Abstract
Mammalian endothelial cells (ECs) display marked phenotypic heterogeneity. Little is known about the evolutionary mechanisms underlying EC heterogeneity. The last common ancestor of hagfish and gnathostomes was also the last common ancestor of all extant vertebrates, which lived some time more than 500 million years ago. Features of ECs that are shared between hagfish and gnathostomes can be inferred to have already been present in this ancestral vertebrate. The goal of this study was to determine whether the hagfish endothelium displays phenotypic heterogeneity. Electron microscopy of the aorta, dermis, heart, and liver revealed ultrastructural heterogeneity of the endothelium. Immunofluorescent studies demonstrated marked differences in lectin binding between vascular beds. Intravital microscopy of the dermis revealed histamine-induced adhesion of leukocytes in capillaries and postcapillary venules, but no such adhesion in arterioles. Together, these data suggest that structural, molecular, and functional heterogeneity of the endothelium evolved as an early feature of this cell lineage.
Introduction
Endothelial cells (ECs) participate in many physiologic processes, including the regulation of vasomotor tone, hemostasis, leukocyte trafficking, angiogenesis, and permeability. These functions are differentially regulated in space and time (reviewed in Aird1 ). Although there have been remarkable advances in our understanding of proximate mechanisms of endothelial structure and function in health and disease, far fewer studies have addressed the evolutionary history of this cell lineage.
ECs are absent in invertebrates, cephalocordates, and tunicates, but are present in the 3 major groups of extant vertebrates: hagfish (myxinoids), lampreys, and jawed vertebrates (gnathostomes) (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). The fact that the endothelium is shared by jawless and jawed vertebrates is evidence that the endothelium was present in the ancestor of these animals. The absence of an endothelium in cephalochordates and tunicates suggests that this structure evolved after the divergence of these groups from the vertebrate lineage, between 540 and 510 million years ago.
The last common ancestor of hagfish and gnathostomes was also the last common ancestor of all extant vertebrates, which lived some time more than 500 million years ago. Features of ECs that are shared between hagfish and gnathostomes can be inferred to have already been present in this ancestral vertebrate. Here, we show that hagfish endothelium displays structural, molecular, and functional heterogeneity, suggesting that phenotypic heterogeneity is an ancestral rather than a derived feature of this cell lineage.
Materials and methods
Atlantic hagfish, Myxine glutinosa, were purchased from a commercial supplier (Huntsman Marine Science Center, St Andrews, NB, Canada) who caught the specimens in the Bay of Fundy, Canada. Hagfish were maintained in the dark in circular tanks with running seawater at 10±2°C. The animals were anesthetized in seawater containing tricaine methanesulfonate (MS 222; Sigma-Aldrich, St Louis, MO). For lectin staining, 5-üm cryosections were fixed with acetone at –20°C for 2 minutes and 80% methanol at 4°C for 5 minutes, followed by multiple PBS washes. Sections were incubated with each lectin at room temperature for 1 hour. A total of 9 different lectins were used (Table S1). Nuclei were stained with diamidino-phenylindol (DAPI). For electron microscopy, standard fixation, dehydration, and embedding were carried out as previously described.2 Plastic sections (1 üm) were stained with alkaline Giemsa stain for study by light microscopy, and 70- to 80-nm thin sections were examined by electron microscopy with Philips 300, 400, and CM10 electron microscopes (Philips, Eindhoven, The Netherlands) by 3 different microscopists. Intravital microscopy was carried out using an IV 500 intravital microscope (Mikron Instruments, San Diego, CA) equipped with water immersion objectives (Carl Zeiss, Thornwood, NY). Prior to anesthetizing hagfish with MS 222, 100 üL fluorescein-conjugated dextran (2 000 000 molecular weight [MW], 2 mg/mL; Invitrogen, Carlsbad, CA) was injected into the hagfish dorsal sinus and was allowed to circulate systemically for 10 minutes. Next, 20 üL of 1 M histamine (Sigma-Aldrich) or PBS was injected intradermally into the ventral fin of hagfish. After 15 minutes, injected areas were observed.
Results and discussion
For purposes of orientation, the gross anatomy of hagfish is shown in Figure S2.
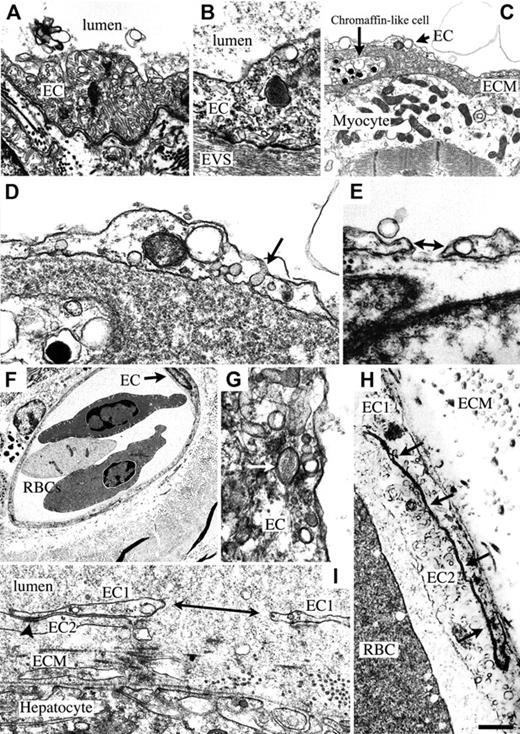
Hagfish have a single aorta and paired posterior cardinal veins (Figure S3A-D). Electron microscopy of the aortic endothelium revealed the presence of elongated tubules, which were relatively uniform in width (Figure 1A). Some of these tubules contained stomata. Similar structures have been previously described in hagfish endothelium in the conus arteriosus, ventral aorta, and cerebral vasculature.3,4 The function of these tubular invaginations is unknown. Weibel-Palade bodies were observed in ECs from the aorta (Figure 1B). Weibel-Palade bodies have not been described in hagfish, and their presence provides strong evidence for the expression of von Willebrand factor (VWF) in these fish.
Ultrastructure of hagfish endothelium. (A) Electron microscopy (EM) of aorta reveals the presence of many tubular structures within an aortic endothelial cell (EC). (B) EM of aorta shows Weibel-Palade body in EC from aorta. EVS indicates extravascular space. Micrographs in panels A and B were acquired with a 60 ×/1.40 NA oil objective lens (Nikon, Melville, NY), an RT slider SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI), and SPOT version 4.0.9 (Diagnostic Instruments). (C) EM of heart shows electron-lucent endothelium overlying a thick extracellular matrix (ECM) containing a chromaffin-like cell, and a cardiomyocyte with electron-lucent cytoplasm, well-preserved mitochondria, and muscle fibrils. (D) Higher magnification of panel C shows an EC with electron-lucent cytoplasm, a mitochondrion next to a vacuole, and caveola with diaphragm (arrow). (E) EM of heart shows gap in endothelium (double-headed arrow). In panels A to E, blood vessel lumen is on top. (F) EM of dermis reveals a microvessel in cross-section containing 2 nucleated red blood cells (RBCs) with multiple vesicles in the periphery of the cell. EC with nucleus is indicated with an arrow. A cell containing melanin granules is seen in the EVS on the left-hand side of the microvessel. (G) EM of skin shows a Weibel-Palade body (arrow) in a dermal microvascular EC. (H) Higher dermal microvascular endothelium shows well-developed lateral borders of 2 adjacent ECs (EC1 and EC2; arrows). (I) EM of liver sinusoid reveals a large gap in a single endothelial cell (double-headed arrow), well preserved junctions between 2 endothelial cells, thick ECM, and a continuum of proteinaceous material from lumen to extravascular space. EC1 indicates 1 endothelial cell (divided by gap); EC2 is a second endothelial cell. Arrowhead indicates tight and adherens junctional area in the lateral borders of 2 apposed endothelial cells (EC1 and EC2). Scale bar in panel H applies to all panels and represents 865 nm (A), 469 nm (B), 898 nm (C), 258 nm (D), 393 nm (E), 5172 nm (F), 261 nm (G), 2305 nm (H), and 492 nm (I).
Ultrastructure of hagfish endothelium. (A) Electron microscopy (EM) of aorta reveals the presence of many tubular structures within an aortic endothelial cell (EC). (B) EM of aorta shows Weibel-Palade body in EC from aorta. EVS indicates extravascular space. Micrographs in panels A and B were acquired with a 60 ×/1.40 NA oil objective lens (Nikon, Melville, NY), an RT slider SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI), and SPOT version 4.0.9 (Diagnostic Instruments). (C) EM of heart shows electron-lucent endothelium overlying a thick extracellular matrix (ECM) containing a chromaffin-like cell, and a cardiomyocyte with electron-lucent cytoplasm, well-preserved mitochondria, and muscle fibrils. (D) Higher magnification of panel C shows an EC with electron-lucent cytoplasm, a mitochondrion next to a vacuole, and caveola with diaphragm (arrow). (E) EM of heart shows gap in endothelium (double-headed arrow). In panels A to E, blood vessel lumen is on top. (F) EM of dermis reveals a microvessel in cross-section containing 2 nucleated red blood cells (RBCs) with multiple vesicles in the periphery of the cell. EC with nucleus is indicated with an arrow. A cell containing melanin granules is seen in the EVS on the left-hand side of the microvessel. (G) EM of skin shows a Weibel-Palade body (arrow) in a dermal microvascular EC. (H) Higher dermal microvascular endothelium shows well-developed lateral borders of 2 adjacent ECs (EC1 and EC2; arrows). (I) EM of liver sinusoid reveals a large gap in a single endothelial cell (double-headed arrow), well preserved junctions between 2 endothelial cells, thick ECM, and a continuum of proteinaceous material from lumen to extravascular space. EC1 indicates 1 endothelial cell (divided by gap); EC2 is a second endothelial cell. Arrowhead indicates tight and adherens junctional area in the lateral borders of 2 apposed endothelial cells (EC1 and EC2). Scale bar in panel H applies to all panels and represents 865 nm (A), 469 nm (B), 898 nm (C), 258 nm (D), 393 nm (E), 5172 nm (F), 261 nm (G), 2305 nm (H), and 492 nm (I).
The bronchial heart is aneural, lacks coronary arteries, and consists of spongy myocardium with blood-filled sinuses (Figure S3E-F). The myocardial cells derive their oxygen from mixed venous blood. As a result, the myocardium depends heavily on anaerobic metabolism.5 In ultrastructural studies, ECs lining the heart sinuses were remarkably attenuated, electron lucent, and contained many vesicular structures, including caveolae and vacuoles (but few tubules) (Figure 1C). Many of the caveolae possessed detectable diaphragms (Figure 1D). Gaps between or holes within ECs were observed in several sections (Figure 1E). Cardiac ECs possessed a well-developed basal lamina, and were separated from cardiomyocytes by a thick basement membrane. The hagfish heart contained many chromaffin-like cells on the abluminal side of the endothelium (Figure 1C). These catecholamine-containing cells have been proposed to modulate cardiac function.5
The epidermis is avascular, while the dermis, which consists of multiple layers of packed collagen fibers, has a rich microvascular network (Figure S3G-I). In ultrastructural studies, dermal vessels demonstrated a well-developed continuous endothelium (Figure 1F) with the presence of occasional Weibel-Palade bodies (Figure 1G). In contrast to a previous study of dermal capillaries, which described the presence of simple “desmosome-like specializations,”6(p683) the lateral plasma membrane borders observed in our studies had specializations characteristic of tight and adherens junctional areas in endothelial cells of other vertebrates (Figure 1H). Compared with the aorta, ECs from the dermal capillaries contained few tubules. Very few pericytes were observed.
There is little information about the structure and function of the hagfish liver. Although the gross structure of the liver appears remarkably similar to other vertebrates, histochemical staining reveals an unusual architecture with a lack of portal triads and the presence of abundant fat (Figure S3J-K). Ultrastructural studies revealed a discontinuous endothelium with many gaps (Figure 1I).
The data in this study provide strong evidence that the endothelium of the hagfish displays structural heterogeneity. At present, few genes have been cloned in hagfish, and no molecular markers exist for the endothelium. To obtain indirect evidence for molecular heterogeneity, we carried out lectin staining of various tissues. These studies demonstrated significant differences in lectin binding to the endothelium (Figure 2A; Table 1).
Lectin staining and intravital microscopy of dermal microvessels. (A) Light microscopy of H&E-stained sections and immunofluorescent microscopy of lectins (LCA indicates Lens culinaris agglutinin; and HP, Helix pomatia) and/or DAPI-stained sections from skeletal muscle (top) or skin (bottom). H&E staining of skeletal muscle shows several muscle fibers with intervening capillaries cut in cross section. Both LCA and HP label capillary endothelial cells between the myocytes. Light microscopy of H&E-stained skin reveals epidermis on top, dermis on bottom. The upper dermis contains several microvessels, which are cut in cross-section. Dermal microvessels bind LCA, but not HP (HP stains nonvascular tissue in upper epidermis). Scale bars represent 55 üm. (B) Hagfish blood smear after injection of fluorescein-conjugated dextran. A distinct population of leukocytes is labeled following intravenous injection of fluorescein-conjugated dextran (left panel). DAPI staining (middle and right panels) shows that the fluorescein-laden cell resembles a circulating neutrophil, as identified by the characteristic nuclear morphology. Scale bars represent 20 üm. (C) The hagfish dermal microvascular network was visualized by intravital microscopy (Video S1). Here, the quantitative analysis of neutrophil rolling (Ci) and firm adhesion (Cii) in response to intradermal injection of histamine or vehicle (PBS) is presented. Mean ± SEM (n = 3); *P<.05.
Lectin staining and intravital microscopy of dermal microvessels. (A) Light microscopy of H&E-stained sections and immunofluorescent microscopy of lectins (LCA indicates Lens culinaris agglutinin; and HP, Helix pomatia) and/or DAPI-stained sections from skeletal muscle (top) or skin (bottom). H&E staining of skeletal muscle shows several muscle fibers with intervening capillaries cut in cross section. Both LCA and HP label capillary endothelial cells between the myocytes. Light microscopy of H&E-stained skin reveals epidermis on top, dermis on bottom. The upper dermis contains several microvessels, which are cut in cross-section. Dermal microvessels bind LCA, but not HP (HP stains nonvascular tissue in upper epidermis). Scale bars represent 55 üm. (B) Hagfish blood smear after injection of fluorescein-conjugated dextran. A distinct population of leukocytes is labeled following intravenous injection of fluorescein-conjugated dextran (left panel). DAPI staining (middle and right panels) shows that the fluorescein-laden cell resembles a circulating neutrophil, as identified by the characteristic nuclear morphology. Scale bars represent 20 üm. (C) The hagfish dermal microvascular network was visualized by intravital microscopy (Video S1). Here, the quantitative analysis of neutrophil rolling (Ci) and firm adhesion (Cii) in response to intradermal injection of histamine or vehicle (PBS) is presented. Mean ± SEM (n = 3); *P<.05.
Profile of endothelial lectin staining in various vascular beds
| Lectins . | Aorta . | Vein . | Muscle . | Heart . | Liver . | Skin . |
|---|---|---|---|---|---|---|
| Arachis hypogaea | ± | − | + | − | − | − |
| Isolectin B4 | + | ± | + | − | − | − |
| Dolichos biflorus agglutinin | + | + | + | − | − | − |
| Glycine max | + | + | + | − | − | − |
| Helix pomatia | − | − | + | − | − | − |
| Lens culinaris agglutinin | + | + | + | − | − | + |
| Lycopersicon esculentum | − | − | − | − | − | − |
| Ricinus communis agglutinin | + | + | + | − | − | − |
| Ulex europaeus agglutinin | + | + | + | − | − | + |
| Lectins . | Aorta . | Vein . | Muscle . | Heart . | Liver . | Skin . |
|---|---|---|---|---|---|---|
| Arachis hypogaea | ± | − | + | − | − | − |
| Isolectin B4 | + | ± | + | − | − | − |
| Dolichos biflorus agglutinin | + | + | + | − | − | − |
| Glycine max | + | + | + | − | − | − |
| Helix pomatia | − | − | + | − | − | − |
| Lens culinaris agglutinin | + | + | + | − | − | + |
| Lycopersicon esculentum | − | − | − | − | − | − |
| Ricinus communis agglutinin | + | + | + | − | − | − |
| Ulex europaeus agglutinin | + | + | + | − | − | + |
Vein indicates posterior cardinal vein; muscle, skeletal muscle; heart, bronchial heart; ±, weak positive; –, negative; +, positive.
To determine whether the hagfish endothelium displays functional heterogeneity, we used intravital microscopy to determine sites of leukocyte adhesion in dermal microvessels. Injection of fluorescein-conjugated dextran as a plasma marker revealed a characteristic network of hagfish skin microvasculature, with arterioles supplying a honeycomb-like capillary network that drains into postcapillary venules (Video S1). Notably, apart from blood plasma, fluorescein-conjugated dextran also labeled a distinct fraction of circulating cells. DAPI staining of blood smears revealed that these fluorescein-dextran–laden cells resembled circulating neutrophils (Figure 2B). Under basal conditions, occasional rolling and neutrophil adhesion of fluorescein-dextran–positive neutrophils was observed in the venous ends of capillaries and postcapillary venules, but not in the arterioles of the skin (Video S1). Following local injection with histamine, there was a substantial increase in neutrophil adhesion in capillaries and postcapillary venules, but not in arterioles (Figure 2C). To our knowledge, this is the first evidence that site-specific rolling and adhesion of leukocytes is conserved between mammals and fish.
Although a small number of previous studies have reported the ultrastructure of ECs in hagfish, these latter investigations neither examined for nor revealed phenotypic heterogeneity. The current study is the first to systematically compare the structure of ECs from different vascular beds. The unequivocal existence of organ-specific properties in the endothelium suggests that structural and functional heterogeneity, including preferential adhesion of leukocytes in postarteriolar segments of the vasculature, evolved as an early, core feature of this cell lineage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: K.Y., D.G., and W.C.A. designed and performed research, analyzed data, and wrote the paper; S.M., R.M.E., and E.S.M. designed and performed research and analyzed data; P.K.C. designed and performed research; D.H. and U.H.v.A. designed research and analyzed data; and A.M.D. analyzed data.
Acknowledgments
This work was supported by a Mount Desert Island Biological Laboratory (MDIBL) New Investigator Award (W.C.A.). W.C.A. is an Established Investigator of the American Heart Association.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal