schizont-stage (S) CS2WT and CS2ΔSBP1-complemented parasites were loaded to detect the level of expression of SBP1. For each cell line, samples were taken from the same culture dish at different time points. The blot was stripped and reprobed with HSP70 to show the amount of parasite material loaded. The cross-reactive band at 80 kDa is shown for the same purpose. Saponin pellet of CS2ΔSBP1 trophozoite-infected erythrocytes was loaded as a control. (B) Immunofluorescence assay to assess trafficking of PfEMP1 in erythrocytes infected with CS2ΔSBP1-complemented parasites. Cells were probed with anti-ATS antibody showing typical PfEMP1 localization with protein detectable within the parasite and in prominent punctate structures in the erythrocyte. These structures were shown to colocalize with the Maurer clefts protein SBP1. The amount and timing of SBP1 expression is different in the CS2ΔSBP1-complemented cells because of the episomal expression under a different promoter. DAPI was used to stain the nuclear DNA.
Discussion
The P falciparum–infected erythrocyte undergoes a remarkable series of modifications after invasion, resulting in new structures in the erythrocyte cytoplasm and protein complexes on the membrane surface. One important result of this host cell remodeling is the ability of infected erythrocytes to adhere to endothelial cells in the host vasculature, a property mediated by the protein family PfEMP1.25 This major variant surface protein consists of a large ectodomain, a transmembrane region, and a short conserved acidic cytoplasmic tail (ATS). Recent results have shown that trafficking to the erythrocyte requires a parasite export element (PEXEL-like) and a transmembrane region for translocation beyond the PVM.2,15 The mechanism of trafficking PfEMP1 through P falciparum–infected erythrocytes to Maurer clefts has not been deciphered, and proteins involved in this process have yet to be elucidated. In this work, we have shown PfSBP1 is required for PfEMP1 export to the infected erythrocyte and that this protein functions in transfer of PfEMP1 to Maurer clefts.
Previous reports suggest that PfEMP1 is transported through the P falciparum–infected erythrocyte as complexes rather than within membranous vesicles.15,33 These findings were also supported by the solubility characteristics of PfEMP1 in the erythrocyte cytosol, which are inconsistent with trafficking in small membranous vesicles.33 The demonstration that PfSBP1 is required for transfer of PfEMP1 from the PVM to Maurer clefts suggests that the protein is most likely loaded directly into these structures rather than being released into the erythrocyte cytosol. It is also possible that PfSBP1 has a chaperone-like function allowing interaction with PfEMP1, which is required for trafficking out of the parasitophorous vacuole and association with Maurer clefts.
The membrane association of PfEMP1 seems to be essential for correct trafficking of PfEMP1 by Maurer clefts onto the surface of infected erythrocytes.15 At least 3 PfEMP1 populations have been described outside the parasite differing in their solubility characteristics: PfEMP1 on the erythrocyte surface, an intracellular peripheral membrane protein pool, and intracellular protein with solubility characteristics of the cell-surface population.33 In CS2ΔSBP1 cells the surface-exposed PfEMP1 is absent (Figures 4 and 5A,E). At the same time the pool of intracellular peripheral membrane protein is increased (Figure 5C). One explanation for the slight increase of soluble PfEMP1 found in the erythrocyte cytosol in the SLO experiment could be the transport of soluble PfEMP1 across the PVM. Dilution in the cytosolic volume would explain the inability of detecting PfEMP1 by IFA. The second possibility is that during SLO treatment the PVM may become marginally leaky (as seen in Figure 5D, CS2 supernatant). This leakiness would have no effect on the membrane-associated PfEMP1 in the wild type, but in the absence of PfSBP1 and consequent loss of membrane association it may leak into the erythrocyte cytosol more readily.
While this manuscript was under review a similar study by Cooke et al36 was published, describing PfSBP1 gene disruption in the 3D7 parasite strain. Although transport of PfEMP1 to the surface is also impaired in these infected erythrocytes, the loading of PfEMP1 into Maurer clefts does not seem to be affected by lack of PfSBP1 function (as determined by IFA). Both the internal pool with solubility characteristics of the cell-surface population and the surface-exposed PfEMP1 populations, however, are lost when the cells were analyzed in the trypsin cleavage assay. Whether the dissimilar results in the 2 studies are due to different strains or different antibodies used remains the subject of further investigation. It has been reported that Maurer clefts are prone to nonspecific labeling by antibodies.37 During our studies we observed a propensity of the mouse anti-ATS antibody to show a punctated staining in infected erythrocytes. Whether this is due to the recognition of the parasite-specific 140-kDa band detected only with the mouse anti-ATS antibody seen in Figure 5A is unclear. To confirm our results, we also studied the fate of PfEMP1 transport by a YFP-tagged fusion gene. In CS2 parental cells (PfSBP1 competent), PfEMP1-YFP was detected in Maurer clefts as demonstrated earlier,2 whereas in CS2ΔSBP1 (PfSBP1 null), trafficking to Maurer clefts was abolished, confirming our immunofluorescence results. This is consistent with the requirement of PfSBP1 function for transfer of PfEMP1 to Maurer clefts.
Differential fractionation of infected erythrocytes suggests that PfSBP1 associates with the cytoskeleton and may be important in anchoring Maurer clefts to the cytoskeleton of the host cell.16 Subsequently, it has been shown that PfSBP1 binds to host LANCL1 and recruits it to Maurer clefts.17 It was hypothesized that PfSBP1 may allow tight binding of Maurer clefts to the cytoskeleton by interaction with LANCL1 and other unknown proteins, resulting in altered membrane properties of the host cell preventing premature release of merozoites. We have demonstrated that PfSBP1 plays an essential role in transfer of PfEMP1 to Maurer clefts. How this transfer occurs is not clear but may involve a direct interaction of PfSBP1 with the translocation machinery involved in exporting PfEMP1 out of the parasitophorous vacuolar space. Involvement of PfSBP1 in PfEMP1 translocation into the erythrocyte cytosol does not rule out an additional role involving interaction with the erythrocyte cytoskeleton; however, lack of this protein function does not significantly affect Maurer clefts topology or development and release of merozoites from the developing schizont. It is therefore likely that the main function of PfSBP1 is transfer of PfEMP1 from the parasitophorous vacuole to Maurer clefts.
Previously, it had been suggested that PfSBP1 was conserved in other malaria species because of the reactivity of anti-PfSBP1 antibodies with other mouse malaria species.16 However, with the availability of genome data for several Plasmodium species, no orthologues of PfSBP1 have yet been identified except in Plasmodium reichenowi. PfSBP1 is located in the subtelomeric region of chromosome 5 in the P falciparum genome as part of a coexpressed cluster of genes. Of these, PFE0050w, PFE0055c, and PFE0060w (together with PfSBP1, PFE0065w) are exported to the erythrocyte as shown by proteomic analysis.34 This is coherent with most of them containing an identifiable PEXEL for transport across the PVM (except PfSBP1).2 The absence of a PEXEL in PfSBP1 is consistent with it being loaded directly into Maurer clefts as suggested for another Maurer cleft resident protein MAHRP.29 However, it is still possible that PfSBP1 crosses the PVM by being escorted with a PEXEL-containing protein, or secondly, that it contains a nontypical transport motif not easily recognizable. Although the cluster of genes surrounding PfSBP1 has no identifiable orthologues in other Plasmodia the chromosomal region located more directly centromeric contains genes showing synteny in other species. This suggests that the cluster of genes, including PfSBP1, encodes roles unique to P falciparum and is likely to be associated with trafficking or function of PfEMP1. Interestingly, one gene in this cluster (PFE0055c) contains a DNAJ domain and is annotated as a putative heat shock protein, suggesting it may function as a cochaperone.
Disruption of PfSBP1 expression resulted in a marked reduction in antibody binding to the surface of P falciparum–infected erythrocytes using sera from exposed donors, which further supports our conclusion that trafficking of PfEMP1 is impaired in the transgenic line. PfEMP1 is believed to be the major target of acquired antibodies to the infected erythrocyte surface.30,38 CS2-infected erythrocytes express var2csa as the dominant var gene transcript,22 and the same PfEMP1 was detected in Western blots of both parental and CS2ΔSBP1 parasites; therefore, var2csa-PfEMP1 is likely to be the major target of antibodies to CS2 measured in our assays. Our data demonstrate that antibody binding to the parasitized erythrocyte surface antigens was greatly reduced using the PfSBP1 knockout cell line. The residual IgG binding to CS2ΔSBP1 may reflect antibody binding to antigens other than PfEMP1. Further studies using parasite lines with disruption of PfSBP1 may facilitate the identification of additional P falciparum–infected erythrocyte surface antigens and delineate the nature and specificity of acquired immunity. In these studies we used sera from multigravid women because antibodies to CSA-binding parasite-infected erythrocytes, such as CS2, are generally absent among persons who have not been exposed to placental malaria.39,40 The results support the hypothesis that the major immune response to P falciparum–infected erythrocytes is directed toward PfEMP1; however, we cannot rule out the possibility that disruption of PfSBP1 has interfered with trafficking of other parasite surface proteins. However, the fact that other exported proteins are trafficked normally makes this possibility unlikely. To rule out the possibility that disruption of PfSBP1 has interfered with trafficking of other parasite erythrocyte proteins, we complemented the PfSBP1 gene in the ko cell line. The trafficking of PfEMP1 to Maurer clefts and its final destination on the surface was restored; therefore, together with the fact that proteins such as KAHRP, PfMAHRP1 and PfREX1 are trafficked normally, we can conclude that the effect of impairment on PfEMP1 trafficking is solely due to loss of PfSBP1.
In conclusion, disruption of PfSBP1 in P falciparum has shown that the protein is required for trafficking of the major virulence protein PfEMP1 from the parasite to Maurer clefts. Consequently, PfEMP1 cannot be displayed on the P falciparum–infected erythrocyte surface resulting in loss of adherence. Although PfSBP1 is the first protein identified in P falciparum directly involved in trafficking of PfEMP1 to the erythrocyte surface, it is likely that a large number of proteins are required for its export, and identification of PfSBP1 opens the way to identify other players in this crucial process pivotal to the pathogenesis of this human pathogen.
Authorship
Contribution: A.G.M. and M.R. designed and performed the experiments, analyzed the data, and wrote manuscript; M.T.O'N. performed the experiments; J.G.B. designed and performed the experiments and analyzed the data; M.M. designed and performed the experiments; J.R. supplied critical reagents and assisted in writing the manuscript; A.F.C. designed the experiments, analyzed the data, and wrote manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.G.M. and M.R. contributed equally to this work.
Correspondence: Alan F. Cowman, The Walter and Eliza Hall Institute of Medical Research, 1G, Royal Parade, Parkville, 3050, Melbourne, Australia; e-mail: cowman@wehi.edu.au.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Catherine Braun-Breton (anti-SBP1), Hans-Peter Beck (anti-MAHRP1), Mike Ryan (anti-GFP), and Mike Duffy (anti-ATS) for antibodies and the Red Cross Blood Service (Melbourne, Australia) for the supply of erythrocytes and serum. We thank Greg Kelly, Andrew Raiko, and Alfred Cortes for sample collection in PNG.
This work was supported by the National Institutes of Health (R01-A144008-04A1), the Wellcome Trust (066742), and the National Health and Medical Research Council of Australia (215 201); by an International Research Fellowship from the Howard Hughes Medical Institute (A.F.C.); and by The Walter and Eliza Hall Institute of Medical Research Miller Fellowship (J.G.B.).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal