Abstract
Most anticancer chemotherapies are immunosuppressive and induce nonimmunogenic tumor cell death. Bortezomib, a specific inhibitor of 26S proteasome, has shown clinical activity in several human tumors, including myeloma. Here we show that the uptake of human myeloma cells by dendritic cells (DCs) after tumor cell death by bortezomib, but not γ irradiation or steroids, leads to the induction of antitumor immunity, including against primary tumor cells, without the need for any additional adjuvants. The delivery of activating signal from bortezomib-killed tumor cells to DCs depends on cell-cell contact between DCs and dying tumor cells and is mediated by bortezomib-induced exposure of heat shock protein 90 (hsp90) on the surface of dying cells. The combination of bortezomib and geldanamycin (an hsp90 inhibitor) leads to greater apoptosis of tumor cells but abrogates their immunogenicity. These data identify drug-induced exposure of endogenous heat shock proteins on the surface of dying cells as a mechanism of immunogenic death of human tumors. Specific targeting of bortezomib to tumors may enhance their immunogenicity and the induction of antitumor immunity.
Introduction
Most antitumor chemotherapies kill tumor cells by the induction of apoptosis. However, in most (but not all) instances, chemotherapy-induced cell death does not lead to enhanced antitumor immunity.1–4 Improved understanding of which specific agents lead to immunogenic cell death and the underlying mechanisms may facilitate the integration of chemotherapy and immunotherapy of cancer. Bortezomib is a specific inhibitor of 26S proteasome subunit that has shown promising clinical activity in several tumors.5 Bortezomib induces apoptosis of human tumor cells by several mechanisms, including the inhibition of NFκB and disruption of unfolded protein response.5 We hypothesized that the mode of induction of tumor cell death may have a major impact on their immunogenicity. Here we show that the uptake of bortezomib-killed human myeloma cells by DCs leads to strong induction of antitumor immunity in culture without the need for any additional adjuvants and characterize the mechanism of enhanced immunogenicity
Patients, materials, and methods
Patients
Peripheral blood and bone marrow samples from patients with multiple myeloma were obtained following informed consent, which was provided in accordance with the Declaration of Helsinki, approved by the institutional review boards of St Vincent's Cancer Center and the Rockefeller University.
Isolation of primary tumor cells
Mononuclear cells from blood or bone marrow were obtained by density gradient centrifugation using Ficoll Hypaque (GE HealthCare, Uppsala, Sweden). Bone marrow mononuclear cells were separated into tumor (CD138+) and nontumor (CD138−) fractions using CD138 magnetic beads (Miltenyi Biotec, Auburn, CA). Tumor cells were cryopreserved in aliquots for use as source of antigen and targets.
Cell lines
Myeloma cell lines were obtained from American Type Culture Collection (U266, HLA-A2 positive; ATCC, Manassas, VA) or provided by J. Epstein, Arkansas Cancer Center, Little Rock, AR (cag, HLA-A2 negative). Other tumor cell lines used were NCEB1 (mantle cell lymphoma; gift of O. O'Connor, Memorial Sloan Kettering [MSKCC], New York, NY) and MCF-7 (breast cancer; gift of P. Livingston, MSKCC). Cell lines were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS)/glutamine/gentamicin.
Generation of tumor-loaded DCs
DCs were generated by culture of purified CD14+ cells isolated from patients peripheral blood mononuclear cells or from buffy coats in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Thousand Oaks, CA) and interleukin-4 (IL-4) (R&D Systems, Minneapolis, MN) as described.6 Tumor cells were killed by culture in the presence of bortezomib (100 nM) (Velcade, Millennium Pharmaceuticals, Cambridge, MA) or dexamethasone (1 μM) for 24 hours or by γ irradiation (20 Gy). Extent of apoptosis was monitored by annexin V/TO-PRO3 staining. Cells were extensively washed prior to feeding DCs. Immature DCs (day 5) were fed tumor cells at a DC/tumor cell ratio of 1:2. In some experiments tumor cells were coated with anti–syndecan-1 antibody (B-B4, 1 μg/mL; Serotec, Raleigh, NC).6,7 In some experiments pulsed DCs were stimulated with 100 ng/mL of lipopolysaccharide (LPS) (Sigma, St. Louis, MO) for 6 hours.
Stimulation of myeloma cells–specific T cells
Unpulsed or tumor-loaded DCs were added to CD56-depleted T cells at a ratio of 1:30 on days 0 and 7 of culture, as described.7 IL-2 (25-50 international units/mL; Chiron, Emeryville, CA) was added on day 2 and 7 of culture. Cultures were tested for the presence of tumor-specific T cells 7 to 9 days after the last stimulation with DCs.
HSPs expression on dying tumor cells
Tumor cells were treated by bortezomib or γ irradiated and expression of inducible hsp70 and hsp90 analyzed 6 to 24 hours later by flow cytometry using specific monoclonal antibodies (Stressgen, Ann Arbor, MI).
FACS analysis of DCs phenotype after interaction with killed tumor cells
Phenotype of DCs cultured with tumor cells was monitored by flow cytometry. Tumor cells were killed by bortezomib, γ irradiation, or dexamethasone and cocultured for 24 hours with immature DCs. For some experiments, DCs and tumor cells were dye labeled before coculture in order to monitor phagocytosis, as described.6 In some experiments, tumor cells were preincubated with geldanamycin (GDC, Sigma) at 100 nM or anti-hsp90 mAb (10 μg/mL) prior to setting up of the coculture. Phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) against the following molecules were used: CD80, CD86, CD83, and HLA-DR (BD Biosciences, San Jose, CA). DCs were stained for 30 minutes at 4°C, washed twice in phosphate buffered saline (PBS) + 0.1% bovine serum albumin (BSA) and analyzed on FACS Calibur (BD Biosciences) using FlowJo software (Tree Star, Ashland, OR). DCs were gated according to their FSC and SSC properties, and dead cells were excluded from the analysis by means of TO-PRO3 (Molecular Probes, Eugene, OR) staining. Appropriate isotype controls were included, and 5000 viable DCs were acquired for each experiment.
Evaluation of tumor-reactive, IFN-γ–producing T cells
The induction of tumor-reactive, interferon (IFN)-γ–producing T cells by tumor-loaded DCs was assessed by an enzyme-linked immunospot (ELISPOT) assay, as described.7 For some experiments, the induction of antimyeloma T-cell responses in HLA-A2+ donors was tested against HLA A2+ U266 tumor cells as targets, with HLA A2-cag cells serving as a control targets. To detect peptide-specific T cells, autologous DCs pulsed with 10 μM of defined HLA A2 restricted peptides from MAGEA3 (271-279, FLWGPRALV) and NYESO1 (157-165, SLLMWITQC), or 2.5 μM of an overlapping peptide library (15-mer peptides overlapping by 11 aa) specific for survivin, were used as antigen-presenting cells (APCs) in some experiments, as described previously.6,7 The peptides were synthesized by the proteomics resource center at the Rockefeller University. For some experiments, tumor-specific IFN-γ–producing T cells also were monitored by intracellular staining for IFNγ μ6
Cytotoxicity assay
Cytotoxicity of antitumor T cells was analyzed with a flow cytometry–based cytotoxicity assay.8 Target cells were labeled by 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) and cocultured with effector T cells at tumor-T cells ratio of 1:20 for 6 hours. Percentage of killed tumor cells was then determined by CFSE/TO-PRO3 double staining by flow cytometry.
Results
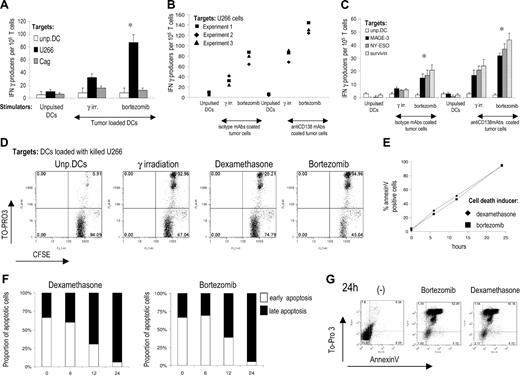
Bortezomib induces apoptosis of myeloma cells in a dose- and time-dependent manner.9,10 Culture of myeloma cell lines (U266 and cag) in the presence of 100 nM bortezomib led to apoptosis (% annexin V+ cells) of more than 95% cells at 24 hours, and this dose was used in initial experiments. Dye (PKH-26)–labeled tumor cells were killed by bortezomib or γ irradiation and fed to DCs.11 Bortezomib-killed U266 myeloma cells were phagocytosed by immature DCs at similar rate as γ-irradiated cells (data not shown). These DCs were then activated by LPS and used to stimulate tumor-specific T cells. DCs pulsed with bortezomib-killed myeloma cells were more potent inducers of tumor-specific IFN γ–producing T cells (Figure 1A). Opsonization of myeloma cells by anti–syndecan-1 monoclonal antibody enhances cross-presentation of tumor antigens by targeting the Fcγ receptors on DCs.7,12 Even in this setting, DCs pulsed with bortezomib-killed U266 cells were more efficient (Figure 1B). Enhanced induction of tumor-specific immune response by DCs pulsed with bortezomib-killed U266 cells also was evident at the level of T cells against specific tumor antigens known to be expressed by myeloma cells, for instance, MAGE-3, NY-ESO1, and survivin (Figure 1C). Tumor cells–specific T cells induced by DCs pulsed with bortezomib-killed U266 also had higher cytotoxic activity than T cells expanded by DCs loaded with either γ irradiation or dexamethasone-killed tumor cells (Figure 1D). Therefore, DC uptake of tumor cells killed by bortezomib leads to greater induction of antitumor T cells than with tumor cells killed by γ irradiation or dexamethasone.
Bortezomib induces immunogenic cell death in human myeloma. (A) Expansion of myeloma-reactive T cells by DCs loaded with U266 tumor cells killed by bortezomib or γ irradiation. Monocyte-derived DCs alone or loaded with U266 tumor cells were matured using LPS as a maturation stimulus. The tumor-loaded and unpulsed DCs were each used to stimulate autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs, U266, or control cag cells as control were analyzed by ELISPOT assay. Data shown are mean/SD of 1 representative experiment of 3. (B) Same set up as in panel A. In addition to using DCs loaded with killed tumor cells, DCs also were pulsed with U266 cell killed by bortezomib and γ irradiation and opsonized with anti-CD138 monoclonal antibody. IFN-γ producers against U266 cells were analyzed by an overnight ELISPOT assay. Data shown are summary of 3 independent experiments with different blood donors. (C) Immature monocyte-derived DCs from HLA-A2+ donors were loaded with U266 myeloma cells coated with isotype control or anti-CD138 antibody. DCs were then used to stimulate autologous T cells. On day 7, T cells were restimulated with same DCs. After 14 days of culture, T cells were stimulated overnight in ELISPOT plates with autologous DCs pulsed with 10 μM HLA-A2 restricted peptides derived from MAGE-A3, NY-ESO-1, or 2.5 μM of an overlapping 15-mer peptide library derived from survivin. IFN-γ producers were quantified by an ELISPOT assay. Data shown are mean/SD of 3 independent experiments on 3 blood donors. *P value for comparison with γ-irradiated tumor cell–loaded DCs, P < .05. (D) Cytotoxic activity of myeloma-reactive T cells expanded by DCs loaded with U266 tumor cells killed by bortezomib, dexamethasone, or γ irradiation. CFSE-labeled target cells were incubated with T cells at the ratio of 1:20 for 6 hours and percentage of dead cells determined by TO-PRO3 staining. Data shown are representative of 3 experiments. (E) Kinetics of apoptosis after bortezomib or dexamethasone treatment. Either annexin V–positive or annexin V/TO-PRO3 double-positive cells was considered to be apoptotic. Live cells are defined as annexin V–negativeTO-PRO3 negative. Data are representative of 3 separate experiments. (F) Proportion of early (annexin V positive/TO-PRO3 negative) or late (annexin V/TO-PRO3 double-positive) tumor cells in the course of apoptosis induced by bortezomib or dexamethasone. Representative result of 3 experiments. (G) Representative FACS plot showing the stage of apoptosis of tumor cells used for pulsing of DCs, 24 hours after the induction of apoptosis.
Bortezomib induces immunogenic cell death in human myeloma. (A) Expansion of myeloma-reactive T cells by DCs loaded with U266 tumor cells killed by bortezomib or γ irradiation. Monocyte-derived DCs alone or loaded with U266 tumor cells were matured using LPS as a maturation stimulus. The tumor-loaded and unpulsed DCs were each used to stimulate autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs, U266, or control cag cells as control were analyzed by ELISPOT assay. Data shown are mean/SD of 1 representative experiment of 3. (B) Same set up as in panel A. In addition to using DCs loaded with killed tumor cells, DCs also were pulsed with U266 cell killed by bortezomib and γ irradiation and opsonized with anti-CD138 monoclonal antibody. IFN-γ producers against U266 cells were analyzed by an overnight ELISPOT assay. Data shown are summary of 3 independent experiments with different blood donors. (C) Immature monocyte-derived DCs from HLA-A2+ donors were loaded with U266 myeloma cells coated with isotype control or anti-CD138 antibody. DCs were then used to stimulate autologous T cells. On day 7, T cells were restimulated with same DCs. After 14 days of culture, T cells were stimulated overnight in ELISPOT plates with autologous DCs pulsed with 10 μM HLA-A2 restricted peptides derived from MAGE-A3, NY-ESO-1, or 2.5 μM of an overlapping 15-mer peptide library derived from survivin. IFN-γ producers were quantified by an ELISPOT assay. Data shown are mean/SD of 3 independent experiments on 3 blood donors. *P value for comparison with γ-irradiated tumor cell–loaded DCs, P < .05. (D) Cytotoxic activity of myeloma-reactive T cells expanded by DCs loaded with U266 tumor cells killed by bortezomib, dexamethasone, or γ irradiation. CFSE-labeled target cells were incubated with T cells at the ratio of 1:20 for 6 hours and percentage of dead cells determined by TO-PRO3 staining. Data shown are representative of 3 experiments. (E) Kinetics of apoptosis after bortezomib or dexamethasone treatment. Either annexin V–positive or annexin V/TO-PRO3 double-positive cells was considered to be apoptotic. Live cells are defined as annexin V–negativeTO-PRO3 negative. Data are representative of 3 separate experiments. (F) Proportion of early (annexin V positive/TO-PRO3 negative) or late (annexin V/TO-PRO3 double-positive) tumor cells in the course of apoptosis induced by bortezomib or dexamethasone. Representative result of 3 experiments. (G) Representative FACS plot showing the stage of apoptosis of tumor cells used for pulsing of DCs, 24 hours after the induction of apoptosis.
To verify that the observed difference between the immunogenicity of dexamethasone and bortezomib-killed tumor cells was not caused by differences in the kinetics or extent of tumor cells' death by these agents, we monitored the kinetics of apoptosis, measured as percent annexin V+ cells (Figure 1E) and the proportion of early (annexin V+TOPRO3-) versus late (annexinV+TOPRO3+) apoptotic cells (Figure 1F) at various time-points after drug exposure. Both dexamethasone and bortezomib induced tumor cells death with similar kinetics. At 24 hours, when tumor cells were used for DCs pulsing, more that 92% of cells were at the stage of late apoptosis for both treatments (Figure 1G). Therefore, in spite of similar kinetics and extent of apoptosis, the tumor cells killed by bortezomib differ significantly in their ability to activate DCs, compared to tumors killed by other therapies.
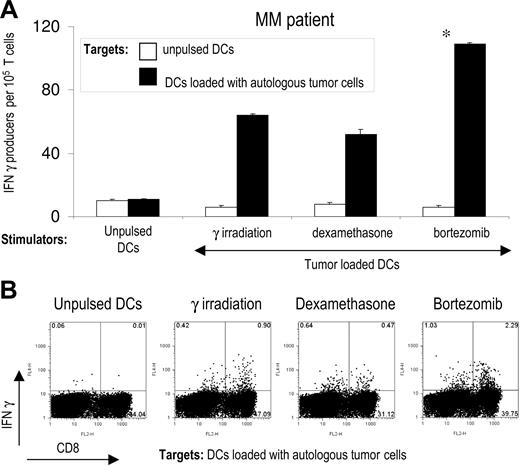
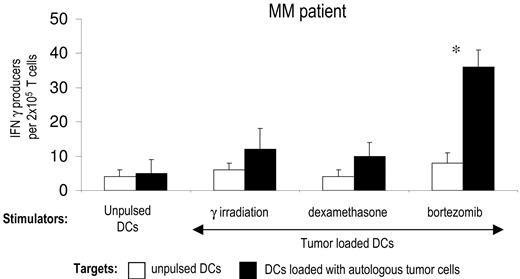
Using the same experimental model, we then tested whether the mode of induction of apoptosis in primary tumor cells isolated from myeloma patients also has an impact on the induction of T-cell immunity. Purified CD138+ tumor cells from the bone marrow of myeloma patients were killed by bortezomib, γ irradiation, or dexamethasone and fed to autologous DCs. DCs fed with bortezomib-killed tumor cells induced greater tumor-specific CD4 and CD8 T-cell responses than DCs fed with tumor cells killed by γ irradiation or dexamethasone (Figure 2A,B). Therefore, the method of induction of apoptosis of primary myeloma cells has a major impact on the generation of antitumor immunity by tumor-loaded DCs.
Enhanced immunogenicity of bortezomib-killed myeloma cells in patients with multiple myeloma. (A) DCs from patients with multiple myeloma (MM) were pulsed with autologous CD138-positive tumor cells killed by γ irradiation, dexamethasone, or bortezomib, matured with LPS, and used for the stimulation of autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs or DCs pulsed with autologous tumor cells were analyzed by ELISPOT assay. Data shown mean/SD of representative patient of 3 patients tested. *P < .05. (B) Intracellular IFNγ production by autologous tumor cell–specific CD4 and CD8 T cells. T cells were expanded as in panel A, and IFNγ production after stimulation with autologous DCs loaded with killed tumor cells was analyzed by flow cytometry.
Enhanced immunogenicity of bortezomib-killed myeloma cells in patients with multiple myeloma. (A) DCs from patients with multiple myeloma (MM) were pulsed with autologous CD138-positive tumor cells killed by γ irradiation, dexamethasone, or bortezomib, matured with LPS, and used for the stimulation of autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs or DCs pulsed with autologous tumor cells were analyzed by ELISPOT assay. Data shown mean/SD of representative patient of 3 patients tested. *P < .05. (B) Intracellular IFNγ production by autologous tumor cell–specific CD4 and CD8 T cells. T cells were expanded as in panel A, and IFNγ production after stimulation with autologous DCs loaded with killed tumor cells was analyzed by flow cytometry.
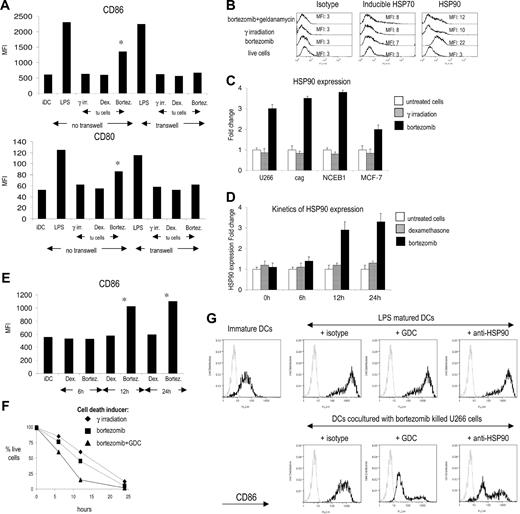
The ability of DCs to induce immunity is strongly linked to their activation or maturation status. Coculture of immature DCs with bortezomib-killed but not γ irradiated or dexamethasone-treated tumor cells led to an increase in the expression of CD83, CD80, and CD86, consistent with the delivery of a DC maturation stimulus by bortezomib-killed tumor cells (Figure 3A, data not shown). Importantly, the ability of bortezomib-killed tumor cells to promote DC maturation was abrogated when the DCs were separated from dying tumor cells by a transwell, suggesting the need for cell-cell contact between tumors and DCs. Prior studies have shown that inhibition of the proteasome leads to increased expression of several heat shock proteins (hsps), including hsp70 and hsp90.13,14 We hypothesized that bortezomib-induced cell death might, in addition, also lead to the translocation of hsps to the cell surface. DCs indeed express receptors for hsps and can be activated by this mechanism.15 Treatment of myeloma cells with bortezomib led to marked increase in the surface expression of hsp90, compared to inducible hsp70 (Figure 3B). Induction of cell surface hsp90 by bortezomib is not unique to myeloma and also was detected on mantle cell lymphoma (NCEB1) and breast carcinoma (MCF-7) cell lines (Figure 3C). The delivery of DC activation stimulus by killed tumors correlated with the degree of hsp90 up-regulation and was not restricted to the late apoptotic cells. We detected significant hsp90 expression 12 to 24 hours after bortezomib treatment, and this finding correlated with increased expression of maturation associated markers on DCs cocultured with bortezomib-killed tumor cells (Figure 3D,E). Preincubation of myeloma cells with geldanamycin (GDC, an hsp90 inhibitor) prior to bortezomib led to greater induction of tumor cell apoptosis (Figure 3F). However, tumor cells killed by this combination expressed reduced levels of surface hsp90 on dying tumor cells (Figure 3B). When myeloma cells killed by the combination of GDC and bortezomib were fed to DCs, they led to less induction of costimulatory molecules than on DCs fed with bortezomib-killed tumor cells at the same ratio of DCs to dying tumor cells (Figure 3G). Simply increasing the tumor/DC ratio does not lead to DC maturation in the setting of tumor cells killed by γ irradiation11 or dexamethasone (data not shown). Therefore, the effect of GDC in these experiments was not due to altered degree of tumor cell death. To further verify that the observed effects of GDC were indeed related to its ability to inhibit hsp90 (versus either hsp90 independent effects or nonspecific effect of enhanced apoptosis), we also analyzed the effect of an anti-hsp90 antibody instead of GDC. Preincubation with this antibody also blocked DC maturation induced by bortezomib-killed tumor cells (Figure 3G). Therefore, the activation signal to DCs from bortezomib-killed tumor cells is mediated by the exposure of hsp90 on the surface of dying cells and requires cell-cell contact between dying cells and DCs.
Mechanism of bortezomib-induced immunogenic cell death: role of cell surface hsp90 on the immunogenicity of bortezomib-induced cell death. (A) Phenotype of dendritic cells after interaction with bortezomib-killed U266 cells. Day 5 immature DCs were cultured for 24 hours with U266 myeloma cells killed by γ irradiation, dexamethasone, or bortezomib. Second set of cocultures was set up in transwells to prevent DCs/tumor cells contact. After 24 hours, expression of CD80 and CD86 on DCs was analyzed by flow cytometry. Mean fluorescence intensity is shown. *P value for comparison with γ-irradiated tumor cell–loaded DCs, P < .05. (B) Induction of inducible HSP70 (iHPS70) and HSP90 expression on U266 treated with bortezomib. Apoptosis of U266 myeloma cells was induced by γ irradiation or bortezomib and surface expression of HSP70 and HSP90 analyzed 24 hours later by flow cytometry. Preincubation of tumor cells with geldanamycin 1 hour prior to the addition of bortezomib inhibits the increase in HSP90 expression. (C) HSP90 expression 24 hours after bortezomib treatment on myeloma (U266 and cag), mantle cell lymphoma (NCEB1), and breast cancer (MCF-7) cell lines. (D) Kinetics of HSP90 expression on U266 cells after dexamethasone or bortezomib treatment analyzed by flow cytometry. (E) Phenotype of dendritic cells after interaction with dexamethasone or bortezomib-killed U266 cells at different stages of apoptosis. U266 cells were killed by bortezomib or dexamethasone, and cocultures with day 5 immature DCs were set up at 6, 12, and 24 hours after induction of apoptosis. After 24 hours, expression of CD86 on DCs was analyzed by flow cytometry. Mean fluorescence intensity is shown. *P value for comparison with immature DCs, P < .05. (F) Kinetics of U266 myeloma cell apoptosis after γ irradiation, bortezomib treatment, or combined bortezomib + geldanamycin (GDC) treatment. Percentage of live cells was followed for 24 hours by flow cytometry. Live cells were defined as annexin V negative/To-Pro3 negative. (G) Inhibition of increase in CD86 expression on DCs after interaction with bortezomib-killed myeloma cells by GDC or anti-HSP90 antibody. Immature DCs were cultured for 24 hours with U266 myeloma cells killed by bortezomib and expression of CD86 analyzed by flow cytometry. Preincubation of tumor cells with geldanamycin or anti-HSP90 mAb prior to the induction of apoptosis by bortezomib abrogates increase in CD86 expression. Staining with isotype control (gray line) and anti-HPS90 mAb (black line) is shown. Error bars represent mean ± SD.
Mechanism of bortezomib-induced immunogenic cell death: role of cell surface hsp90 on the immunogenicity of bortezomib-induced cell death. (A) Phenotype of dendritic cells after interaction with bortezomib-killed U266 cells. Day 5 immature DCs were cultured for 24 hours with U266 myeloma cells killed by γ irradiation, dexamethasone, or bortezomib. Second set of cocultures was set up in transwells to prevent DCs/tumor cells contact. After 24 hours, expression of CD80 and CD86 on DCs was analyzed by flow cytometry. Mean fluorescence intensity is shown. *P value for comparison with γ-irradiated tumor cell–loaded DCs, P < .05. (B) Induction of inducible HSP70 (iHPS70) and HSP90 expression on U266 treated with bortezomib. Apoptosis of U266 myeloma cells was induced by γ irradiation or bortezomib and surface expression of HSP70 and HSP90 analyzed 24 hours later by flow cytometry. Preincubation of tumor cells with geldanamycin 1 hour prior to the addition of bortezomib inhibits the increase in HSP90 expression. (C) HSP90 expression 24 hours after bortezomib treatment on myeloma (U266 and cag), mantle cell lymphoma (NCEB1), and breast cancer (MCF-7) cell lines. (D) Kinetics of HSP90 expression on U266 cells after dexamethasone or bortezomib treatment analyzed by flow cytometry. (E) Phenotype of dendritic cells after interaction with dexamethasone or bortezomib-killed U266 cells at different stages of apoptosis. U266 cells were killed by bortezomib or dexamethasone, and cocultures with day 5 immature DCs were set up at 6, 12, and 24 hours after induction of apoptosis. After 24 hours, expression of CD86 on DCs was analyzed by flow cytometry. Mean fluorescence intensity is shown. *P value for comparison with immature DCs, P < .05. (F) Kinetics of U266 myeloma cell apoptosis after γ irradiation, bortezomib treatment, or combined bortezomib + geldanamycin (GDC) treatment. Percentage of live cells was followed for 24 hours by flow cytometry. Live cells were defined as annexin V negative/To-Pro3 negative. (G) Inhibition of increase in CD86 expression on DCs after interaction with bortezomib-killed myeloma cells by GDC or anti-HSP90 antibody. Immature DCs were cultured for 24 hours with U266 myeloma cells killed by bortezomib and expression of CD86 analyzed by flow cytometry. Preincubation of tumor cells with geldanamycin or anti-HSP90 mAb prior to the induction of apoptosis by bortezomib abrogates increase in CD86 expression. Staining with isotype control (gray line) and anti-HPS90 mAb (black line) is shown. Error bars represent mean ± SD.
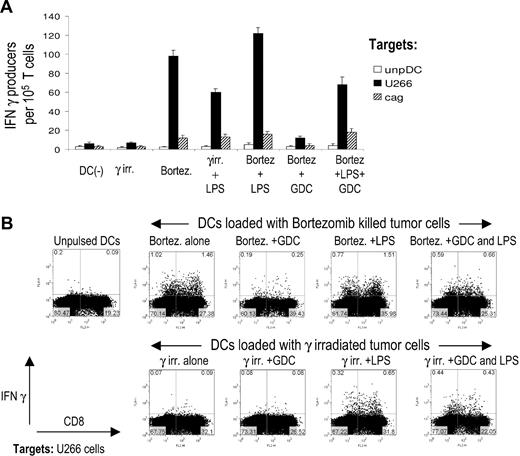
Next we evaluated the impact of tumor-induced DC activation on the induction of antitumor immunity by these DCs. Tumor cells were killed with bortezomib or irradiation, either alone or with GDC. DCs were fed with these tumor cells, with or without subsequent maturation with LPS, and used to stimulate T cells. Generation of tumor-specific T cells was monitored by interferon-γ ELISPOT (Figure 4A), as well as by intracellular cytokine flow cytometry (Figure 4B). Importantly, DCs loaded with bortezomib-killed tumor cells efficiently induced antitumor immunity, without the need for additional maturation stimulus, while DCs loaded with irradiated tumor cells were ineffective, unless an exogenous DC maturation was provided (Figure 4A,B). DCs fed with tumor cells killed by the combination of GDC and bortezomib also led to less induction of antitumor T cells, compared to DCs fed with bortezomib-killed tumors (Figure 4A,B). Therefore, the enhanced immunogenicity of bortezomib-killed tumor cells is strongly linked to the induction of hsp90 on dying tumor cells and cell contact–mediated DC activation.
Geldanamycin abrogates increased immunogenicity of bortezomib-killed myeloma cells. (A) DCs were pulsed with U266 myeloma cells killed by γ irradiation, bortezomib alone, or with myeloma cells preincubated with geldamamycin for 1 hour before treatment by bortezomib. Tumor-loaded DCs with or without additional maturation stimulus with LPS were then used as stimulators of autologous T cells for 2 weeks. On day 7, T cells were restimulated with fresh tumor cell–pulsed DCs. On day 14, IFNγ production by tumor-reactive T cells in response to U266 myeloma cells was quantified by an overnight ELISPOT. Response to an HLA-A2–negative cell line cag or unpulsed autologous DCs was used as a control. Data shown are mean/SD of 3 independent experiments on 3 blood donors. (B) DCs were fed with apoptotic U266 tumor cells killed by γ irradiation or bortezomib, with or without 1 hour preincubation of U266 cells with geldanamycin. Pulsed DCs then were activated with LPS or left untreated. DCs then were used to stimulate T cells as in panel 2A. On day 14 of culture, IFNγ production in response to U266 tumor cells was monitored by intracellular cytokine flow cytometry. Data are representative of similar experiments on 3 donors. Response to HLA-A2 negative cag cells was used as a negative control and did not exceed 0.1% (not shown).
Geldanamycin abrogates increased immunogenicity of bortezomib-killed myeloma cells. (A) DCs were pulsed with U266 myeloma cells killed by γ irradiation, bortezomib alone, or with myeloma cells preincubated with geldamamycin for 1 hour before treatment by bortezomib. Tumor-loaded DCs with or without additional maturation stimulus with LPS were then used as stimulators of autologous T cells for 2 weeks. On day 7, T cells were restimulated with fresh tumor cell–pulsed DCs. On day 14, IFNγ production by tumor-reactive T cells in response to U266 myeloma cells was quantified by an overnight ELISPOT. Response to an HLA-A2–negative cell line cag or unpulsed autologous DCs was used as a control. Data shown are mean/SD of 3 independent experiments on 3 blood donors. (B) DCs were fed with apoptotic U266 tumor cells killed by γ irradiation or bortezomib, with or without 1 hour preincubation of U266 cells with geldanamycin. Pulsed DCs then were activated with LPS or left untreated. DCs then were used to stimulate T cells as in panel 2A. On day 14 of culture, IFNγ production in response to U266 tumor cells was monitored by intracellular cytokine flow cytometry. Data are representative of similar experiments on 3 donors. Response to HLA-A2 negative cag cells was used as a negative control and did not exceed 0.1% (not shown).
Next we tested whether bortezomib-induced apoptosis of primary tumor cells from myeloma patients could lead to induction of antitumor T-cell immunity, without the need for additional stimuli. Purified CD138+ tumor cells from myeloma patients were killed with bortezomib, dexamethasone, or γ irradiation and fed to autologous monocyte-derived immature DCs. These DCs were then used to stimulate tumor specific T cells without any additional maturation stimulus. DCs fed with tumors killed by bortezomib, but not with irradiation, led to the activation of tumor-specific T cells in 3 of 3 patients tested (Figure 5). Therefore, bortezomib-mediated cell death provides a distinct immunogenic stimulus and leads to the activation of antitumor immunity.
Induction of antitumor immunity by bortezomib-killed primary myeloma cells without the need for exogenous DC maturation stimulus. Monocyte-derived DCs from patients with MM were pulsed with autologous CD138-positive tumor cells killed by γ irradiation, dexamethasone, or bortezomib in the absence of any DC maturation stimuli and used for the stimulation of autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs or DCs pulsed with autologous tumor cells were analyzed by an ELISPOT assay. Data shown are representative (mean/SD) of 3 tested patients.
Induction of antitumor immunity by bortezomib-killed primary myeloma cells without the need for exogenous DC maturation stimulus. Monocyte-derived DCs from patients with MM were pulsed with autologous CD138-positive tumor cells killed by γ irradiation, dexamethasone, or bortezomib in the absence of any DC maturation stimuli and used for the stimulation of autologous T cells for 2 weeks. IFN-γ producers against unpulsed DCs or DCs pulsed with autologous tumor cells were analyzed by an ELISPOT assay. Data shown are representative (mean/SD) of 3 tested patients.
Discussion
Interactions between dying cells and DCs are a focus of intense investigation.3 DCs are specialized for uptake and presentation of antigens from dying cells and play an important role in the maintenance of immune tolerance during tissue homeostasis. Acquisition and cross-presentation of tumor antigens by DCs is thought to also play a critical role in the generation of T-cell immunity against tumors. Therefore, whether cell death induced by different chemotherapies has differential effects on cross-presentation of antigens from human tumors has fundamental implications for combining chemotherapy and immunotherapy of cancer. Here we show that myeloma cell death induced by bortezomib differs from other common myeloma therapies (dexamethasone and irradiation) in that it delivers a distinct immune-activating stimulus. The finding that bortezomib induces immunogenic death of human myeloma, including primary tumor cells, has implications for harnessing the immune system against this and possibly other tumors. To our knowledge, these data represent the first example wherein drug-induced apoptosis leads to enhanced autologous antitumor T-cell response to primary human tumor cells without the need for any additional stimulus.
The mechanism of immunogenicity of bortezomib-killed tumor cells was dependent on cell-cell contact and linked to the expression of hsp90 on the surface of dying cells. Prior studies have documented an increase in several hsps in bortezomib-treated tumor cells,14 however, their translocation to the cell surface and resulting immunologic effects have not been examined. Demaria et al observed up-regulation of maturation markers in murine DCs fed with bortezomib-killed carcinoma cell lines, although the underlying mechanism and induction of T-cell immunity were not investigated.16 Several soluble factors from necrotic cells have been implicated as danger signals that mediate DC activation.3,17 Our data, however, indicate the need for cell contact between DCs and dying cells in this setting and support the murine studies that hsps on the cell surface represent a distinct immunogenic stimulus.18,19 Bortezomib also may impact cross-presentation by inhibiting antigen processing in the donor cell.20,21 However, the effects of bortezomib were abrogated by geldanamycin, suggesting that enhanced cross-presentation in these experiments was largely hsp dependent.
There is considerable interest in understanding the biochemical features of immunogenic versus nonimmunogenic death of tumor cells induced by anticancer therapies. Our data, using primary human tumor cells, suggest that the exposure of endogenous hsps on the surface of dying tumor cells may represent one such stimulus. In our case, the dominant signal is delivered via hsp90. However, the nature of specific hsp exposed on dying cells may be cell type, tissue, or stimulus/drug-specific, and the underlying mechanisms require further study.
These data also have several implications for the use of bortezomib in the clinic. The finding that the mode of induction of apoptosis has a major impact on cross-presentation by DCs also has implications for improving DC-mediated immunotherapy of cancer. Heat shock proteins are under active evaluation for immunotherapy of cancer, in view of their adjuvant properties.22 This has been attempted generally via the injection of purified hsps or induction of hyperthermia.22–24 Bortezomib may therefore be a useful tool for the induction of endogenous hsps on dying tumor cells and enhance their immunogenicity in vivo. These findings were not restricted to myeloma but also observed in lymphoma and breast cancer cell lines. Drug-induced expression of hsps also may be responsible for some of the observed clinical effects of bortezomib, such as a vasculitic skin rash that correlates with antitumor activity.25 Bortezomib, however, also may be toxic to DCs and activated T cells.26,27 Indeed, disruption of tumor-DC interactions10,28 may contribute to the antimyeloma effects of this drug. Targeted delivery of this agent to tumor cells (as opposed to systemic administration, as currently practiced) may therefore be needed to fully harness the immunogenic properties of bortezomib described here. This may be accomplished via direct injection into tumors (in the case of solid tumors, lymphoma, or plasmacytoma) or coupling to antitumor antibodies. The finding that bortezomib-mediated immunogenicity is dependent on hsp90 also urges caution for ongoing trials of combination with hsp90 inhibitors. The rationale for designing these studies was based on enhanced killing of tumor cells with the combination.29 However, while these combinations might lead to enhanced responses in the short term, reduced immunogenicity of the dying tumor cells might ultimately limit the durability of such responses. Alternately, the combination of proteasome inhibitors with other drugs thought to mediate immunogenic death (such as anthracyclines)4 might be of special interest.30 Optimal combination of antitumor therapies may depend not just on their effects on tumor cells, but also the host.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr R. M. Steinman and K. M. Dhodapkar for critical reading of the manuscript, J. Adams for help with illustrations, and P. Matthews for technical assistance.
M.V.D. is supported in part by funds from the National Institutes of Health (CA106802, CA109465, P50-AT02779), Damon Runyon Cancer Research Fund, Irene Diamond Foundation, Dana Foundation, and Irma T. Hirschl Foundation.
National Institutes of Health
Authorship
Contribution: R.S. performed research, analyzed data, and wrote the paper; A.C. analyzed data; A.M., D.V., and S.J. performed clinical research; and M.V.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, Laboratory of Tumor Immunology and Immunotherapy, The Rockefeller University, 1230 York Ave, New York, NY, 10021; e-mail: dhodapm@rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal