Abstract
Trafficking of dendritic cells (DCs) to peripheral tissues and to secondary lymphoid organs depends on chemokines and lipid mediators. Here, we show that bone marrow–derived DCs (BM-DCs) express functional leukotriene B4 (LTB4) receptors as observed in dose-dependent chemotaxis and calcium mobilization responses. LTB4, at low concentrations, promoted the migration of immature and mature DCs to CCL19 and CCL21, which was associated with a rapid (30-minute) increase of CCR7 expression at the membrane level. At longer incubation times (6 hours), gene array analysis revealed a promoting role of LTB4, showing a significant increase of CCR7 and CCL19 mRNA levels. BM-DCs cultured from BLT1−/− or BLT1/2−/− mice showed a normal phenotype, but in vivo BLT1/2−/−DCs showed dramatic decrease in migration to the draining lymph nodes relative to wild-type (WT) DCs. Consistent with these observations, BLT1/2−/− mice showed a reduced response in a model of 2,4-dinitro-fluorobenzene (DNFB)–induced contact hypersensitivity. Adoptive transfer of 2,4-dinitrobenzene sulfonic acid (DNBS)–pulsed DCs directly implicated the defect in DC migration to lymph node with the defect in contact hypersensitivity. These results provide strong evidence for a role of LTB4 in regulating DC migration and the induction of adaptive immune responses.
Introduction
Dendritic cells (DCs) play a unique role in the activation of antigen-specific naive T lymphocytes.1,2 To perform this function, antigen-loaded DCs travel from peripheral tissues to lymph nodes. This migration is dependent on the expression of CCR7 by DCs and the production of CCR7 ligands, namely CCL19, by stromal cells and mature DCs in the lymph node and CCL21 by afferent lymphatic cells.3-7 However, the expression of CCR7 is not sufficient to ensure the migration of mature DCs.8-10 Experimental evidence generated in vitro and in vivo has shown that CCR7 function is dependent on the presence of costimulatory signals, including cysteinyl-leukotrienes and their membrane transporter (MRP1), as well as prostaglandin E2.9,10 These results indicate that in vivo, the local inflammatory microenvironment acts critically in regulating the migration of maturing DCs.
Inflammatory cytokines and microbial agents are known to induce phospholipid metabolism and the activation of arachidonic acid cascade.11 Leukotriene B4 (LTB4) is a potent chemoattractant generated by sequential actions of cytosolic phospholipase A2, 5-lipoxygenase and leukotriene A4 hydrolase on membrane phospholipids.11,12 Previous studies have shown that LTB4 is a mediator of innate immunity, based on its chemotactic effect for phagocytic leukocytes.13,14 Two distinct G protein–coupled receptors, the high affinity BLT115 and the low affinity BLT2,16 have been identified as LTB4 receptors. In this study, we show that LTB4 is also a key mediator of adaptive immunity through the regulation of DC migration to secondary lymphoid organs.
Materials and methods
All animal studies and procedures were approved by the Animal Care and Use Committee of University of Louisville Research Resources Center.
Reagents
Murine CCL3, CCL19, and CCL21 were from PeproTech (Rocky Hill, NJ). Recombinant murine granulocyte macrophage–colony-stimulating factor (rmGM-CSF), human Fms-like kinase-3 (Flt-3) ligand (hFlt3L), and recombinant murine tumor necrosis factor α (rmTNF-α) were from R&D Systems (Minneapolis, MN). Cytokines were endotoxin free as assessed by Limulus amebocyte assay (BioWhittaker, Walkersville. MD). LTB4 was purchased from Cayman Chemical (Ann Arbor, MI).
Dendritic cell culture
BLT1−/− and BLT1/2−/−17 mice backcrossed onto B6 background for 7 generations and C57/B6 mice from the National Cancer Institute (NCI) (wild-type [WT]) were used at 8 to 12 weeks of age. CD34+-derived myeloid DCs were generated by positive immunoselection using the rat monoclonal antibody (mAb) MEC 14.7 to mouse CD34 (kindly provided by Dr Vecchi, Milan, Italy) followed by MACS micro beads coated with goat anti–rat IgG (Miltenyi Biotec, Auburn, CA) as previously described.18 DCs were characterized in terms of membrane phenotype (expression of FITC-MHC II, PE-CD11c, FITC-CD86, and PE-CD80 from BD-Pharmingen, San Diego, CA; PE-CCR7 from E-Bioscience, San Diego, CA19), pinocytosis, and antigen presentation in allogeneic mixed leukocyte reaction (MLR) were determined as previously described.18 To induce maturation, DCs were cultured with 20 ng/mL murine TNF-α for the last 24 hours of culture.
Chemotaxis assay
Cell migration was evaluated using a 48-well chemotaxis chamber (Neuroprobe, Pleasanton, CA) and polycarbonate filters (5-μm pore size; Neuroprobe) for a 90-minute incubation as previously described.4 Results are expressed as number of migrated cells in an average of 5 high-power fields (100×).
Calcium mobilization
Calcium mobilization was monitored in Indo-I–loaded cells stimulated with various concentrations of LTB4 as previously described.13 Briefly, 1 × 106 cells were loaded with 1.2 μL Indo-1 (1 mM solution) in the presence of 0.6 μL pluronic acid (200 mg/mL in Me2SO) for 30 minutes at 37°C. The response to various concentrations of ligand was recorded using a spectrofluorometer (F2500; Hitachi, San Jose, CA).
Gene array analysis
LTB4-induced changes in cytokine and chemokine mRNA levels were determined using Gene arrays (SuperArray, Frederick, MD) according to the manufacturer's protocol. Briefly, 2 μg RNA was used to synthesize biotinylated cDNA probes to be hybridized with mouse cytokine and chemokine arrays (SuperArray). A streptavidin-labeled alkaline phosphatase reporter antibody was added to the array, followed by development with a chemiluminescent substrate (CDP, Star; SuperArray). Chemiluminescence was detected on photographic film (Biomax Light, GBX fixer/developer; Eastman Kodak, Rochester, NY) with multiple exposures taken with different exposure time, which were normalized by GAPDH saturation. These films were scanned and quantified using GEArray Analyzer software (SuperArray).
Confocal laser scanning microscopy
Immature DCs (iDCs) were washed with ice-cold phosphate-buffered saline (PBS)/1% fetal bovine serum (FBS) (washing buffer) and fixed in 4% paraformaldehyde for 10 minutes on ice, and additionally for 30 minutes at room temperature. The cells were then permeabilized by incubation in washing buffer containing 0.1% saponin for 3 minutes at room temperature. Mouse CCL19-hIgG fusion protein (eBioscience) followed by PE-conjugated anti–human IgG antibody was used for mouse CCR7 detection.20 Cell morphology and fluorescence intensity were analyzed using a Zeiss LSM510 Meta confocal laser scanning microscope (Zeiss, Jena, Germany) equipped with a Plan Neo Fluar 100 ×/1.3 numerical aperture (NA) oil objective and a 10 × eyepiece. Imaging medium is PBS; images were acquired using LSM510 Meta software, and were processed using LSM image examiner.
In vivo migration of murine DCs
WT and BLT1/2−/− mature DCs were labeled with 0.5 μM of the vital dye 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester, mixed isomer (5-(6)-CFDA, SE [CFSE]; Molecular Probes, Eugene, OR) and injected subcutaneously in the hind leg footpad as previously described.18 Briefly, popliteal lymph nodes were recovered at the indicated time points, mechanically disaggregated, and treated with a collagenase A (1 mg/mL; Boehringer Mannheim, Indianapolis, IN) and DNase (0.4 mg/mL; Roche, Indianapolis, IN) mixture for 30 minutes; the enzymatically treated cell suspension was evaluated by FACScan (Becton Dickinson, San Jose, CA).
Contact hypersensitivity (CHS)
The hapten 2,4-dinitro-fluorobenzene (DNFB; Sigma, St Louis, MO) was freshly prepared before CHS assays as previously described.18 For sensitization, mice were painted once (day −5) on the shaved abdominal skin with 50 μL of 0.5% DNFB in 4:1 acetone/olive oil (vol/vol) and 5 μL on each footpad. Five days later (day 0) mice were challenged by the application of 10 μL DNFB (0.2%) on each side of the right ear, while the left ear received the vehicle alone. CHS response was determined by measuring the degree of ear swelling of the antigen-painted ear compared with that of the vehicle-treated contra lateral ear at the indicated times after challenge using a dial thickness gauge (Mitutoyo, Cardiff, United Kingdom). In some experiments CHS response was evaluated after in vivo inoculation of DCs loaded with 2,4-dinitrobenzene sulfonic acid (DNBS; ICN Biomedical, Aurora, OH).18,21 Briefly, bone marrow (BM)–derived DCs from WT and BLT1/2−/− mice were resuspended in Hanks balanced salt solution (HBSS) without fetal calf serum (FCS) containing 100 μg/mL DNBS and incubated at 37°C for 30 minutes. For sensitization (day −5) 1 × 106 DNBS-pulsed BM-DCs from WT and BLT1/2−/− mice were injected subcutaneously into the flank of WT recipient mice, in 200 μL saline. Five days later (day 0), mice were challenged by the application of DNFB (0.2%) on each side of the right ear. A group of mice injected with the same number of PBS-treated DCs served as a negative control.
Statistical analysis
Experimental groups include at least 5 mice. All experiments were performed at least 3 times. Statistical significance was evaluated using the 2-tailed Student t test.
Results
Functional expression of LTB4 receptors in murine DCs
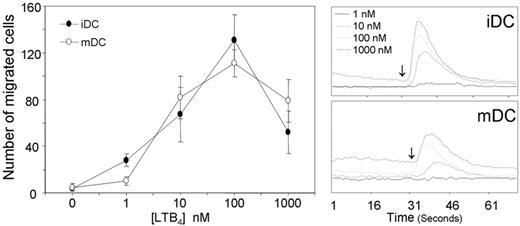
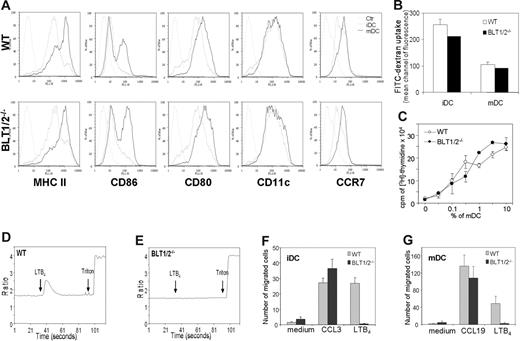
Dendritic cells cultured from BM precursors express functional LTB4 receptors as evaluated by chemotactic response (Figure 1, left panel) and calcium fluxes (Figure 1, right panel), regardless of their state of maturation (ie, iDCs and TNF-mature DCs [mDCs]). Both in chemotaxis and in calcium mobilization assays the peak response was observed at the concentration of 100 nM. BM-derived DCs from BLT1/2−/− mice behaved in a similar manner to WT DCs in terms of membrane phenotype (Figure 2A), antigen-uptake (Figure 2B), and induction of allogenic T-cell proliferation (Figure 2C). In contrast, BLT1/2−/− DCs were not able to respond to LTB4 in terms of calcium mobilization (Figure 2D-E). Similarly, BLT1/2−/− DCs did not migrate in response to LTB4, whereas a normal chemotactic response was detected in response to CCL3 (iDCs) and CCL19 (mDCs), indicating the absence of functional LTB4 receptors in BLT1/2−/− DCs (Figure 2F-G). Chemotaxis or calcium responses to LTB4 were also not observed in DCs cultured from BLT1−/− (data not shown), indicating that BLT1 is the LTB4 responsive receptor in DCs.
Functional expression of LTB4 receptors on murine BM-DCs. Immature (iDCs) and mature DCs (20 ng/mL TNF-α for 24 hours) were generated from BM CD34+ precursors in vitro. Chemotaxis (left panel): Ligand-dependent chemotaxis of both iDCs and mDCs was measured using a 48-well micro chemotaxis chamber, as described in “Chemotaxis assay.” Data are the mean ± SE of cells from 3 individual fields for each concentration from a representative experiment of at least 3 repetitions. Calcium mobilization (right panel): Both iDCs and mDCs loaded with Indo-1 were induced with various concentrations of LTB4, and Ca2+ mobilization was measured. The y-axis indicates the fluorescence ratio (F340/380). Arrows indicate LTB4 addition.
Functional expression of LTB4 receptors on murine BM-DCs. Immature (iDCs) and mature DCs (20 ng/mL TNF-α for 24 hours) were generated from BM CD34+ precursors in vitro. Chemotaxis (left panel): Ligand-dependent chemotaxis of both iDCs and mDCs was measured using a 48-well micro chemotaxis chamber, as described in “Chemotaxis assay.” Data are the mean ± SE of cells from 3 individual fields for each concentration from a representative experiment of at least 3 repetitions. Calcium mobilization (right panel): Both iDCs and mDCs loaded with Indo-1 were induced with various concentrations of LTB4, and Ca2+ mobilization was measured. The y-axis indicates the fluorescence ratio (F340/380). Arrows indicate LTB4 addition.
Characterization of BM-derived WT and BLT1/2−/− DCs. (A) FACS analysis of membrane phenotype in WT and BLT1/2−/− DCs. Gray line indicates isotype control; broken line, iDCs; and black line, mDCs. (B) FITC-dextran uptake. (C) Mixed leukocyte reaction induced by WT and BLT1/2−/− DCs. (D,E) Calcium mobilization measurement in response to LTB4 in WT (D) and in BLT1/2−/− (E) DCs. (F, G) In vitro chemotaxis of iDCs (F) and mDCs (G) toward CCL3 (100 ng/mL), CCL19 (100 ng/mL), and LTB4 (100 nM). Error bars indicate SE.
Characterization of BM-derived WT and BLT1/2−/− DCs. (A) FACS analysis of membrane phenotype in WT and BLT1/2−/− DCs. Gray line indicates isotype control; broken line, iDCs; and black line, mDCs. (B) FITC-dextran uptake. (C) Mixed leukocyte reaction induced by WT and BLT1/2−/− DCs. (D,E) Calcium mobilization measurement in response to LTB4 in WT (D) and in BLT1/2−/− (E) DCs. (F, G) In vitro chemotaxis of iDCs (F) and mDCs (G) toward CCL3 (100 ng/mL), CCL19 (100 ng/mL), and LTB4 (100 nM). Error bars indicate SE.
Priming effect of LTB4 on CCR7
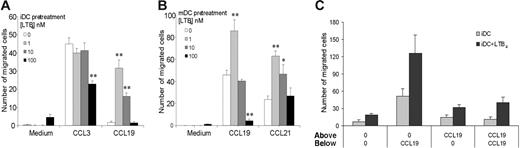
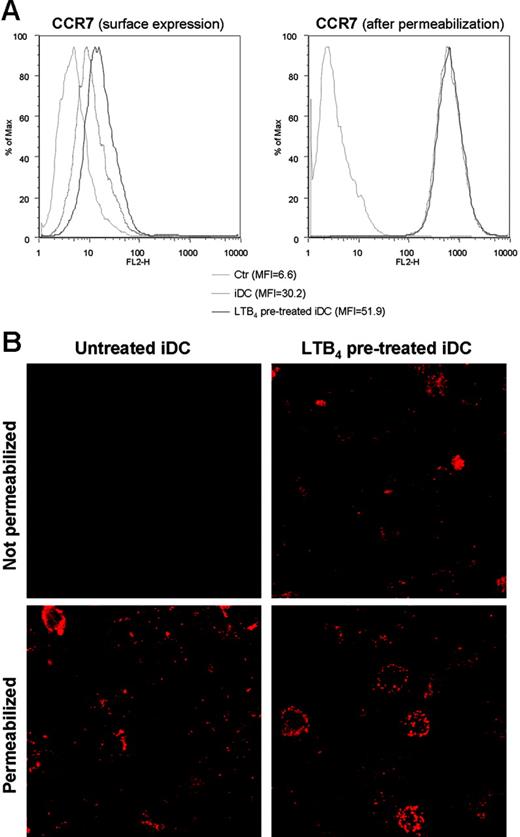
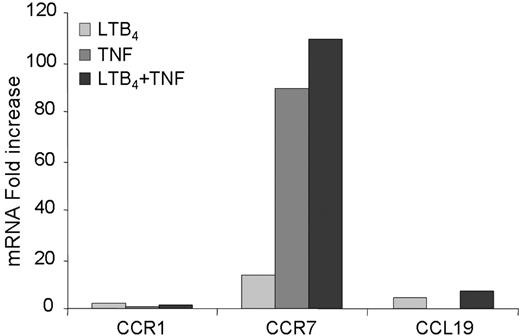
To elucidate the role of LTB4 in DC biology we evaluated the effect of LTB4 in modulating DC migration to both inflammatory and homeostatic chemokines, in vitro. A short exposure (15-30 minutes) of DCs to LTB4 did not alter the migration of iDCs in response to CCL3. However, the migration of iDCs to CCR7 ligands, CCL19 and CCL21, was markedly increased (Figure 3A). A similar but less pronounced effect was also observed in mDCs (Figure 3B). The effect was maximal at 1 nM LTB4 and declined at higher concentrations (ie, 100 nM LTB4). In order to clarify whether LTB4 pretreatment induced an increased chemokinetic rather than chemotactic response toward CCR7 ligands, checkerboard experiments were performed. The migratory capacity of iDCs was evaluated in the presence of a positive chemotactic gradient (100 ng/mL CCL19 in the lower well), in the presence of a negative gradient (ligand only in upper well), and in the absence of a gradient (equal concentration of the ligand in the upper and the lower wells). Figure 3C shows that the LTB4 pretreatment increased iDC chemotactic response (directional migration) to an optimal concentration of CCL19, with only a minor effect on chemokinesis (activated random migration). Similar results were observed using mDCs (data not shown). Fluorescence-activated cell sorting (FACS) analysis revealed that LTB4 pretreatment induced a rapid (30-minute) up-regulation of CCR7 membrane expression. No differences were observed in CCR7 staining in permeabilized LTB4-treated DCs compared with control cells (Figure 4A). Conversely, the expression of CCR7 in mDCs was apparently unaffected by LTB4 treatment (data not shown). Similar results were obtained by confocal microscopy analysis using a CCL19-Fc fusion protein to detect CCR7 expression (Figure 4B). To gain further insights into the regulation of CCR7 expression/function by LTB4, we investigated the effect of LTB4 on chemokine and chemokine receptor expression using a SuperArray system. These experiments revealed that LTB4 pretreatment (1 μM; 6 hours) increased CCR7 expression both in iDCs (14.1-fold increase) and mDCs (1.2-fold increase; Figure 5). Conversely, in the same experimental system, LTB4 had no effect on CCR1 mRNA levels. The expression of CCL19 mRNA was also up-regulated by LTB4 in both iDCs and mDCs (4.1- and 7.5-fold increase, respectively).
Chemotaxis of LTB4-pretreated DCs. BM-derived WT DCs, both iDCs (A) and mDCs (B), were pretreated with LTB4 as indicated for 30 minutes at 37°C. The chemotactic response toward a fixed concentration (100 ng/mL) of CCL3 and CCL19 (iDCs) and toward CCL19 and CCL21 (mDCs) was determined. Significant difference in chemotaxis levels between untreated and LTB4-treated DCs was indicated (*P < .05, *P < .01 by t test). Data are the mean ± SE of cells from 3 individual fields for each concentration from a representative experiment of at least 3 repetitions. (C) Checkerboard analysis of DC migration across polycarbonate filters toward CCL19.
Chemotaxis of LTB4-pretreated DCs. BM-derived WT DCs, both iDCs (A) and mDCs (B), were pretreated with LTB4 as indicated for 30 minutes at 37°C. The chemotactic response toward a fixed concentration (100 ng/mL) of CCL3 and CCL19 (iDCs) and toward CCL19 and CCL21 (mDCs) was determined. Significant difference in chemotaxis levels between untreated and LTB4-treated DCs was indicated (*P < .05, *P < .01 by t test). Data are the mean ± SE of cells from 3 individual fields for each concentration from a representative experiment of at least 3 repetitions. (C) Checkerboard analysis of DC migration across polycarbonate filters toward CCL19.
Effect of LTB4 treatment on surface expression of CCR7. (A) FACS analysis of CCR7 expression on the surface and after permeabilization in iDCs after 30 minutes of LTB4 (1 nM) treatment. Gray line indicates isotype control; broken line, iDCs; and black line, LTB4-treated iDCs. (B) CCL19-Fc fusion protein staining of untreated and LTB4-treated iDCs, both before and after permeabilization, analyzed by confocal microscopy (×100).
Effect of LTB4 treatment on surface expression of CCR7. (A) FACS analysis of CCR7 expression on the surface and after permeabilization in iDCs after 30 minutes of LTB4 (1 nM) treatment. Gray line indicates isotype control; broken line, iDCs; and black line, LTB4-treated iDCs. (B) CCL19-Fc fusion protein staining of untreated and LTB4-treated iDCs, both before and after permeabilization, analyzed by confocal microscopy (×100).
LTB4-induced changes in gene expression profiles. A cytokine and chemokine SuperArray was performed using 2 μg/mL RNA for each sample as described in “Methods.” The graph indicates the fold increase of each group over iDCs showing changes in CCR1, CCR7, and CCL19.
LTB4-induced changes in gene expression profiles. A cytokine and chemokine SuperArray was performed using 2 μg/mL RNA for each sample as described in “Methods.” The graph indicates the fold increase of each group over iDCs showing changes in CCR1, CCR7, and CCL19.
Reduced DC migration in vivo and inhibition of CHS reactions in BLT1/2−/− mice
To evaluate in vivo the biologic relevance of LTB4 action(s) on CCR7 expression and function, BM-derived mDCs from WT and BLT1/2−/− mice were CFSE-labeled and injected into the footpads of WT recipients. Cell migration was evaluated as the number of fluorescent cells migrated to popliteal lymph nodes. In comparison to WT DCs, a significant defect in migration of DCs from BLT1/2−/− mice was observed at early times, that is, at 20 and 48 hours after the inoculation (53% and 72.7% inhibition, respectively; Figure 6). However, at longer time points (72 hours), the migration of BLT1/2−/− DCs was restored, suggesting the existence of compensatory mechanisms (Figure 6). Similar results were obtained with DCs from BLT1−/−-deficient mice (data not shown).
Defective in vivo migration of BM-BLT1/2−/− DCs. Mature DCs from WT and BLT1/2−/− mice were labeled with the vital dye CFSE and injected subcutaneously in the hind leg footpad of WT mice. Popliteal lymph nodes were recovered at the indicated times and the cell suspension was evaluated by flow cytometry. One experiment representative of 3 is shown (*P < .05, *P < .01 by t test, vs respective WT control group). Error bars indicate SE.
Defective in vivo migration of BM-BLT1/2−/− DCs. Mature DCs from WT and BLT1/2−/− mice were labeled with the vital dye CFSE and injected subcutaneously in the hind leg footpad of WT mice. Popliteal lymph nodes were recovered at the indicated times and the cell suspension was evaluated by flow cytometry. One experiment representative of 3 is shown (*P < .05, *P < .01 by t test, vs respective WT control group). Error bars indicate SE.
Previous studies showed that defective DC migration affects the induction of adaptive immunity in a model of CHS.18 To test whether this observation might also be applicable to the LTB4 pathway, BLT1/2−/− and WT mice were sensitized on day 0 and challenged with the antigen on the right ear after 5 days; the contra lateral ear received only the vehicle, as a control. CHS response in BLT1/2−/− mice was strongly decreased compared with WT mice at all the time points investigated (24 to 72 hours; P < .02 vs WT mice; Figure 7A). Histopathologic examination of ear sections showed that BLT1/2−/− mice had a reduced thickness, inflammatory reaction, and cell infiltration compared with WT mice (Figure 7B). Since recent work has reported the expression of functional LTB4 receptors in T-effector cells,22 experiments were performed to directly evaluate the relevance of the LTB4 pathway in DC function in vivo. An adoptive transfer model of CHS was performed using BLT1/2−/− and WT DCs loaded in vitro with the antigen (DNBS) and injected subcutaneously into naive WT recipients. Mice were subsequently challenged at day 5 with DNFB on the right ear. Figure 7C shows that, similar to the observations in the “classic” CHS model (Figure 7A), DNBS-pulsed BLT1/2−/− DCs were poorly effective in inducing CHS response, compared with WT DCs. This response was specific since it could not be induced using an unrelated antigen (oxazolone; data not shown) or PBS-loaded DCs (Figure 7C). Taken together, these results strongly implicate a role for LTB4 in the regulation of DC migration and function in vivo.
Defective CHS in BLT1/2−/− mice. (A) WT and BLT1−/− mice were sensitized on the shaved abdominal skin with 50 μL of 0.5% DNFB in 4:1 acetone/olive oil (vol/vol) and 5 μL on each footpad. Five days later, mice were challenged on the right ear (10 μL of 0.2% DNFB on each side). The left ear was painted with vehicle as control. Increases in ear swelling were measured at different time points. Mean values ± SE for each group (5 mice) are presented. (*P < .05, **P < .01 by t test, vs respective WT control group). One experiment representative of 3 is shown. (B) Histology, hematoxylin and eosin–stained tissue sections from WT and BLT1/2−/− mice after 24 hours read-out CHS. The top row represents a ×100 magnification; an enlarged region of the same pictures is shown in the bottom row. Images were acquired using a Carl Zeiss Axioskope Optical Microscope equipped with an Achroplan 10 ×/0.25 NA objective and a 10 × eyepiece. Images were captured using a Nikon CoolPix 3.34 Megapixel camera (Nikon, Tokyo, Japan) at a fine resolution of 1600 × 1200 pixels in JPEG format and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). (C) Defective capacity of BLT1/2−/− DCs to elicit CHS. WT mice were immunized (on day −5) by subcutaneous injection of DNBS-loaded DCs (106/mouse) obtained from WT or BLT1/2−/− mice. Mice were challenged 5 days later (day 0) by ear painting with DNFB. Figure represents the values for ear swelling (mean ± SE) of 5 mice per group, and is representative of 3 experiments. **P < .01 by t test.
Defective CHS in BLT1/2−/− mice. (A) WT and BLT1−/− mice were sensitized on the shaved abdominal skin with 50 μL of 0.5% DNFB in 4:1 acetone/olive oil (vol/vol) and 5 μL on each footpad. Five days later, mice were challenged on the right ear (10 μL of 0.2% DNFB on each side). The left ear was painted with vehicle as control. Increases in ear swelling were measured at different time points. Mean values ± SE for each group (5 mice) are presented. (*P < .05, **P < .01 by t test, vs respective WT control group). One experiment representative of 3 is shown. (B) Histology, hematoxylin and eosin–stained tissue sections from WT and BLT1/2−/− mice after 24 hours read-out CHS. The top row represents a ×100 magnification; an enlarged region of the same pictures is shown in the bottom row. Images were acquired using a Carl Zeiss Axioskope Optical Microscope equipped with an Achroplan 10 ×/0.25 NA objective and a 10 × eyepiece. Images were captured using a Nikon CoolPix 3.34 Megapixel camera (Nikon, Tokyo, Japan) at a fine resolution of 1600 × 1200 pixels in JPEG format and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). (C) Defective capacity of BLT1/2−/− DCs to elicit CHS. WT mice were immunized (on day −5) by subcutaneous injection of DNBS-loaded DCs (106/mouse) obtained from WT or BLT1/2−/− mice. Mice were challenged 5 days later (day 0) by ear painting with DNFB. Figure represents the values for ear swelling (mean ± SE) of 5 mice per group, and is representative of 3 experiments. **P < .01 by t test.
Discussion
The present study shows that murine DCs express functional LTB4 receptors, as observed in chemotactic response and calcium mobilization assays. The maturation status of DCs does not affect this expression, since both iDCs and TNF-mDCs showed the same level of response to LTB4. Moreover, we demonstrate that LTB4 is capable of controlling DC functions in multiple ways. First, LTB4 can directly promote the migration of circulating immature and maturing DCs to the sites of inflammation and to the draining lymph nodes, respectively. Second, LTB4 up-regulates CCR7 expression and functions. At low concentrations, LTB4 promotes the migration of iDCs to CCR7 ligands; this effect is the induction of true chemotaxis with only a minor effect on chemokinesis. LTB4-induced migration of iDCs to CCL19 and CCL21 is associated with an increase of CCR7 membrane expression. Similarly, a short preincubation to LTB4 also up-regulates the migration of mDCs to CCR7 ligands. Although the exact mechanism(s) involved in this effect remain unclear, it is likely that LTB4 exerts multiple effects both at the level of CCR7 membrane expression and at the signaling level. The observation that CCL19-Fc fusion protein binding sites appear on the cell surface following LTB4 pretreatment suggests a novel mechanism for rapid modulation of DC responsiveness. The mobilization to plasma membrane of intracellular pools of chemokine receptors was previously reported for CXCR4.23,24 Furthermore, at a longer time of activation, LTB4 may influence at the transcriptional level the expression of CCR7 and CCL19, one of its ligands. The LTB4-induced DC migration to CCR7 ligands and the induction of CCL19 might also be relevant for the entering of maturing DCs into afferent lymphatic vessels.5,6 Since granulocytes are major producers of LTB4, DC migration might be regulated at the early phases of the inflammatory response when large numbers of activated granulocytes are recruited at the pathologic site. In this context, it is interesting to note that neutrophils were recently described to regulate certain DC functions.25 Finally, LTB4 promotes the migration of mDCs to regional lymph nodes by increasing their migration in response to CCL19 and CCL21 in vitro and in vivo.
The severe reduction of in vivo migration of BLT1/2-deficient DCs strongly suggests a role for LTB4 receptors in DC trafficking. This defect was present at relatively early time points (20-48 hours) and declined at later time points, suggesting the existence of compensatory mechanisms. In BLT1−/− mice, similar kinetic effects were observed for macrophage recruitment in a model of zymosan-induced peritonitis13 and in the development of atherosclerosis.26 Previous studies have described cysteinyl leukotrienes and PGE29 as regulators of DC functions. The current study identifies LTB4 as a new lipid mediator able to regulate CCR7-dependent migration of DCs in vitro and in vivo. Since CCR7 has been described as a regulator of several DC functions,27 LTB4 might contribute to modulate the immune response by regulating CCR7 functions.
We previously described that DC migration is tightly associated with the induction of adaptive immunity, in a model of contact hypersensitivity.18 Consistent with this finding, the current study also shows that defective DC migration due to the lack of LTB4 receptors causes a dramatic impairment of DNFB-induced CHS. A similar defect was also observed in an adoptive transfer experiment in which DNBS-pulsed BLT1/2−/− and WT DCs showed significant reduction in CHS induced by DNBS-pulsed DCs from BLT1/2−/− mice.
Based on the effects on granulocytes, LTB4 has long been identified as an important mediator of innate immunity.13,15,28 Recent studies also highlight the importance of LTB4 and BLT1 in T-cell responses and asthma.29,30 The findings reported in this paper propose LTB4 at the interface of innate and adaptive immunity. These results strongly suggest that the LTB4/BLT1 axis may have an important role in the regulation of adaptive immunity through the modulation of DC migration and function. Further studies will be required to define in a more detailed manner the molecular mechanisms of the leukotriene receptor functions in DCs and specific pathologic scenarios under which LTB4 regulates immune functions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.D.P. designed and performed the research and wrote the manuscript; W.-H.S. and S.M. participated in research; G.S. assisted in the design of research; and S.S. and B.H. designed research, analyzed data, and wrote the manuscript.
Acknowledgments
This work was supported by National Institutes of Health grant AI-52381 and the James Graham Brown Cancer Center, University of Louisville. S.S. was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) and MIUR (Ministero dell'Istruzione Università e Ricerca, Italy).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal