Abstract
The antiphospholipid syndrome (APS) is an important cause of acquired thrombophilia. It is characterized by the core clinical manifestations of thrombosis, either venous or arterial, and in women it can also be associated with recurrent fetal loss. The detection of persistently elevated levels of antiphospholipid antibodies (aPL Abs) is a requisite laboratory feature for the diagnosis to be made. The dominant antigenic targets in APS are beta 2-glycoprotein I (β2-GPI) and prothrombin. There is an accumulating body of experimental evidence that suggests that specific subgroups of aPL Abs may directly contribute to disease pathogenesis. This review critically examines the experimental evidence underlying the various propositions made to explain how these antibodies may predispose to disease in humans. Furthermore, it also examines the evidence relating to the immunologic mechanisms that may contribute to the breakage of peripheral tolerance in this disorder. Delineating the strengths and limitations of the experimental evidence accumulated thus far will hopefully stimulate further experimentation toward achieving the ultimate goal of precisely defining the dominant pathogenic mechanisms operational in APS. This may pave the way for the development of improved therapies.
Introduction
The antiphospholipid syndrome (APS) is characterized by the core clinical manifestations of thrombosis, venous or arterial, and recurrent fetal loss.1 The detection of persistently elevated levels of antiphospholipid antibodies (aPL Abs) is a requisite laboratory feature for the diagnosis to be made.2 APS can occur in isolation or in association with other autoimmune conditions, particularly systemic lupus erythematosus (SLE).1
The predominant antibodies in this disorder are directed against protein antigens that bind to anionic phospholipids, such as beta 2-glycoprotein I (β2-GPI)3 and prothrombin.4
The physiological function of prothrombin has been well described.5 It is a proenzyme that upon cleavage by the prothrombinase complex leads to the generation of thrombin.5
β2-GPI is a 54-kDa protein, with a plasma concentration of 4 μM, composed of 5 complement control protein modules, which are termed domains I through to V.5 Domain V is responsible for binding anionic phospholipids.5 The in vivo function of β2-GPI is not definitively known. As discussed in this review, in vitro it interacts with diverse cell types, receptors, and enzymes. In vivo significance of these interactions needs to be established.
Antibodies directed against phospholipids per se, such as cardiolipin (CL) and phosphatidylserine (PS), are also detected in patients with APS.1 They appear to be part of the natural antibody repertoire and increase during certain infections.1 In this latter context they do not tend to be associated with the clinical manifestations of APS.6
Antigenic targets other than β2-GPI and prothrombin have been identified in APS patients, including tissue plasminogen activator (tPA),7 plasmin,8 annexin A2,9 and thrombin10 to name a few. β2-GPI 3 and prothrombin4 are the main antigenic targets in APS, and it would seem reasonable to assert that the dominant pathopsychological mechanisms are likely to involve antibodies directed against these antigens.
The generic term “aPL Abs” encompasses antibodies that target protein antigens that bind anionic phospholipids and those that bind anionic phospholipid antigens directly (Table 1).
The nomenclature applied to antiphospholipid antibodies
| Abbreviation . | Description . |
|---|---|
| aCL | Anticardiolipin antibodies detected with a cardiolipin ELISA |
| LA | Lupus anticoagulant antibodies detected by prolongation of in vitro clotting tests; specificities: αβ2-GPI and/or αFII |
| αβ2-GPI | Anti-β2-GPI antibodies detected with a β2-GPI ELISA |
| αFII | Antiprothrombin Abs detected with prothrombin ELISA |
| aPL | aCL, LA, αβ2-GPI, αFII |
| Abbreviation . | Description . |
|---|---|
| aCL | Anticardiolipin antibodies detected with a cardiolipin ELISA |
| LA | Lupus anticoagulant antibodies detected by prolongation of in vitro clotting tests; specificities: αβ2-GPI and/or αFII |
| αβ2-GPI | Anti-β2-GPI antibodies detected with a β2-GPI ELISA |
| αFII | Antiprothrombin Abs detected with prothrombin ELISA |
| aPL | aCL, LA, αβ2-GPI, αFII |
Studies into mechanisms predisposing to thrombosis
Complement
The possible role of complement activation in APS pathogenesis is suggested by the demonstration of increased complement activation products in the plasma of patients with APS who have had a cerebral ischemic event, compared with patients suffering from non-APS–related cerebral ischemia.11
The animal model of APS used to demonstrate that complement activation may be an important mediator in thrombosis pathogenesis involved the transfer of polyclonal IgG aPL Abs from patients with APS to rats pretreated with lipopolysaccharide (LPS).12 Thrombus was induced in the rodents receiving aPL Abs but not in the rodents receiving polyclonal IgG Abs from healthy volunteers.12 The effect was dependent on the activation of C5 and C6.12 When the β2-GPI–reactive antibody fraction was removed, thrombus was not induced.12
In patients with APS, aPL Abs tend to be of the IgG isotype, though the IgM and IgA isotypes can be present concurrently.1 The IgG subclasses tend to be IgG1, IgG2, and IgG4.1 The IgG2 subclass is more prevalent in APS.13 IgG2 and IgG4 antibody subclasses have a relatively weak ability to fix complement via the classical pathway in humans.14 This argues for the necessity to consider additional mechanisms to the classical complement pathway by which anti–β2-GPI Abs may enhance and accelerate thrombus formation in patients with APS.
Prothrombotic mechanisms involving the dysregulated activation of platelets, endothelial cells, and monocytes by the anti–β2-GPI Ab/β2-GPI complex bound to the cell surface
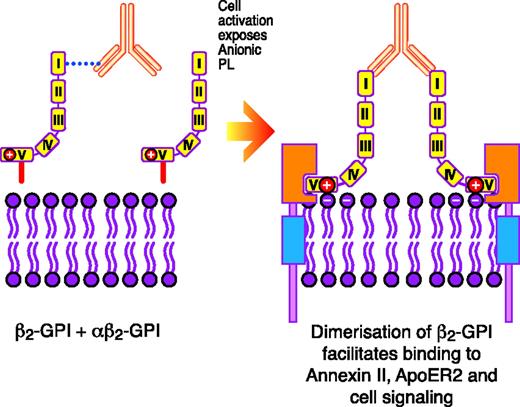
In vitro, submaximal prestimulation of platelets by agonists such as thrombin15 or collagen16 appears to be a prerequisite for β2-GPI–reactive Abs in the presence of β2-GPI to be able to potentiate activation. Prestimulation with an agonist leads to the exposure of PS on the cell outer membrane.15,16 It is hypothesized that the anti–β2-GPI Ab/β2-GPI complex has to initially form on the exposed PS before interacting with specific platelet receptors to potentiate activation.16 This was derived from the observation that when the PS binding site was blocked, potentiation of platelet activation via apolipoprotein E receptor 2′ (ApoER2′) using artificially constructed dimers of β2-GPI, which it is hypothesized mimic the in vivo biological properties of the anti–β2-GPI Ab/β2-GPI complex, did not occur16 (Figure 1). The reason that dimerized β2-GPI cannot directly interact with the platelet receptor ApoER2′ without an initial independent priming stimulus to the platelet to lead to the exposure of PS is not known.16 It needs to be ascertained whether the priming stimulus, in addition to leading to the exposure of PS, may also lead to the up-regulated expression or structural modification of the ApoER2′ receptor on the platelet surface, perhaps increasing the probability that the anti–β2-GPI Ab/β2-GPI complex will interact with it.
Schematic representation of how anti–β2-GPI Abs in complex with β2-GPI may interact with certain surface receptors on platelets (eg, ApoER2′) and endothelial cells (eg, annexin II, also known as annexin A2) to induce cellular activation. Reprinted from Miyakis et al17 with permission.
Schematic representation of how anti–β2-GPI Abs in complex with β2-GPI may interact with certain surface receptors on platelets (eg, ApoER2′) and endothelial cells (eg, annexin II, also known as annexin A2) to induce cellular activation. Reprinted from Miyakis et al17 with permission.
β2-GPI has been shown in vitro to bind nonstimulated endothelial cells, which then enables anti–β2-GPI Abs to bind the cells and to induce a procoagulant and proinflammatory phenotype.18 Clinical evidence of endothelial dysfunction is supported by findings of elevated endothelial microparticles in the circulation of APS patients.19 Annexin A220 and receptors of the Toll-like receptor (TLR) family21 have been suggested to interact with the anti–β2-GPI Ab/β2-GPI complex on the endothelial cells surface, mediating activation. Antibodies that directly bind annexin A2 independently of β2-GPI have also been shown to associate with the thrombotic manifestations seen in APS patients.9 They are able to directly activate nonstimulated endothelial cells, similar to the effect of the anti–β2-GPI Ab/β2-GPI complex.9
Fischetti et al have demonstrated that in vivo the assembly of anti–β2-GPI Ab/β2-GPI complexes on the endothelial cell surface does not induce the development of thrombus without an additional priming factor.12 In their model the priming factor was LPS.12 Support for the need for a priming factor in vivo is also derived from other models, which necessitated either a photochemical injury or mechanical injury to the blood vessel to allow β2-GPI–reactive Abs to promote thrombus formation.22,23
It is not clear why the formation of the anti–β2-GPI Ab/β2-GPI complex on the endothelial surface cannot induce thrombosis without a priming factor in vivo.12 One possibility that needs to be assessed is whether the various methods of priming12,22,23 may lead to the unregulated expression or structural modification of the endothelial receptors annexin A2 and perhaps TLRs, potentially allowing for pathophysiologically significant interactions with the anti–β2-GPI Ab/β2-GPI complex to occur. Studying the variables that influence the interaction between the anti–β2-GPI Ab/β2-GPI complex and the relevant receptors on platelets and endothelial cells may give clues to the nonantibody-related factors that may determine where and when thrombosis occurs in patients with APS.
Monocytes can be activated to express tissue factor (TF) and various proinflammatory cytokines24,25 by the anti–β2-GPI Ab/β2-GPI complex in vitro. The relevant cell surface receptor has not been identified.24,25
In vitro studies looking at endothelial cells,20,21 platelets,15,16 and monocytes25 have suggested that involvement of the Fcγ receptor is not necessary for cellular activation. This is supported by an in vivo study that showed that the F(ab′)2 fragments of a murine monoclonal anti–β2-GPI Ab are able to induce platelet-rich thrombus to the same extent as the whole IgG molecule.22
Platelet receptors
A possible role of dysregulated platelet activation contributing to the thrombotic manifestations of APS has been suggested by the demonstration of elevated levels of platelet-derived thromboxane metabolic breakdown products in the urine of patients diagnosed with APS compared with controls.26 The importance of platelets in thrombosis pathogenesis is further supported by an in vivo study that has demonstrated that the thrombi resulting from the infusion of anti–β2-GPI Abs into hamsters whose carotid arteries were primed with a photochemical injury were rich in platelets.22
Lutters et al demonstrated that when they blocked the ApoER2′ receptor on platelets using receptor-associated protein, the increased adhesion by platelets to collagen induced by the anti–β2-GPI Ab/β2-GPI complex was lost.16 These findings suggest that the ApoER2′ receptor, the only receptor of the low-density lipoprotein (LDL) family described on platelets, mediates a role in the activation of platelets.16 The ApoER2′ receptor was able to coprecipitate with dimerized β2-GPI, providing evidence for a direct interaction between β2-GPI and the receptor.16
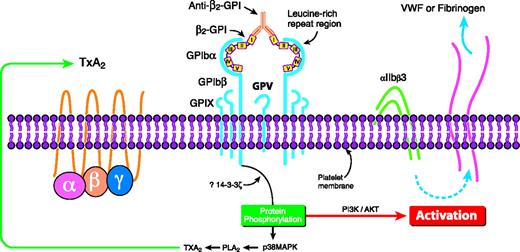
The GPIbα subunit of the GPIb/IX/V receptor has been shown to bind β2-GPI (via domains II to V) in vitro.27 GPIbα is located on platelets,28 its most important ligand being von Willebrand factor,28 though it also serves to colocalize FXI and thrombin on the platelet surface.29 The interaction with β2-GPI in vitro allowed a murine monoclonal anti–β2-GPI Ab to activate platelets, leading to thromboxane production and the activation of the phosphoinositide-3 kinase/Akt pathway27 (Figure 2). This pathway contributes to the activation of the αIIbβ3 receptor on platelets.30 APL Abs with β2-GPI reactivity can also activate the p38MAPK/phospholipase A2 (PLA2) pathway, leading to the potentiation of thromboxane production by platelets prestimulated with low-dose thrombin.31 The p38MAPK/PLA2 pathway can be activated downstream of GPIbα32 and ApoER2′.33
Proposed mechanism of how anti–β2-GPI Abs in complex with β2-GPI may crosslink the GPIbα subunit of the GPIb-IX-V receptor on platelets to induce activation of the p38 MAPK/PLA2 and the PI3K/Akt intracellular pathways, potentially leading to thromboxane A2 production and the activation of αIIbβ3. TxA2 indicates thromboxane. Reprinted from Shi et al27 with permission.
Proposed mechanism of how anti–β2-GPI Abs in complex with β2-GPI may crosslink the GPIbα subunit of the GPIb-IX-V receptor on platelets to induce activation of the p38 MAPK/PLA2 and the PI3K/Akt intracellular pathways, potentially leading to thromboxane A2 production and the activation of αIIbβ3. TxA2 indicates thromboxane. Reprinted from Shi et al27 with permission.
Endothelial cell receptors
Zhang et al demonstrated that β2-GPI binding to annexin A2 enabled anti–β2-GPI Abs to activate endothelium, presumably via a crosslinking mechanism, inducing the expression of a procoagulant phenotype.20 The annexin A2 receptor does not have a transmembrane region, and it needs to be determined by what means it may mediate activation.20 This receptor can also be directly targeted and the endothelial cells activated by anti–annexin A2 Abs, which have been described in association with APS.9
Meroni et al have noted that anti–β2-GPI Abs, in the presence of β2-GPI, are able to activate the nuclear factor-κB (NF-κB) pathway in endothelial cells, leading to the expression of a procoagulant and proinflammatory phenotype.35 TLRs may mediate a role, though direct binding between β2-GPI and TLRs has not been demonstrated yet.21 It has been speculated that TLR4 36 and TLR2 37 may be the responsible receptors in this family.
Other receptors and cell surfaces that interact with β2-GPI
As well as the receptors discussed in “Platelet receptors” and “Endothelial cell receptors,” in the context of mechanisms related to thrombosis, β2-GPI in vitro has also been shown to bind a number of receptors of the LDL family that are found on multiple other cell types.38 Megalin is one such receptor.38 It is an endocytotic receptor responsible for the uptake of vitamin D–binding protein and vitamin D in the renal tubules39 and of sex hormone–binding protein and either androgen or estrogen in the reproductive tissues.40 Perhaps β2-GPI may serve additional functions in vivo that are not purely related to hemostasis.
Prothrombotic mechanisms based on the disruption of the interaction between anticoagulant factors and the phosphatidylserine surface
Activated protein C.
Activated protein C (APC) serves an anticoagulant role by binding and inactivating the procoagulant factors Va (FVa) and VIIIa.43 A number of murine monoclonal anti–β2-GPI Abs in the presence of β2-GPI have been shown to be able to inhibit APC anticoagulant activity in vitro.44 It is hypothesized that the anti–β2-GPI Ab/β2-GPI complex may either compete with components of the APC complex for limited phospholipid binding sites or disrupt an interaction within the APC complex.44 The presence of oxidized phosphatidylethanolamine (PE) containing phospholipids allowed the anti–β2-GPI Ab/β2-GPI complex to selectively disturb APC function.44 This, it is hypothesized, may explain why the APC complex may be disturbed relative to the PS-dependent procoagulant pathways in vivo.44
The anti–β2-GPI Ab/β2-GPI complex has also been shown in vitro to disturb other PS-dependent anticoagulant pathways, including inhibition of activated FX by the protein Z/protein Z–dependent protease inhibitor45 and the TF pathway inhibitor.46 Determining how these pathways may be disturbed relative to the PS-dependent procoagulant pathways will be a key step in providing support for the in vivo relevance of these pathways.
Annexin A5.
The proposition made by this theory is that annexin A5, a protein that binds anionic phospholipids with high affinity, may form a protective anticoagulant shield on vascular cells and that anti–β2-GPI Abs in complex with β2-GPI may disturb the shield and hence predispose to placental thrombosis and fetal loss.47,48 It needs to be clarified whether the annexin A5 shield exists in vivo. Doubts have been raised in this regard with the study of the reproductive capacity of annexin A5 knockout mice in which it was demonstrated that annexin A5 deficiency did not have an impact on litter size and fetus viability.49
Prothrombotic mechanisms based on the disruption of fibrinolysis
Patient-derived polyclonal IgG aPL Abs can impair fibrinolysis on endothelial cells in vitro.50 The annexin A2 receptor is responsible for colocalizing tissue plasminogen activator (tPA) and plasminogen on endothelial cells, catalyzing the generation of plasmin.51 In vivo disturbance of the annexin A2 receptor is characterized by poor clot dissolution.51 Annexin A2 antibodies have been demonstrated in patients with APS.9 In vitro these antibodies can impair fibrinolysis on the endothelial surface.9 Anti-tPA Abs7 and antiplasmin Abs8 have also been described in a few patients. They may contribute to the impairment of fibrinolysis in APS.
Distinct mechanisms pertaining to antiprothrombin antibodies
Antiprothrombin Abs in APS bind with low affinity to prothrombin.52 One hypothesis by which they may predispose to thrombosis in APS is via the interruption of APC function by the antiprothrombin Ab/prothrombin complex competing for PS binding sites.53
The lupus anticoagulant (LA) is an in vitro test that is characterized by the prolongation of clotting.1 Paradoxically, it is the laboratory test that is most strongly associated with the thrombotic complications associated with APS.54 However, LA can also be detected in nonthrombotic situations.55 The LA effect can be caused either by anti–β2-GPI antibodies in complex with β2-GPI competitively inhibiting the formation of the prothrombinase complex in vitro or by antiprothrombin antibodies in complex with prothrombin.55 The contribution of antiprothrombin antibodies with LA activity to APS pathogenesis has been recently questioned.55 In patients with thrombosis who are positive for LA, it was found using novel methodology that the antibodies that strongly correlate are anti–β2-GPI Abs with LA activity, specifically those directed against an epitope on domain I, rather than antiprothrombin Abs with LA activity.55,56 Although antiprothrombin Abs can coexist with anti–β2-GPI Abs in APS, both contributing to the LA effect, the former antibodies on their own did not correlate with thrombosis.55
Novel in vitro findings regarding β2-GPI's possible physiological function that may have relevance to further understanding APS pathophysiology
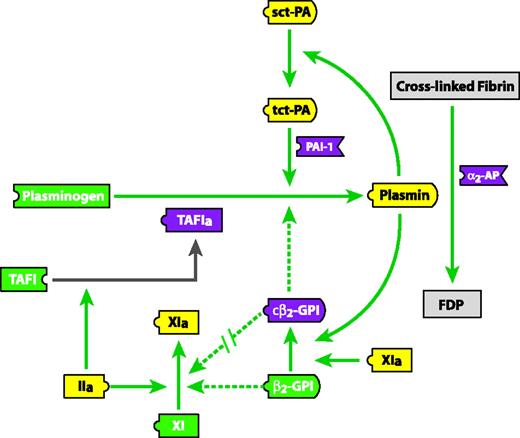
In vitro studies have suggested that β2-GPI may regulate the activity of FXI 57 and plasminogen.58 β2-GPI was able to directly bind to FXI and inhibit its activation by thrombin and FXIIa.57 β2-GPI clipped by plasmin at Lys317-Thr318 was able to bind FXI, though it lost its ability to inhibit FXI activation.57 Yasuda et al have demonstrated that clipped β2-GPI can directly bind to plasminogen and inhibit its conversion to plasmin by tPA,58 suggesting that β2-GPI may potentially provide a regulatory link between the fibrinolysis and the FXI/thrombin pathways (Figure 3).
β2-GPI proteolytically clipped at Lys317-Thr318 inhibits plasmin generation but not FXI activation. A schematic representation of the effect of clipped β2-GPI (cβ2-GPI) and β2-GPI on FXI activation and plasmin generation is shown. Continuous green line indicates activation; segmented green line, inhibition; \\, attenuation of inhibition; FDP, fibrin degradation products; sct-PA, single-chain tissue plasminogen activator; tct-PA, 2-chain tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; α2-AP, α2-antiplasmin; TAFI, thrombin-activatable fibrinolysis inhibitor; TAFIa, activated TAFI. Reprinted from Shi et al59 with permission.
β2-GPI proteolytically clipped at Lys317-Thr318 inhibits plasmin generation but not FXI activation. A schematic representation of the effect of clipped β2-GPI (cβ2-GPI) and β2-GPI on FXI activation and plasmin generation is shown. Continuous green line indicates activation; segmented green line, inhibition; \\, attenuation of inhibition; FDP, fibrin degradation products; sct-PA, single-chain tissue plasminogen activator; tct-PA, 2-chain tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; α2-AP, α2-antiplasmin; TAFI, thrombin-activatable fibrinolysis inhibitor; TAFIa, activated TAFI. Reprinted from Shi et al59 with permission.
FXI 60 and FXII 61 activation in mice plays an important role in thrombus propagation. It appears in murine models, at least, that a distinction exists between coagulation factors involved in physiological hemostasis, as assessed by bleeding time, and those involved in pathological thrombus propagation, with FXI and FXII being relatively redundant in the former situation but critical in the latter.60,61
GPIbα allows thrombin and FXI to colocalize on the platelet surface,62 and annexin A2 allows tPA and plasminogen to colocalize on the endothelial cell surface.63 Both of these receptors are known to bind β2-GPI.27,64 It is tempting to speculate that this may occur to allow β2-GPI to regulate the relevant enzymatic process on the appropriate cell surface.
Cardiac valve disease in APS
One possible mechanism by which arterial thrombosis may occur in APS is as result of cardioembolic disease from damaged heart valves.65 Valvular lesions have been well documented in association with APS,66 and the issue that needs to be resolved is by what means are the valves damaged. Perhaps the formation and subsequent organization of clot on the heart valve, occurring as a result of the prothrombotic mechanisms discussed, may lead to damage.66
APL abs and atherosclerosis
Antibodies directed against prothrombin have been detected in asymptomatic dyslipidemic middle-aged men using a solid-phase enzyme-linked immunosorbent assay (ELISA) and have been shown to be predictive of an increased risk of myocardial infarction related to atherosclerosis.67 Antibodies directed against oxidized LDL (ox-LDL) were also predictive of an increased risk of myocardial infarction in middle-aged men.68 Anti–ox-LDL Abs may enhance the uptake of ox-LDL by macrophages in vitro, suggesting they may directly contribute to atheromatous disease progression.68 Antiprothrombin Abs from this patient group cross-react with plasminogen, raising the possibility that they may impair fibrinolysis, though this latter point awaits to be definitively demonstrated.69 The other possibility that has to be considered is that antiprothrombin Abs and anti–ox-LDL Abs detected in this setting are purely surrogate markers of atheromatous disease severity, arising as a result of prothombin and ox-LDL binding on the damaged vessel wall and exposing neoantigens, without playing a direct role in atherothrombotic disease causation.68
It is interesting that outside the context of myocardial infarction associated with dyslipidemia in middle-aged men,67 antiprothrombin Abs detected either by ELISA70 or using novel methodology (which can distinguish their contribution to the LA effect from that caused by anti–β2-GPI Abs55 ) do not appear to correlate with thrombosis.
Whether patients with APS experience an increased incidence of atherosclerosis compared with the general community is a pertinent question.71 The possibility of accelerated atherosclerosis is raised by the observation that anti–β2-GPI Abs can bind to β2-GPI/ox-LDL complexes found in APS patients and lead to enhanced uptake of ox-LDL by macrophages in vitro.71 Vlachoyiannopoulos and Samarkos, on reviewing the literature, have suggested that there is evidence to indicate that some APS patients develop premature asymptomatic atherosclerosis in the absence of other risk factors.72 They did indicate, however, that the numbers studied are small and, hence, to confidently incorporate atherosclerosis within the vista of vascular pathology directly attributed to antibodies associated with APS, independent of traditional cardiovascular risk factors, much larger epidemiologic studies are required.72
Proposed nonthrombotic mechanisms predisposing to fetal loss in APS
The spectrum of obstetric manifestations associated with APS is wide, ranging from recurrent miscarriages during the first trimester to fetal growth restriction or death in the second or third trimester.73 There are also a group of healthy pregnant women in whom elevated aPL Ab levels are detected in a research setting or during the investigation for false positive syphilis serology, whose obstetric outcome is unremarkeable.73 These findings raise a number of issues that are not fully resolved. Are aPL Abs responsible for the various manifestations described, or do they merely represent an epiphenomenon?73 If the aPL Abs are responsible, are multiple antibody subgroups responsible or only one? Are concurrent, aPL Ab–independent genetic and environmental factors affecting the maternal-fetal interface responsible for influencing the potential pathogenicity of the aPL Abs and hence the spectrum of clinical manifestations? Finally, the placental tissue from APS patients shows heterogeneous findings.74 Not all show thrombosis.74 This raises the question: What is the nature of the pathogenic insult leading to fetal loss in these latter cases?
Despite the differences between murine and human placentae,75,76 the passive administration of polyclonal and monoclonal Abs from patients with APS and healthy controls to pregnant mice has been an important model in establishing that aPL Abs do display biologic properties in vivo that can lead to adverse pregnancy outcomes, thus establishing tentative validitity for the concept that they are unlikely to merely represent an epiphenomenon in humans.77,78 The difficulties that arise, however, are knowing how far we can extrapolate the delineated mechanisms for fetal loss arising from such models to humans without having a complete grasp of the functional differences in terms of responses to endogenous and exogenous noxious stimuli between murine and human placentae and the patient-specific variables that lead to the spectrum of clinical disease highlighted. This is compounded by not understanding the possible similarities and differences of physiological function of the major APS antigen β2-GPI in mice and humans.77
It has been hypothesized that complement activation may contribute to recurrent fetal loss associated with APS, particularly in situations characterized by an inflammatory infiltrate in the placenta.79,80 Trophoblasts in placental tissue can express antigens such as PS and β2-GPI, which can potentially be targeted by the relevant aPL Ab subgroups.81
The passive transfer of patient IgG polyclonal Abs and patient-derived monoclonal antibody targeting phospholipids per se to pregnant mice was the in vivo model used to assess the possible involvement of complement activation to adverse fetal outcome in these models.79,80 Pregnant mice that had the complement C5a receptors knocked out did not display fetal resorption, which is hypothesized to be the murine equivalent of miscarriage in humans.80 Activation of C3 was also important.79 Neutrophils80 and TNF-α82 were intermediates in the process. The F(ab′)2 fragments of the antibodies were unable to induce fetal resorption, suggesting that the classical pathway is initially involved.80 This is in contrast to the thrombosis model developed by Jankowski et al.22
Disruption of trophoblast function independent of mechanisms involving thrombosis and complement activation can also potentially occur. Anti–β2-GPI Abs interacting with β2-GPI on the trophoblast surface can inhibit trophoblast gonadotropin secretion and invasiveness in vitro.81 This mechanism has been hypothesized to contribute to early pregnancy loss.81 There is evidence of a significant reduction in intradecidual endovascular trophoblast invasion on analysis of the products of conception (first-trimester failure) from APS patients.83
The physiological role of β2-GPI in murine pregnancy has been studied by Robertson et al.77 Compared with wild-type, β2-GPI knockout mice have subtle changes in their placenta.77 Furthermore, absence of β2-GPI in progeny of β2-GPI+/− mice appeared to significantly decrease fecundity.77 These findings suggest that in mice β2-GPI may mediate a role in embryo implanation.77 The passive administration of patient IgG polyclonal Abs with β2-GPI reactivity to wild-type mice did not lead to fetal resorption but, rather, impairment of embryo implanation.77 The relevance of this finding to humans is not clear, because we do not know what if any physiological purpose β2-GPI serves in human placental functioning.77
Proposed mechanisms contributing to autoantibody production in APS
Issues regarding the physiological importance of the relationship between β2-GPI and apoptotic cells and PS-expressing membranes
Monomeric β2-GPI in vitro binds with low affinity to PS membranes in the absence of anti–β2-GPI Abs.84 Antibodies increase the affinity of β2-GPI for PS by 1000-fold.84 In view of this low affinity in vitro, questions have been raised about β2-GPI's ability to serve a physiologically relevant role in vivo via mechanisms involving its binding to PS, such as an opsonin for apoptotic cells, and as a natural anticoagulant by binding PS on activated cell surfaces.84 In vivo β2-GPI may exist not only in the monomeric form but also in the multimeric form, which has a greater affinity for binding PS.85 Hence, a physiologically relevant in vivo role involving the interaction between multimeric β2-GPI and PS is not excluded.84 Furthermore, in vivo apoptotic cells express on their surface, in addition to PS, apoptotic blebs that contain intracellular debris.86 It is possible that components of the debris may be able to bind monomeric β2-GPI. β2-GPI–deficient mice have been generated77 ; however, the role of β2-GPI in apoptotic cell clearance using these mice has not been addressed yet and is an area worthy of pursuit in the future.
Impaired apoptotic cell clearance hypothesis
In SLE it has been proposed that there may be impaired clearance of apoptotic cells, which may predispose a genetically susceptible individual to mount an immune response against the molecules bound to the apoptotic blebs, leading to autoantibody production against these antigens.86-88 Similarly, in APS it has been suggested that β2-GPI 89,90 and prothrombin91 may bind to exposed PS on apoptotic blebs. If there is impairment in apoptotic cell clearance or an increase in apoptotic cell generation, this may predispose an individual to developing antibodies directed against β2-GPI and prothrombin bound to the apoptotic blebs.86 Similar mechanisms may be operational in the generation of anti–annexin A2 Abs, because annexin A2 also binds PS.9
Not all autoantigens described in APS, such as thrombin,10 bind PS5 ; hence, this raises the question of what the mechanism is that leads to their generation. An avenue that needs to be assessed is the possibility of a shared epitope, either linear or conformational, between thrombin and other antigens targeted in APS that do bind PS.92
Activation of autoreactive CD4 T cells hypothesis
Autoantibodies in APS tend to be of the IgG2 isotype and are of low affinity, which suggests they may arise from T-cell–independent immune responses, perhaps from the activation of marginal zone (MZ) B cells.93 β2-GPI–reactive antibodies from APS patients do show evidence of having undergone affinity maturation94 and, even though this is not inconsistent with the Abs arising from MZ B cells, it does support the role of T-cell involvement in their generation. Autoreactive T cells against β2GPI have been identified in patients with APS.95 They have also been detected in healthy individuals.95
Autoreactive CD4+ and HLA class II–restricted T cells directed against β2-GPI in vitro were be able to be activated by antigen-presenting cells when native β2-GPI was complexed to phospholipid.96 They did not become activated when presented with native β2-GPI alone or phospholipid alone.96 The intracellular processing of β2-GPI may differ when it is complexed to phospholipid compared with when it is on its own, allowing for the presentation of a cryptic epitope in the former situation.96 It was hypothesized that in states associated with impaired clearance of apoptotic cells, β2-GPI bound to surface-exposed PS on apoptotic cell surfaces may provide the substrate for this reaction.96
Interleukin-6 and the CD40-CD40L interaction mediate an important role in anti–β2-GPI Ab production by self-reactive B cells in vitro.97
The Toll-like receptor hypothesis
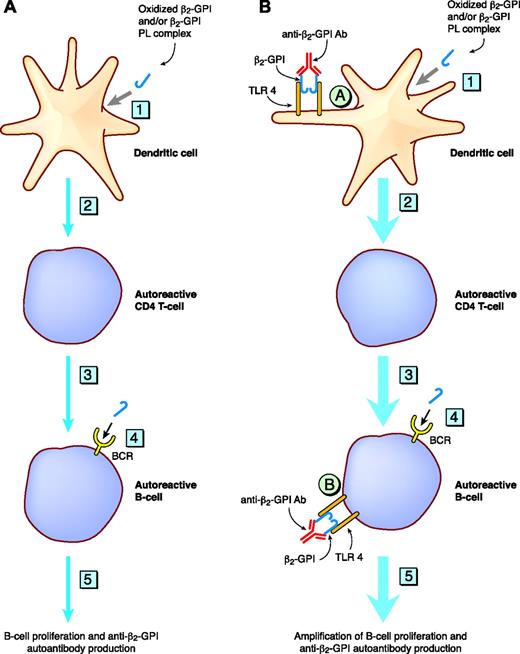
Oxidized β2-GPI is able to bind to dendritic cells (DCs) and induce them to mature, subsequently priming naive T cells and causing Th1 polarization.98 DC activation led to a signaling cascade resembling that triggered by TLR4.98 This raises the possibility that oxidized β2-GPI alone98 or in complex with anti–β2-GPI Ab may act as an endogenous immunologic adjuvant driving additional antibody generation via TLR4 (Figure 4). This would be somewhat analogous to the antichromatin Ab/chromatin immune complex driving additional autoantibody production in an SLE murine model via a crosslinking mechanism involving TLR9 and the B-cell receptor.99
Schematic representation of the hypothesis that anti–β2-GPI Abs in complex with β2-GPI may be able to amplify the production of autoantibodies via their ability to bind and crosslink TLR4. (A) The steps that have been delineated in in vitro experiments to be important in the generation of autoantibodies directed against β2-GPI. Step 1: the uptake and intracellular processing of the autoantigen β2-GPI (complexed to phospholipid or in the oxidized form) via as yet poorly delineated mechanisms. Step 2: the presentation (by activated DCs) of the β2-GPI cryptic epitope to autoreactive CD4+ HLA class II–restricted T cells. DCs have been shown to release an array of cytokines that lead to Th1 polarization. Step 3: activated autoreactive CD4+ T cells providing help to autoreactive B cells, which have also received a signal via the B-cell receptor (BCR) (see step 4). Step 4: the ligation by β2-GPI of the B-cell receptor on autoreactive B cells. Step 5: anti–β2-GPI autoantibody production. (B) It is hypothesized that oxidized β2-GPI or the anti–β2-GPI Ab/β2-GPI complex may function as an immunologic adjuvant by providing a costimulatory signal via TLR4 on DCs (circled A) and B cells (circled B), leading to the amplification of the signals delineated in panel A. The thick blue line denotes the amplification of steps 1 to 5.
Schematic representation of the hypothesis that anti–β2-GPI Abs in complex with β2-GPI may be able to amplify the production of autoantibodies via their ability to bind and crosslink TLR4. (A) The steps that have been delineated in in vitro experiments to be important in the generation of autoantibodies directed against β2-GPI. Step 1: the uptake and intracellular processing of the autoantigen β2-GPI (complexed to phospholipid or in the oxidized form) via as yet poorly delineated mechanisms. Step 2: the presentation (by activated DCs) of the β2-GPI cryptic epitope to autoreactive CD4+ HLA class II–restricted T cells. DCs have been shown to release an array of cytokines that lead to Th1 polarization. Step 3: activated autoreactive CD4+ T cells providing help to autoreactive B cells, which have also received a signal via the B-cell receptor (BCR) (see step 4). Step 4: the ligation by β2-GPI of the B-cell receptor on autoreactive B cells. Step 5: anti–β2-GPI autoantibody production. (B) It is hypothesized that oxidized β2-GPI or the anti–β2-GPI Ab/β2-GPI complex may function as an immunologic adjuvant by providing a costimulatory signal via TLR4 on DCs (circled A) and B cells (circled B), leading to the amplification of the signals delineated in panel A. The thick blue line denotes the amplification of steps 1 to 5.
Direct binding experiments, as have been performed to study the interaction between β2-GPI and other receptors such ApoER2′,100 annexin A2,64 and GPIbα,27 are required to solidify the involvement of TLR4.
TLR2 has also been implicated to interact with polyclonal aPL Abs.37 However, once again, direct binding experiments await to be performed.
Molecular mimicry hypothesis
It has been suggested that anti–β2-GPI Abs may be generated as a result of molecular mimicry between human β2-GPI and molecules similar to β2-GPI in invading bacteria.101 This framework was developed with the discovery that mice immunized with certain bacteria (Haemophilus influenzae and Neisseria gonorrhoeae) or tetanus toxoid can develop anti–β2-GPI Abs, which when extracted and passively transferred to pregnant mice are able to induce fetal resorption.101 Extrapolating this result to humans has some limitations. Anti–β2-GPI Abs have not been documented to arise on exposure to these specific organisms or toxin in humans. Anti–β2-GPI Abs have been detected in patients suffering from leprosy, but in this case they were directed against distinct epitopes on the β2-GPI molecule compared with the epitopes targeted in APS.102 In leprosy, which is not associated with prothrombotic manifestations, anti–β2-GPI Abs are directed against domain V,102 whereas in APS multiple studies have demonstrated that they are directed against epitopes on domain I.103-105 This suggests that distinct mechanisms may be responsible for nonpathogenic anti–β2-GPI Ab generation in the context of certain bacterial infections (domain V) and pathogenic anti–β2-GPI Ab generation in the context of APS (domain I).
Synthesis
Anti–β2-GPI Abs closely associate with the clinical manifestations of APS. Multiple mechanisms have been delineated by which they may predispose to thrombosis. At this time it cannot be discerned whether one dominant mechanism is responsible for venous, arterial, and placental thrombosis or whether distinct mechanisms come into play depending on patient background traits.
Other aPL Abs subgroups may contribute to the thrombotic manifestations associated with APS. It is not known whether combinations of aPL Ab subgroups acting synergistically may predispose to thrombosis in the various vascular beds.
Fetal morbidity and mortality in APS may be due not only to placental thrombosis but also placental inflammation due to complement activation and to impairment of trophoblast function. The factors that determine whether aPL Abs induce a thrombotic or nonthrombotic disease phenotype in the placenta are not known. It is likely that an interplay between patient background traits and distinct aPL Ab subgroups determines disease manifestation.
Some perspectives for future research
An important issue is to determine which of the multiple in vitro–delineated β2-GPI interactions serve an in vivo physiological purpose. Establishing this will likely give insights into the dominant pathophysiological mechamism(s) by which the anti–β2-GPI Ab/β2-GPI complex predisposes to the clinical manifestations associated with APS.
Large multicenter, multipatient studies looking at the association between the various clinical manifestations of APS and the distinct aPL Ab subgroups are a key area in assessing the contribution of the latter to the various manifestations. The development of internationally standardized assays to clearly distinguish between the various antibody subgroups will be critical in allowing their individual pathogenic importance to be assessed.
Finally, considerations for the development of therapies that lead to the lowering of specific pathogenic antibody levels in patients with established APS would seem a reasonable path to pursue.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: B.G. conceived the ideas and wrote the article; F.P. and S.R. reviewed the article and made suggestions; and S.K. conceived the ideas, reviewed the article, and made suggestions.
Acknowledgments
This work was supported by a project research grant (S.A.K.) and a medical research scholarship (B.G.) from the National Health and Medical Research Council of Australia (NHMRC).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal