Abstract
Signals through the B-cell antigen receptor (BCR) are important for the survival of chronic lymphocytic leukemia (CLL) cells. Therefore, factors that influence these signals have important pathophysiological roles in this disease. One key mediator of BCR signaling is protein kinase C β (PKCβ), which regulates the activation of I-κB kinases and the deactivation of Bruton tyrosine kinase within the signaling pathways initiated by BCR engagement. The present study demonstrates that overexpression of the PKCβII isoform is a feature of CLL cells and that activity of this enzyme strongly correlates with CLL cell response to BCR engagement. Thus, intracellular Ca2+ release and increases in cell survival after BCR cross-linking were significantly greater in CLL patients with low levels than in CLL patients with high levels of active PKCβII. Furthermore, BCR-induced Ca2+ fluxes could be restored in CLL patients with high levels of active PKCβII by pretreating the cells with the PKCβ-specific inhibitor LY379196. Conversely, BCR-mediated intracellular Ca2+ release could be inhibited in CLL cells with low levels of active PKCβII by pretreatment with the PKC agonist bryostatin. Taken together, these results demonstrate that overexpressed active PKCβII plays a role in the regulation and outcome of BCR signals that can be important for the progression of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a malignant disorder of mature B cells that is characterized by monoclonal expansion in blood, bone marrow, and lymphoid organs of cells arrested in the G0/G1 stage of the cell cycle.1 Factors involved in the clonal expansion of malignant CLL cells are not yet completely understood.
One approach to understanding CLL is to investigate the nature of intracellular signals responsible for the development and prolonged survival of the malignant cells.2 In this regard, signals generated by B-cell receptor (BCR) engagement are known to play an important role.1 A key mediator of BCR-induced signaling is protein kinase C (PKC).3-9 In CLL cells, this class of enzymes has been identified as a possible target of therapeutic intervention based on in vitro studies demonstrating that inhibition of these enzymes induces apoptosis.10-15 Given the role of BCR signals in the survival and clonal expansion of CLL cells and the role of PKC(s) in the signaling pathways induced by BCR engagement, it follows that PKC(s) may play an important role in the BCR-induced survival of CLL cells.
The PKC family is divided into 3 subgroups: the classical, which includes PKCα, βI, βII, and γ; the novel, which includes PKCδ, ϵ, η, and θ; and the atypical, which includes PKCλ and ζ. These enzymes are activated by the presence of Ca2+, diacylglycerol, or other activating factors,16 and they function in an array of cellular processes that can be specific for a particular cell type. In B cells, PKCζ,5 PKCβ,3,4,7,8 PKCδ,6 and PKCϵ9 play important roles in regulating signals generated by the BCR. With respect to CLL, active PKCδ is thought to maintain cell survival downstream of phosphoinositol 3′-kinase.15 Despite the potential of PKCs as therapeutic targets in CLL,10-15 little is known about the relative levels and activities of the different isoforms known to be expressed within the malignant cells of this disease.
In the present study, we show that PKCβII is overexpressed in CLL cells and that the activity of this enzyme inversely correlates with CLL cell response to BCR engagement. Therefore, by regulating BCR signals important for malignant cell survival, PKCβII may be a key factor in CLL progression.
Materials and methods
Materials
Mouse monoclonal and rabbit polyclonal anti-PKCβII, monoclonal anti-PKCβI, -PLCγ2, and -CD40 antibodies, rabbit anti-PKCα, -PKCδ, -PKCζ, Mcl-1 and procaspase-8, and horseradish peroxidase–conjugated anti–mouse and anti–rabbit immunoglobulin antibodies were purchased from Santa Cruz Biotechnology (Insight Biotechnology, Middlesex, United Kingdom). Monoclonal anti–PKCδ and anti–PKCϵ antibodies were purchased from BD Biosciences (Oxford, United Kingdom). F(ab2)′ fragments of goat anti–human IgM were purchased from Jackson ImmunoResearch Laboratories (Stratech, Soham, United Kingdom). Mouse anti–pS180-Bruton tyrosine kinase (Btk) and rabbit anti-Btk and anti–pY759-PLCγ2 antibodies were purchased from Cell Signaling Technology (New England Biolabs, Hitchin, Herts, United Kingdom). Purified recombinant PKCα, PKCβI, PKCβII, PKCδ, PKCϵ, and PKCζ proteins and Ro32-0432 were purchased from Merck Biosciences (Nottingham, United Kingdom). Purified recombinant PKCι and PKCμ proteins and mouse anti–ZAP-70 antibody were purchased from Upstate (Milton Keynes, United Kingdom). Indo-1AM, and Alexa-Fluor 630 antimouse and antirabbit antibodies were purchased from Molecular Probes (Invitrogen, Paisley, United Kingdom), and 3,3′-dihexyloxacarbocyanine iodide (DiOC6), propidium iodide (PI), and polyHEMA were from Sigma-Aldrich (Gillingham, United Kingdom). Enhanced chemiluminescence (ECL) reagents were purchased from Amersham Biosciences (Chalfont, United Kingdom). Immobilon membranes were from Millipore (Watford, United Kingdom). The PKCβII-specific inhibitor LY379196 was kindly provided by Lilly Research Laboratories (Indianapolis, IN).
Purification of cells
Normal B cells were purified from buffy coats obtained from the British Transfusion Service (Liverpool, United Kingdom). Hairy cell (HC) leukemia and CLL cells were obtained from the peripheral blood of patients after they gave informed consent and with the approval of the Liverpool Research Ethics Committee. Purified cells were cryopreserved. When required, the cells were thawed, resuspended in culture media, and equilibrated at 37°C, as described.17 In some experiments, the cells were further purified using anti-CD19 antibodies coupled to magnetic beads within a MiniMacs system (Miltenyi Biotech, Bisley, United Kingdom) according to the manufacturer's instructions. Cell purity was assessed by flow cytometry with a FACScalibur (Becton Dickinson, Mountain View, CA) and consisted of at least 90% B cells for all the patients used.
Diagnoses of HCL (n = 5 patients) and CLL (n = 63 patients) were based on standard morphologic, immunophenotypic, and cytogenetic criteria.18 CLL cell samples were from patients selected at random from our departmental tissue bank, in which patients with high malignant cell counts and advanced disease stage are overrepresented (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article).
Quantitation of PKC isoforms in cells
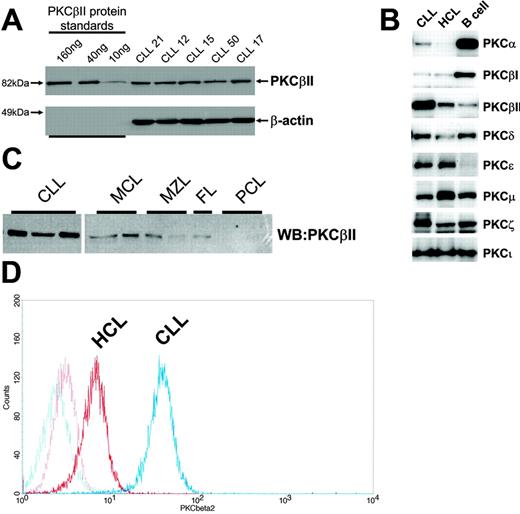
B lymphocytes were lysed with buffer A (125 mM Tris, pH 6.8, 5 mM EDTA, 1% SDS, 10% glycerol), sonicated to disrupt released DNA, and incubated for 10 minutes at 95°C. The protein concentration in the cell lysates was quantitated using a Bio-Rad (Hercules, CA) DC protein assay kit. Ten micrograms protein was loaded on 10% SDS-PAGE gels together with known amounts of purified recombinant proteins (PKCβII, PKCβI, PKCδ, PKCα, PKCζ, and PKCϵ), and the separated proteins were then electroblotted to Immobilon (Millipore, Bedford, MA) membranes. Membranes were blocked in 5% milk T-TBS (150 mM NaCl, 25 mM Tris, pH 7.5, 0.1% Tween-20) and were probed with anti-PKC antibodies. The membranes were further probed with second-layer antibodies conjugated with Alexa-Fluor 633 (Invitrogen, Eugene, OR) fluorochromes or with HRP. Results were read with an FLA-5000 imager (Fujifilm, Tokyo, Japan) for measurement of immunofluorescence or with an LAS-1000 (Fujifilm) for measurement of chemoluminescence after treatment of the membrane with ECL-Plus (Amersham Biosciences) substrate reagents. Specific protein quantitation was achieved by comparing the immunofluorescence and chemiluminescence of PKC bands within CLL cell lysates with those of recombinant protein bands used as standards on the same Western blot, as depicted for PKCβII in Figure 1A. Protein quantities are reported as percentage values of total loaded protein (10 μg). Equal protein loading was validated by Western blot analysis of β-actin in the CLL cell lysates (Figure 1A).
Profile of PKC isoform expression in mature B lymphocytes. (A) Quantitation of PKCβII expression in CLL cells by Western blotting. Equal amounts of protein were used from each CLL patient, and the intensity of the PKCβII-reactive band was compared with that generated from known amounts of recombinant PKCβII. β-Actin was used to indicate equal protein loading. (B) Western blot analysis of PKC isoform expression by CLL, HCL, and CD19-purified normal B cells. Equal amounts of protein were analyzed for each PKC isoform and cell type. This figure is representative of 3 separate experiments with different patients. (C) Western blot analysis of PKCβII expression in CLL cells, mantle cell lymphoma (MCL) cells, marginal zone lymphoma (MZL) cells, follicular lymphoma (FC) cells, and plasma cell leukemia (PCL) cells. Equal amounts of protein were loaded for each cell type. (D) Flow cytometric analysis of PKCβII expression in permeabilized CLL and HCL cells. This figure is representative of 2 separate experiments in different patients with CLL and HCL.
Profile of PKC isoform expression in mature B lymphocytes. (A) Quantitation of PKCβII expression in CLL cells by Western blotting. Equal amounts of protein were used from each CLL patient, and the intensity of the PKCβII-reactive band was compared with that generated from known amounts of recombinant PKCβII. β-Actin was used to indicate equal protein loading. (B) Western blot analysis of PKC isoform expression by CLL, HCL, and CD19-purified normal B cells. Equal amounts of protein were analyzed for each PKC isoform and cell type. This figure is representative of 3 separate experiments with different patients. (C) Western blot analysis of PKCβII expression in CLL cells, mantle cell lymphoma (MCL) cells, marginal zone lymphoma (MZL) cells, follicular lymphoma (FC) cells, and plasma cell leukemia (PCL) cells. Equal amounts of protein were loaded for each cell type. (D) Flow cytometric analysis of PKCβII expression in permeabilized CLL and HCL cells. This figure is representative of 2 separate experiments in different patients with CLL and HCL.
Real-time reverse transcription–polymerase chain reaction analysis of PKCβ mRNA expression
Total RNA was extracted from B cells with the RNeasy mini kit (QIAGEN, Crawley, United Kingdom) according to the manufacturer's instructions. One microgram purified RNA was reverse transcribed using Molony murine leukemia virus reverse transcriptase (Promega, Southampton, United Kingdom) and an oligo(dT)15 primer. Purified cDNA was mixed with DyNAmo SYBR green I qPCR master mix (Finnzymes, Espoo, Finland), a consensus PKCβ forward primer (5′-TGGGGTGACAACCAAGACATTC-3′) and reverse primers specific for PKCβI 5′-TGGAGGTGTCTCTCTTGTCTC-3′) or PKCβII (5′-GTCAATAATCCTGATGACTTCCTG-3′). We used β-actin and glyceraldehyde-3-phosphate dehydrogenase (gapdh) as internal controls, and these were amplified from each cDNA sample using the forward primer (5′-CCTCGCCTTTGCCGATCC-3′) and the reverse primer (5′-GGATCTTCATGAGGTAGTCAGTC-3′) for β-actin and the forward primer (5′-AGCCACATCGCTCAGAACAC-3′) and reverse primer (5′-GACGCATTGCTGATGATCTTG-3′) for gapdh.19 All polymerase chain reactions (PCRs) were performed on a DNA engine Opticon 2 system (Bio-Rad) under optimized, identical cycling conditions consisting of a 10-minute initial denaturing step at 95°C, followed by 45 cycles of amplification (denaturing at 94°C for 20 seconds, annealing at 60°C for 20 seconds, extension at 72°C for 20 seconds, and collecting fluorescence data at 80°C). After a final 10-minute extension at 72°C, a melting curve was measured from 65°C to 98°C. PCR products were reannealed at 72°C for 10 minutes if they were to be analyzed on agarose gels. The specificity of each of these PCR products was confirmed as a single band with the expected molecular size on agarose gels and as a narrow peak that appeared in the melting curve when temperatures rose higher than 80°C. Transcript levels of PKCβI and PKCβII were presented as a ratio to the transcript level of gapdh.
CLL cell viability assays
CLL cells, at 5 × 106 cells/mL, were cultured for 5 days with RPMI 1640 plus 0.5% BSA supplemented with L-glutamine and streptomycin in 24-well culture plates (Falcon, BD Biosciences, Cowley, United Kingdom) coated with polyHEMA. Cell viability was assessed by flow cytometry using DiOC6 to measure mitochondrial integrity and propidium iodide incorporation (as a measure of dead cells) according to established protocol.20 In some experiments, BCR was crosslinked with 10 μg/mL F(ab′)2 fragments of goat anti–human IgM antibody to promote CLL cell survival.
Measurement of intracellular Ca2+
Intracellular calcium concentration was determined by measuring fluorescence emission at 410 nm and at 485 nm during excitation at 350 nm according to a published protocol21 using an Aminco Bowman Series 2 spectrofluorometer (Thermo Electron, Middlewich, Cheshire, United Kingdom) and Indo-1AM as a fluorescent ratiometric probe.
Intracellular Ca2+ release in CLL cells was stimulated with the addition of 20 μg/mL goat anti–human IgM antibodies to the Indo-1–loaded cells. Fluorescence was monitored continuously, and intracellular [Ca2+] was determined using the software provided with the spectrofluorometer.21
Determination of membrane-associated PKCβII in CLL cells
Determination of membrane-associated PKCβII in CLL cells was performed by subcellular fractionation to obtain membrane and cytosolic fractions, as described.22 The protein concentration of each fraction was determined, and equal amounts of protein from each fraction were separated by SDS-PAGE. The amount of PKCβII in each fraction was determined by Western blotting. The percentage of membrane-associated PKCβII was taken as the amount of PKCβII in the membrane fraction divided by the amount of total PKCβII in both the membrane and the cytosolic fractions. Purity of the membrane and cytosolic fractions was verified by Western blot analysis for CD40 (membrane fraction)23 and procaspase-8 (cytosolic fraction)24,25 (data not shown).
PKC kinase assays
CLL cells (2 × 107 cells) were resuspended in buffer B (20 mM Tris, pH 7.4, 2 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, 1 μg/mL aprotinin) and incubated on ice for 30 minutes. The cells were disrupted by 10 passages through a 25-gauge needle, nuclei and other cellular debris were removed by centrifugation at 1000g for 10 minutes, and the supernatant was loaded on a HiTrap-DEAE-FF column (GE Healthcare, Little Chalfont, United Kingdom). The column was washed with 5 mL buffer B, and the PKC- containing fraction was eluted from the column with buffer B containing 200 mM NaCl. PKC activity was assessed in the eluate using a SignaTECT PKC assay system according to the manufacturer's instructions (Promega, Southampton, United Kingdom). PKCβ activity was calculated by subtracting PKC activity in the presence of 50 nM LY379196 from PKC activity in the absence of this PKCβ inhibitor.
Statistical analysis
Data sets were compared for statistical significance using either the Student t test or the Mann-Whitney U test. Comparisons were performed by computer with Microsoft Excel (Redmond, WA) and SPSS (Chicago, IL) version 13.0 software, respectively.
Results
Because PKC expression in CLL cells has not been fully characterized, the profile of PKC isoforms expressed in these cells was determined first and compared with PKC isoform expression in normal peripheral blood B cells, HCs, and a number of other mature malignant B cells.
Profiles of PKC isoforms expressed in CLL cells, HCs, and normal peripheral blood B cells
Analysis of PKC isoforms showed that CLL cells express PKCα, βI, βII, δ, ϵ, μ, ζ, and ι. These isoforms are also expressed in HCs (cells similar to CLL cells in having memory B-cell gene expression profiles26-28 ) and in normal peripheral blood B cells, but the expression levels differed between cell types (Figure 1B). Thus, CLL cells and HCs expressed less PKCα and βI and more PKCϵ than did normal B cells. Moreover, an overexpression of PKCβII clearly distinguished CLL cells from HCs and normal B cells (Figure 1B) and from mature B-lymphoid malignancies such as mantle cell lymphoma, marginal zone lymphoma, follicular lymphoma, and plasma cell leukemia (Figure 1C).
Quantitation of PKC isoform expression in CLL cell lysates using the method depicted in Figure 1A shows that PKCβII comprises 0.53% ± 0.25% of total cellular protein (n = 63). This, in comparison with other PKC isoforms (Table 1), establishes PKCβII as the dominant PKC isoform expressed in CLL cells.
PKC expression in CLL cells
| PKC isoform . | Total cellular protein, % . | No. cases used . |
|---|---|---|
| PKCα | 0.0028 ± 0.0009 | 5 |
| PKCβII | 0.53 ± 0.23 | 63 |
| PKCβI | 0.0096 ± 0.0012 | 6 |
| PKCδ | 0.13 ± 0.020 | 5 |
| PKCϵ | 0.021 ± 0.0088 | 5 |
| PKCμ | 0.12 ± 0.047 | 8 |
| PKCζ | 0.0097 ± 0.0036 | 6 |
| PKCι | 0.0082 ± 0.0035 | 8 |
| PKC isoform . | Total cellular protein, % . | No. cases used . |
|---|---|---|
| PKCα | 0.0028 ± 0.0009 | 5 |
| PKCβII | 0.53 ± 0.23 | 63 |
| PKCβI | 0.0096 ± 0.0012 | 6 |
| PKCδ | 0.13 ± 0.020 | 5 |
| PKCϵ | 0.021 ± 0.0088 | 5 |
| PKCμ | 0.12 ± 0.047 | 8 |
| PKCζ | 0.0097 ± 0.0036 | 6 |
| PKCι | 0.0082 ± 0.0035 | 8 |
Expression levels of various PKC isoforms in CLL cells are presented as the percentage fraction of total cellular protein.
PKCβII is overexpressed in CLL cells
PKCβII is an important mediator of BCR-induced signaling in B cells.3,4,7,8 To further explore the role of this PKC isoform in CLL cells, its expression in these cells was compared with that in HCs and normal B cells. Figure 2A shows that the amount of PKCβII protein in CLL cells is approximately 7-fold greater than in HCs or in normal B cells. PKC levels in CLL cells were measured in relation to known amounts of recombinant protein and were expressed as a percentage of total cellular protein to provide quantitative data for the expression of each isoform (Figure 1A). The validity of this approach was confirmed by FACS analysis of permeabilized cells showing that CLL cells express considerably more of this enzyme than do HCs (Figure 1D). Further analysis revealed pronounced variability in expression of PKCβII in CLL cells that was not observed in HCs or normal B cells. This variability, however, had no clear relationship with VH gene mutation or with CD38 expression (Table S1); however, higher PKCβII expression levels were observed in the malignant cells from patients with 11q23 and 17p13 abnormalities (responsible for ATM and p53 gene deletions, respectively, in CLL29 ) (Figure 2B; Table S1). Moreover, there was an apparent link with disease stage and tumor load at the time of sampling. Thus, cells from CLL patients with stage A disease had significantly lower PKCβII levels than did cells from CLL patients with stage C disease (Figure 2C; Table S1), and there was a positive relationship between PKCβII expression and white blood cell count (Figure 2D; Table S1). Taken together, these data demonstrate an overexpression of PKCβII that distinguishes CLL cells from HCs and normal B cells but do not provide insight into the underlying cause(s) of the variability in the expression of this protein in different CLL cell clones.
PKCβII is overexpressed by CLL cells. Quantitative analysis of PKCβII protein and mRNA expression. (A) Analysis of PKCβII protein in CLL, HCL, and CD19-purified normal B cells. (B) Analysis of PKCβII protein in CLL cells from patients with the indicated chromosomal abnormalities. 11q23 and 17p13 deletions were combined because these abnormalities are typically associated with severe disease.29 13q14 deletion and trisomy 12 chromosomal abnormalities were grouped with normal karyotypes because these abnormalities are not associated with severe disease.29 (C) Analysis of PKCβII protein in CLL cells from patients with different disease stages at the time of sampling. (D) Analysis of PKCβII protein in relation to white blood cell count. (A-D) PKCβII protein levels are expressed as a percentage of total cellular protein. (E) Analysis of PKCβII mRNA by quantitative RT-PCR in CLL cells and HCs. Results are expressed relative to β-actin mRNA in the same cell. Tests for statistical significance were performed with the Mann-Whitney U test.
PKCβII is overexpressed by CLL cells. Quantitative analysis of PKCβII protein and mRNA expression. (A) Analysis of PKCβII protein in CLL, HCL, and CD19-purified normal B cells. (B) Analysis of PKCβII protein in CLL cells from patients with the indicated chromosomal abnormalities. 11q23 and 17p13 deletions were combined because these abnormalities are typically associated with severe disease.29 13q14 deletion and trisomy 12 chromosomal abnormalities were grouped with normal karyotypes because these abnormalities are not associated with severe disease.29 (C) Analysis of PKCβII protein in CLL cells from patients with different disease stages at the time of sampling. (D) Analysis of PKCβII protein in relation to white blood cell count. (A-D) PKCβII protein levels are expressed as a percentage of total cellular protein. (E) Analysis of PKCβII mRNA by quantitative RT-PCR in CLL cells and HCs. Results are expressed relative to β-actin mRNA in the same cell. Tests for statistical significance were performed with the Mann-Whitney U test.
The PRKCB gene is transcribed as a single mRNA that is then variably spliced to yield the coding sequences for PKCβI and PKCβII.30 Reverse transcription–PCR (RT-PCR) analysis of RNA isolated from CLL cells showed that both splice variants were present and that mRNA for PKCβII was present in far greater quantities than mRNA for PKCβI (data not shown). This suggests that PKCβII is the preferred splice variant in CLL cells, a finding supported by the observation that PKCβII protein was present in 35.7 ± 15.2-fold greater quantities (n = 4) than was PKCβI.
A comparison of PKCβII mRNA expression in CLL cells, HCs, and normal B cells by quantitative RT-PCR mirrored the protein data. Figure 2E shows that PKCβII mRNA was more highly expressed in CLL cells than in HCs. In Figure 2E, PKCβII mRNA levels in normal B cells are not included because, although PKCβII mRNA was present in these cells (n = 4), its expression levels were too low to be reliably quantitated using the same method that was used for CLL cells and HCs (data not shown). Taken together, these data demonstrate that PKCβII is overexpressed in CLL cells at both the mRNA and the protein levels.
PKCβII is active in CLL cells
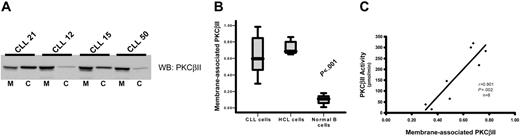
To measure PKCβII activity in CLL cells, subcellular fractions were prepared to establish the ratio between membrane-bound and cytosolic fractions of the total enzyme. This method was used because membrane association is an established paradigm of PKC activation.16,31 Furthermore, immune-complex kinase assays cannot be used to directly measure PKCβII activity because of an inability of specific antibodies to reliably immunoprecipitate active enzyme (data not shown) and because of a reduction of activating lipids by the detergents used to lyse the cells during the immunoprecipitation procedure. Measurement of the ratios of membrane and cytosolic PKCβII clearly demonstrated that the degree of PKCβII activation varied between different CLL patients (Figure 3A-B). This variation in the degree of PKCβII activation, as measured by membrane association, was confirmed using a second technique by which PKCβ activity was assessed in kinase assays of crude PKC extracts from CLL patients. Thus, a near perfect correlation between PKCβ kinase activity and PKCβII membrane association was observed (Figure 3C). Therefore, we used membrane association as a measure of PKCβII activity for the rest of this study. In HCs, PKCβII was almost entirely membrane associated, in contrast to that in normal B cells, in which the enzyme was predominantly cytosolic (Figure 3B), reflecting the respective “activated” and “resting” states of these 2 cell types. In CLL cells, PKCβII activity was much higher than in normal B cells (P < .001), but not all patients showed the same high degree of membrane association as observed in HCs (Figure 3B). This suggested that stimuli that activate PKCβII in some CLL patients may not be present in others.
PKCβII is active in CLL cells. Subcellular fractions from CLL, HCL, and CD19-purified normal B cells were isolated using ultracentrifugation. (A) Western blot analysis of the distribution of PKCβII between membrane, designated M, and cytosol, designated C, fractions in the malignant cells from representative CLL patients. (B) Quantitative representation of membrane-association of PKCβII in CLL cells (n = 25), HCL cells (n = 5), and normal B cells (n = 3). Results are presented as the ratio of the band density of PKCβII in the membrane fraction over the combined densities within the membrane and cytosolic fractions. Tests for statistical significance were performed using the Mann-Whitney U test. (C) Correlation between PKCβII membrane association in CLL cells with an in vitro kinase assay of enzyme activity in crude preparations of total cellular PKC.
PKCβII is active in CLL cells. Subcellular fractions from CLL, HCL, and CD19-purified normal B cells were isolated using ultracentrifugation. (A) Western blot analysis of the distribution of PKCβII between membrane, designated M, and cytosol, designated C, fractions in the malignant cells from representative CLL patients. (B) Quantitative representation of membrane-association of PKCβII in CLL cells (n = 25), HCL cells (n = 5), and normal B cells (n = 3). Results are presented as the ratio of the band density of PKCβII in the membrane fraction over the combined densities within the membrane and cytosolic fractions. Tests for statistical significance were performed using the Mann-Whitney U test. (C) Correlation between PKCβII membrane association in CLL cells with an in vitro kinase assay of enzyme activity in crude preparations of total cellular PKC.
Effect of PKCβ inhibition on CLL cell survival
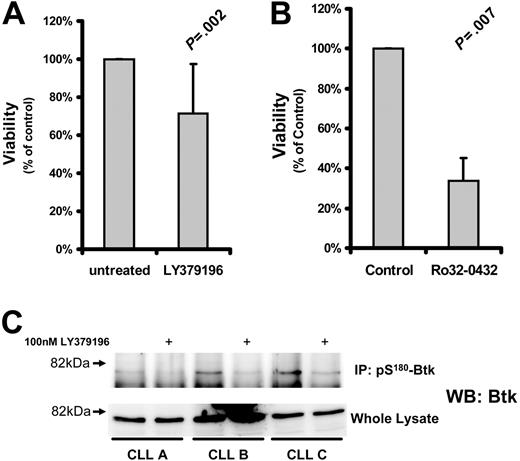
Several studies have suggested that PKCs play an important role in CLL cell cytoprotection.10-15 To determine the role of PKCβII in CLL cell survival, we used the PKC inhibitor LY379196 at concentrations that specifically inhibit PKCβI and PKCβII while having little effect on other PKC isozymes32 and protein kinases.33 Incubation of CLL cells for 5 days with this inhibitor resulted in a relatively small reduction in cell viability (Figure 4A) although the phosphorylation of Btk, a known substrate of PKCβII,3 was clearly reduced by this treatment (Figure 4C). In contrast, treatment of CLL cells with more general PKC inhibitors such as Ro32-0432 (Figure 4B) or safingol (data not shown) led to a much greater reduction in cell viability. These results indicate that PKCβII does not play a decisive role in protecting CLL cells from spontaneous apoptosis.
Effect of PKC inhibition on CLL cell survival. CLL cells were treated with the indicated inhibitors and measured for viability. Viability of the treated cells was normalized to that of untreated cells and is reported as a percentage thereof. (A) Viability (mean ± SD) of CLL cells incubated with 1 μM LY379196 for 5 days (n = 15). (B) Viability of CLL cells incubated with 10 μM Ro32-0432 for 3 days (n = 4). Tests for statistical significance were performed using Student t test for paired data. (C) pS180-Btk was immunoprecipitated from RIPA lysates of CLL cells incubated in the presence or absence of 100 nM LY379196. Immunoprecipitated proteins and whole cell lysates were separated by SDS-PAGE, and Western blots were probed with anti-Btk antibodies. CLL A, CLL B, and CLL C are new patients not represented in Table S1.
Effect of PKC inhibition on CLL cell survival. CLL cells were treated with the indicated inhibitors and measured for viability. Viability of the treated cells was normalized to that of untreated cells and is reported as a percentage thereof. (A) Viability (mean ± SD) of CLL cells incubated with 1 μM LY379196 for 5 days (n = 15). (B) Viability of CLL cells incubated with 10 μM Ro32-0432 for 3 days (n = 4). Tests for statistical significance were performed using Student t test for paired data. (C) pS180-Btk was immunoprecipitated from RIPA lysates of CLL cells incubated in the presence or absence of 100 nM LY379196. Immunoprecipitated proteins and whole cell lysates were separated by SDS-PAGE, and Western blots were probed with anti-Btk antibodies. CLL A, CLL B, and CLL C are new patients not represented in Table S1.
Role of PKCβII in BCR signaling
In normal B cells, the phosphorylation of Btk by PKCβII inhibits the ability of Btk to associate with the membrane, resulting in the feedback inhibition of BCR signals.3 It was, therefore, important to establish whether, in CLL cells, the levels of membrane-associated Btk correlate with active PKCβII. Figure 5A shows that Btk in CLL cells with high PKCβII activity was mainly in the cytosol, whereas in CLL cells with low PKCβII activity Btk was mainly at the membrane, resulting in a negative correlation between these 2 parameters. The main substrate of Btk in B cells is phospholipase Cγ2 (PLCγ2), which is activated by Btk through the phosphorylation of tyrosine 759.34 Analysis of (pY759)-PLCγ2 showed a similar negative correlation with active PKCβII (Figure 5B). Taken together, these data indicate that active PKCβII in CLL cells can influence these cells through the down-regulation of BCR-mediated signals.
Levels of active PKCβII correlate with membrane-associated Btk and with pY759-phosphorylated PLCγ2. (A) Plot of membrane-associated Btk against membrane-associated (active) PKCβII in CLL cells (n = 12). The percentage membrane association of Btk in CLL cells was determined from membrane and cytosolic subcellular fractions, as described for PKCβII. (B) Plot of pY759-PLCγ2 against membrane-associated (active) PKCβII in CLL cells (n = 12). The degree of pY759-PLCγ2 was determined in Western blot analysis of whole cell lysates, taking the ratio of the densities of the bands recognized by antibodies specific for nonphosphorylated PLCγ2 and for pY759-PLCγ2. Results are reported in arbitrary units.
Levels of active PKCβII correlate with membrane-associated Btk and with pY759-phosphorylated PLCγ2. (A) Plot of membrane-associated Btk against membrane-associated (active) PKCβII in CLL cells (n = 12). The percentage membrane association of Btk in CLL cells was determined from membrane and cytosolic subcellular fractions, as described for PKCβII. (B) Plot of pY759-PLCγ2 against membrane-associated (active) PKCβII in CLL cells (n = 12). The degree of pY759-PLCγ2 was determined in Western blot analysis of whole cell lysates, taking the ratio of the densities of the bands recognized by antibodies specific for nonphosphorylated PLCγ2 and for pY759-PLCγ2. Results are reported in arbitrary units.
After BCR stimulation, activated PLCγ2 hydrolyzes phosphatidylinositol bisphosphate to inositol trisphosphate (IP3) and diacylglycerol, which are required for the release of intracellular Ca2+ and activation of PKC(s), respectively. Because feedback inhibition of BCR signaling by overexpressed PKCβII would be expected to influence this process, the BCR-induced Ca2+ fluxes in cells with different levels of PKCβII activity were examined next. Figure 6A-B shows that cells with high levels of PKCβII activity responded to BCR crosslinking with a significantly lower Ca2+ flux than cells with low levels of PKCβII activity. This difference could not be ascribed to effects of VH mutation on the cell response because the cells used in Figure 6A contained unmutated VH genes. In addition, the variation in Ca2+ flux could not be ascribed to differences in the expression of ZAP-70, a protein known to influence BCR signaling in CLL cells,35,36 because the CLL cells used in Figure 6A had similar levels of this enzyme (Figure 6A, inset). In addition, in CLL cells with high PKCβII activity, pretreatment of the cells with 100 nM LY379196 (to inhibit the activity of PKCβII) restored BCR-induced Ca2+ fluxes (Figure 6C). In contrast, in cells with low PKCβII activity, pretreatment of the cells with 10 nM bryostatin to activate PKCβII inhibited BCR-induced Ca2+ fluxes (Figure 6D). The fact that 100 nM LY379196 inhibited the PKCβII-mediated serine phosphorylation of Btk (Figure 4C) shows that this compound indeed acted to inhibit PKCβII in CLL cells. Taken together, these results demonstrate that overexpressed active PKCβII in CLL cells has an important modulatory influence on BCR signaling.
PKCβII activity regulates BCR-induced Ca2+ release in CLL cells. (A) CLL patients with high and low PKCβII activity (defined, respectively, as containing amounts of active PKCβII greater than or less than 1 SD from the mean value) were stimulated with 20 μg/mL anti-IgM antibody (BCR-XL), and intracellular Ca2+ release was measured using the dye Indo-1. Comparison of BCR-induced Ca2+ release in CLL patients with high and low levels of active PKCβII. Western blot analysis of ZAP-70 expression in these patients is shown in the inset. CLL21 is the CLL patient with low PKCβII activity. CLL50 is the CLL patient with high PKCβII activity. (B) Comparison of peak Ca2+ levels (mean ± SD) during BCR stimulation of CLL patients with high (n = 5) and low (n = 5) levels of active PKCβII. (C) One-hour pretreatment with 100 nM LY379196 restores BCR-induced Ca2+ release in CLL patients with high levels of active PKCβII. (D) Thirty-minute pretreatment with 10 nM bryostatin suppresses BCR-induced Ca2+ release in CLL patients with low levels of active PKCβII. (C-D) Representative examples of 3 separate experiments using cells from different patients.
PKCβII activity regulates BCR-induced Ca2+ release in CLL cells. (A) CLL patients with high and low PKCβII activity (defined, respectively, as containing amounts of active PKCβII greater than or less than 1 SD from the mean value) were stimulated with 20 μg/mL anti-IgM antibody (BCR-XL), and intracellular Ca2+ release was measured using the dye Indo-1. Comparison of BCR-induced Ca2+ release in CLL patients with high and low levels of active PKCβII. Western blot analysis of ZAP-70 expression in these patients is shown in the inset. CLL21 is the CLL patient with low PKCβII activity. CLL50 is the CLL patient with high PKCβII activity. (B) Comparison of peak Ca2+ levels (mean ± SD) during BCR stimulation of CLL patients with high (n = 5) and low (n = 5) levels of active PKCβII. (C) One-hour pretreatment with 100 nM LY379196 restores BCR-induced Ca2+ release in CLL patients with high levels of active PKCβII. (D) Thirty-minute pretreatment with 10 nM bryostatin suppresses BCR-induced Ca2+ release in CLL patients with low levels of active PKCβII. (C-D) Representative examples of 3 separate experiments using cells from different patients.
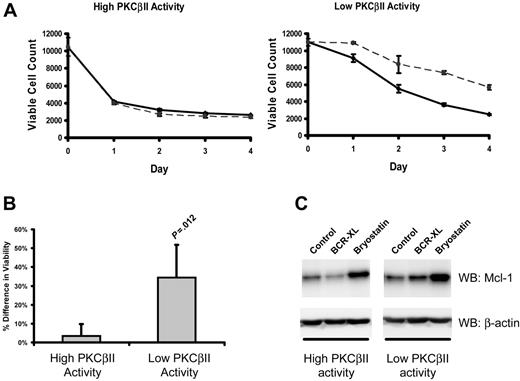
In CLL cells, BCR stimulation provides important prosurvival signals.1 Therefore, to examine how the survival of CLL cells is affected by different levels of active PKCβII, the viability of different CLL cell clones was measured after culture with a BCR-crosslinking agent. Figure 7A-B shows that BCR crosslinking enhanced the survival of malignant cells from CLL cells with low levels of PKCβII activity, whereas such stimulation had little or no effect on the malignant cells from CLL cells with high levels of PKCβII activity.
PKCβII activity regulates BCR-induced survival of CLL cells. Cells from the same CLL patients with high and low PKCβII activity, as described in Figure 6, were incubated with 10 μg/mL F(ab′)2 fragments of goat anti–human IgM. (A) Aliquots of stimulated (dashed line) and control (solid line) CLL cells were taken at 1-day intervals and were stained with PI and DiOC6 to determine viability using flow cytometry with a fixed time setting of 30 seconds to quantitate the number of cells per unit volume.37 The data presented in each graph are representative of a single experiment using 4 CLL patients with high and 4 CLL patients with low levels of PKCβII activity. Each experiment was performed in triplicate. (B) Percentage difference (mean ± SD) in cell viability between stimulated and control cells with high and low PKCβII activity in CLL patients after BCR stimulation. Cell viability was measured at day 3 of culture. Tests for statistical significance were performed using a Mann-Whitney U test (n = 5). (C) Influence of BCR crosslinking (BCR-XL) and bryostatin on Mcl-1 expression in CLL cells with different levels of PKCβII activity (representative Western blots of experiments with cells from 3 CLL patients with high and 3 CLL patients with low levels of PKCβII activity). CLL cells were stimulated with 10 nM bryostatin or with BCR crosslinking for 24 hours.
PKCβII activity regulates BCR-induced survival of CLL cells. Cells from the same CLL patients with high and low PKCβII activity, as described in Figure 6, were incubated with 10 μg/mL F(ab′)2 fragments of goat anti–human IgM. (A) Aliquots of stimulated (dashed line) and control (solid line) CLL cells were taken at 1-day intervals and were stained with PI and DiOC6 to determine viability using flow cytometry with a fixed time setting of 30 seconds to quantitate the number of cells per unit volume.37 The data presented in each graph are representative of a single experiment using 4 CLL patients with high and 4 CLL patients with low levels of PKCβII activity. Each experiment was performed in triplicate. (B) Percentage difference (mean ± SD) in cell viability between stimulated and control cells with high and low PKCβII activity in CLL patients after BCR stimulation. Cell viability was measured at day 3 of culture. Tests for statistical significance were performed using a Mann-Whitney U test (n = 5). (C) Influence of BCR crosslinking (BCR-XL) and bryostatin on Mcl-1 expression in CLL cells with different levels of PKCβII activity (representative Western blots of experiments with cells from 3 CLL patients with high and 3 CLL patients with low levels of PKCβII activity). CLL cells were stimulated with 10 nM bryostatin or with BCR crosslinking for 24 hours.
Mcl-1 up-regulation is an important component of BCR-induced survival of CLL cells.38 Therefore, we next examined the effect of BCR crosslinking on Mcl-1 expression in CLL cells with high and low levels of PKCβII activity. BCR stimulation induced a 1.4 ± 0.1-fold (P = .045; n = 3) increase in Mcl-1 expression in PKCβII low-activity CLL cells (Figure 7C). In contrast, in PKCβII high-activity cells, BCR crosslinking did not lead to a significant change in Mcl-1 expression (Figure 7C). This modulation of BCR-induced survival of malignant cells by PKCβII suggests that the enzyme may influence CLL progression through its effects on BCR signaling and its outcomes.
Discussion
Although it is clear that BCR signals are important for CLL cell survival and that PKCs may play a role in the regulation of these signals, neither the identity nor the role(s) of the involved PKC(s) were known. Therefore, the aim of the present study was to identify and characterize the PKC isozymes that participate in the regulation of BCR-mediated CLL cell survival. The data presented here demonstrate that CLL cells overexpress PKCβII and show an important role for this isozyme in the regulation of BCR signaling and its outcomes.
The overexpression of PKCβII in CLL cells is important for several reasons. In mature and developing B cells, PKCδ is the dominantly expressed PKC isoform.39 Moreover, during B-cell maturation to the memory stage, which is thought to correspond to the CLL development stage,27,28,40 PKCβ expression remains constant41 or may be downregulated.42 In addition, the levels of PKCβII are higher in CLL cells than in HCs, which, like CLL cells, have also reached a memory cell stage of differentiation.26,28 In diffuse large B-cell lymphoma (DLBCL), high PKCβII expression identifies cells with an activated B-like phenotype43,44 ; however, the overall level of PRKCB gene expression in DLBCL is lower than that observed in CLL.40,45 Thus, PKCβII overexpression may be a feature unique to CLL that could be used as an aid in disease diagnosis. Whether high PKCβII expression reflects the cell of origin in CLL is unknown. Further studies comparing gene expression in B cells differentiating under the influence of different microenvironments will have to be performed before this question can be addressed.
Overexpression of PKCβII in CLL cells could also be a specific feature of cell malignancy. This notion is supported by observations that the clinical course of activated B-like DLBCL, in which PKCβII is overexpressed, is more aggressive than GC-like DLBCL,43-46 and by reported high PKCβII levels in cancers of the colon, in which it is suggested that overexpressed enzyme can promote carcinogenesis in these cells.47,48
In CLL, expansion of the malignant cell clones is thought to involve chronic antigenic stimulation1 whereby PKCβII may contribute to expansion through its established role in BCR and Toll-like receptor (TLR) signaling.3,4,7,8,49-51 PKCβII initially participates in positive BCR-induced signaling for cell proliferation and survival,52 but it is also an important component of feedback inhibition of such signaling through a mechanism involving phosphorylation-induced dissociation of Btk from the cell membrane.3 Therefore, we examined the correlation between the levels of active PKCβII in CLL cells and cell signals thought to originate from BCR. This showed that constitutively active PKCβII levels negatively correlated with levels of membrane-associated Btk and with tyrosine phosphorylation of its substrate PLCγ2. The notion that PKCβII may influence CLL cells through the down-regulation of BCR-mediated signals is further supported by its inhibitory effects on BCR-induced Ca2+ release and cell survival. Thus, CLL cells with high levels of active PKCβII responded to BCR crosslinking with a significantly lower Ca2+-flux than did those with low levels of PKCβII activity. Moreover, BCR crosslinking had a greater prosurvival effect on CLL cells with low PKCβII activity than on cells with high PKCβII activity. The decrease in prosurvival effect of IgM crosslinking observed in cases with high PKCβII activity levels could be explained by the pronounced feedback inhibition of BCR signaling seen under these circumstances. A possibility that differences in BCR responsiveness reflected an abnormality upstream of PKCβII53 was excluded by the demonstration that the defect in BCR-induced Ca2+ release was overcome by inhibiting PKCβII with LY379196 and that high Ca2+ fluxes in CLL cells with low levels of active PKCβII were reduced when this enzyme was activated by bryostatin. Taken together, these results demonstrate that active PKCβII in CLL cells has a regulatory influence on BCR signaling and its outcomes and provide a mechanistic explanation for frequently observed variation in BCR-induced Ca2+ release and survival.53-56
The heterogeneity of CLL cell responses to BCR-induced signals is well documented36,37,53-58 and may involve the induction of cell death56,58,59 and a variable degree of cell protection from spontaneous apoptosis.38,53,54 Heterogeneity in response to BCR stimulation is related to the degrees of CD38 and ZAP-70 expression36,56-58 and the degree of mutation of VH genes.53,57,58 Furthermore, one report has shown that ZAP-70 expression enhances IgM signaling in CLL cells.35 Here we show that by causing feedback inhibition of upstream signals, PKCβII has a ZAP-70–independent effect on the cell responses to BCR stimulation. This effect was clearly not caused by variation in ZAP-70 because expression levels of this kinase were equal in the CLL cells with high and low levels of PKCβII activity (Figure 6A). Thus, heterogeneity in the levels of expression and activation of PKCβII in CLL observed in the present study could at least partially explain the differences in the effects of BCR stimulation on CLL cell survival.
In the present study we showed that, unlike other PKC isoforms,10-15 PKCβII does not play a decisive role in the cytoprotection of CLL cells from spontaneous apoptosis. Thus, the inhibition of PKCβII activity with LY379196 had only a minimal effect on the survival of unstimulated CLL cells, whereas the effects of more general PKC inhibitors were more profound. In addition, we confirmed that one of the candidate survival factors induced by BCR crosslinking is Mcl-1.38 The up-regulation of this survival factor was also suppressed in CLL cells with high PKCβII activity.
It is thought that the survival of malignant CLL cells, like that of normal B-1 cells and T cells, depends on a low-affinity interaction of BCR with self-antigens.1 However, when drawing a parallel between normal mature B cells and CLL cells, additional properties unique to the malignant cells must be considered. Thus, in contrast to quiescent normal B cells, CLL cells are thought to be subjected to chronic antigenic stimulation.1 Moreover, unlike normal B cells, antigen-stimulated CLL cells fail to undergo terminal differentiation into antibody-secreting cells.1 Although turnover studies show that malignant CLL cells have relatively limited lifespans, the balance between cell proliferation and cell death favors clonal expansion of these cells.60 Therefore, BCR signals, known to protect CLL cells from apoptosis, are likely to play an important role in the progression of CLL. The results of the present study support a hypothesis by which, in CLL cells, overexpressed and activated PKCβII functions as a feedback switch that keeps cell activation below the potentially proapoptotic level responsible for negative cell selection. Below this level, BCR signaling is involved in cell rescue, which is also kept in balance by PKCβII in a way that activation of this overexpressed enzyme may limit these prosurvival signals. Presumably, the expansion of the malignant cell clone is then principally driven by proliferative and cell rescue stimuli mainly originating from sources other than BCR. The likely candidates are receptor tyrosine kinases for autocrine growth factors such as VEGF,61 TNF receptor families including CD4062 and BAFF receptor,63 and receptors for cytokines such as IL-4.64 Our observation that PKCβII is more highly expressed in the malignant cells of patients with advanced disease suggests that these other factors, rather than BCR, provide signals for disease progression in the late stages of CLL.
In conclusion, the present study demonstrates that PKCβII is overexpressed in CLL cells and that the activity level of this enzyme is inversely related to the ability of these cells to respond to BCR stimulation. Given that antigenic stimulation is a major factor involved in the selection, clonal expansion, and survival of CLL cells, the results of the present study suggest an important role for PKCβII in the modulation of the consequences of this stimulation.
Authorship
Contribution: S.T.A., T.L., and J.R.S. designed the research. S.T.A., T.L., G.M.J., K.L., A.T.T., M.F., M.H., and J.R.S. performed the research. S.T.A., T.L., M.Z., and J.R.S. analyzed the data. M.Z. and J.R.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
S.T.A. and T.L. contributed equally to this study.
Correspondence: Joseph R. Slupsky, Department of Haematology, University of Liverpool, Duncan Building, Daulby Street, Liverpool, United Kingdom, L69 3GA; e-mail: jslupsky@liverpool.ac.uk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by Leukaemia Research Fund UK.
We thank Drs J.C. Cawley, P.M. Johnson, and P.D. Sherrington of the University of Liverpool for critical reading of this manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal