Abstract
Growing evidence points to the potential of agonistic anti-CD40 mAbs as adjuvants for vaccination against cancer. These appear to act by maturing dendritic cells (DCs) and allowing them to prime CD8 cytotoxic T lymphocytes (CTLs). Although it is well established that optimal T-cell priming requires costimulation via B7:CD28, recent studies emphasize the contribution of TNF receptors to this process. To understand how anti-CD40 mAbs trigger effective antitumor immunity, we investigated the role of TNFR superfamily members CD27 and 4-1BB in the generation of this immunity and showed that, although partially dependent on 4-1BB:4-1BBL engagement, it is completely reliant on CD27:CD70 interactions. Importantly, blocking CD70, and to some extent 4-1BBL, during anti-CD40 treatment prevented accumulation of tumor-reactive T cells and subsequent tumor protection. However, it did not influence changes in DC number, phenotype, nor the activity of CTLs once immunity was established. We conclude that CD27:CD70 and 4-1BB:4-1BBL interactions are needed for DC-driven accumulation of antitumor CTLs following anti-CD40 mAb treatment. Finally, in support of the critical role for CD70:CD27, we show for the first time that agonistic anti-CD27 mAbs given without a DC maturation signal completely protect tumor-bearing mice and provide a highly potent reagent for boosting antitumor T-cell immunity.
Introduction
Interactions between members of the TNF receptor (TNFR) superfamily and their ligands play an important role in providing costimulation at several stages during the development of an effective antigen-specific CD8 T-cell response.1–3 Early in the response, the ligation of CD40 on dendritic cells (DCs) by its ligand, CD154, induces the maturation of DCs and potentiates their ability to stimulate antigen-specific naive CD8 T cells.4–6 Conversely, the absence of DC maturation, for example, during presentation of self- or tumor-associated antigens, leads to the induction of T-cell tolerance.7 Thus, antigen presentation by immature DCs maintains peripheral tolerance to self-tissues as well as tumors. Agonistic anti-CD40 mAb, which is a potent mimic of the natural ligand, CD154, has been shown to promote T-cell–mediated immunity in a number of settings, including vaccination, and treatment of tumors.8–11 The success achieved with agonistic anti-CD40 mAbs in preclinical models has recently led to clinical evaluation of anti–human CD40 mAbs as a potential treatment for cancer.12,13 It is assumed that anti-CD40 mAbs trigger the maturation, or licensing of DCs which subsequently leads to the priming of tumor-specific CD8 T cells. Identifying the critical changes in DCs during their CD40-triggered maturation is therefore key to understanding the mechanism of action of anti-CD40 mAbs. CD40-induced maturation of DCs is characterized by an increase in their expression of adhesion and costimulatory molecules, including ICAM-1, B7.1, B7.2, CD70, and 4-1BB ligand (4-1BBL) as well as cytokines.14–19 Although initial antigen-specific cytotoxic T lymphocyte (CTL) activation and proliferation depends on the CD28:B7 engagement,20,21 subsequent expansion and survival of effector and memory T cells are controlled by additional costimulatory interactions and cytokines. Two receptors that appear central in maintaining CD8 T-cell responses are the TNFR superfamily members 4-1BB (CD137)3,22,23 and CD27.14,15,19,24–27 Analysis of mice deficient in 4-1BBL or CD27 has demonstrated that these costimulatory receptors make a nonredundant contribution to the accumulation of antigen-specific CD8 T cells during infection with influenza virus.27 Interestingly, influenza virus–specific CD8 T cells present in these mutant mice were competent in lysing target cells, suggesting that differentiation into cytotoxic T cells (CTLs) is independent of 4-1BB and CD27 signals.3,24
We have previously demonstrated that anti-CD40 mAb stimulates a CD4 T-helper cell–independent CTL response against a number of syngeneic lymphomas which successfully eradicates existing tumors, and leaves the mice resistant to rechallenge.9,10 Our interests now are to uncover the events downstream of CD40 signaling that lead to the induction of the antitumor CTL response. Here, we show that blocking costimulation via CD27, but not 4-1BB, completely prevents anti-CD40 mAb lymphoma therapy as a result of a severe impairment in CD8 T-cell expansion. Furthermore, a new agonistic mAb against mouse CD27 can itself generate strong antilymphoma T-cell immunity.
Materials and methods
Animals and cells
Mice were supplied by Harlan (Blackthorn, Oxon, United Kingdom) and maintained in local facilities. The BCL128 B-lymphoma line was maintained by passage in BALB/c mice, and the A31 line was maintained by passage in C57Bl/6 mice. Animal experiments were conducted under license according to the UK Home Office license guidelines and approved by the University of Southampton Ethical Committee.
Antibodies and reagents
The 3/23 (anti-CD40) (originally provided by G. Klaus, NIMR, London), AT113-2 (anti–4-1BBL), and anti–TAN1-6 (anti-CD70)14 were raised in house by immunizing rats with 4-1BBL-Fc or soluble recombinant CD70. For flow cytometric analysis, ID3 (anti-CD19),29 N418 (anti-CD11c) (ATCC, Manassas, VA), Mc10-6A5 (anti-BCL1 Id mAb),30 LOB12/3 (anti–4-1BB),14 YTS169 (anti-CD8) were all prepared and PE- or FITC-labeled in house; PE-labeled anti-CD27 and APC-labeled anti-CD8 (Pharmingen, Oxford, United Kingdom). Soluble fusion proteins, sCD70-Fc and s4-1BBL-Fc, are as described previously.26
BIACore
Analysis of TAN-6 and AT113-2 binding was performed as described previously.31
Changes in number and phenotype of splenic lymphocytes and DCs
Spleen suspensions were analyzed for BCL1 tumor cells (PE–anti-CD19 and FITC–anti-BCL1 Id) and changes in number and phenotype of CD8 lymphocytes (APC–anti-CD8α and PE–anti–4-1BB and anti-CD27). For DC analysis, spleens were digested in 1 mg/mL collagenase D (Roche, Lewes, United Kingdom) and 0.05 mg/mL DNaseI (Sigma, Poole, United Kingdom) for 30 minutes at 37°C, and samples were labeled using PE–anti-CD11c and FITC–anti-B7.1, B7.1, ICAM, 4-1BB, 4-1BBL, and CD70, in the presence of the FcγR-blocking mAb, 2.4G2.
Immunotherapy
Groups of age-matched mice (n = 5-6) were injected intravenously with 107 BCL1 or A31 cells on day 0 followed by anti-CD40 or anti-CD27 intravenously on days 4 to 7 (250 μg/d) plus, where indicated, blocking mAb intraperitoneally on days 4, 7, 9, and 11 (500 μg/d). To monitor tumor growth and the development of the T-cell response in the spleen, mice received 5 × 107 BCL1 cells on day 0 followed by anti-CD40 mAb (1 mg) on day 4 plus, where indicated, blocking mAb intraperitoneally on days 4, 7, and 9 (500 μg/d). Survival period to the humane end point was plotted using the Kaplan-Meier method with analysis for significance by the log-rank test using Prism version 4.0 for Windows (GraphPad Software, San Diego, CA).
In vivo killing assay
Mice were injected with 2 × 107 BCL1 cells intraperitoneally on day 0, and 1 mg anti-CD40 intraperitoneally on day 1. On day 8, groups of 3 were injected intraperitoneally with 1 mg of the appropriate blocking mAb and 5 hours later with 2 × 107 CFSE-labeled splenocytes from terminal BCL1 tumor-bearing mice intraperitoneally. Twenty-four hours later, peritoneal cavity cells were harvested and stained with PE–anti-BCL1 Id and APC–anti-CD8.13
Results
Phenotypic changes in splenic CD8 T cells during anti-CD40 treatment of BCL1 lymphoma
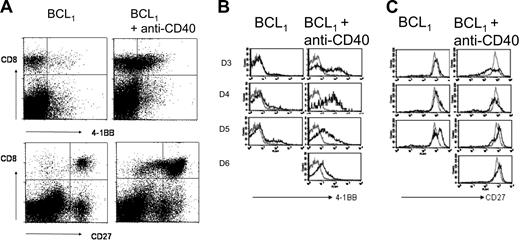
To gain insight into the mechanism by which CD40 mAb promotes the expansion of antitumor CD8 T cells during the immunotherapy of BCL1 lymphoma, we assessed the expression of the costimulatory receptors 4-1BB and CD27 on splenic CD8 T cells during the response. Animals were injected with 5 × 107 BCL1 cells intravenously followed 4 days later by 1 mg anti-CD40 mAb.9 As seen previously (and as will be shown in this article), the tumor continued to grow in the spleens of these mice for 2 to 3 days, but then, concomitant with a marked increase in the number of CD8 T cells, the number of tumor cells fell dramatically and the mice remained healthy. We saw little or no change in the number of CD4 T cells, confirming that this is a helper-independent immune response and observed long-term protection when treated mice were rechallenged with the same tumor.9,10 We next examined the expression of the costimulatory receptor 4-1BB which is known to be induced on activation of CD8 T cells (Figure 1A, top, and 1B). By 2 to 3 days after anti-CD40 mAb treatment, approximately 40% of the CD8 T cells expressed 4-1BB compared with less than 2% in naive mice or mice having received either BCL1 (left) or anti-CD40 mAb (not shown) alone. This expression peaked at day 4 after CD40 treatment, when approximately 70% of CD8 cells expressed 4-1BB, before falling on days 5 and 6. These findings were confirmed in a second lymphoma model, A31 in CBA mice (results not shown), which gave similar results. Interestingly, spleens from untreated mice taken at a late stage of tumor development (equivalent to day 5 after CD40 mAb, Figure 1B) showed a minor population of 4-1BB+ CD8 T cells, suggesting the existence of a weak, ineffectual response to tumor in the absence of anti-CD40 mAb. CD27, unlike 4-1BB, is constitutively expressed on the surface of naive CD8 cells.32 Figure 1A (bottom) and 1C show the expression of CD27 on CD8 cells during the time course. By day 3 after treatment the CD8 cells appear to split into 2 subpopulations on the basis of CD27 expression, with a small population expressing a slightly higher level than in naive animals and a major population expressing a lower level. The CD27high cells may represent primed cells with transient up-regulation of CD27,32,33 whereas the CD27low cells may correspond to a population of senescent terminal effectors.34 Tumor cells alone cause a slight increase in CD27 on a subpopulation of splenic CD8 T cells, whereas the anti-CD40 mAb alone had no effect on expression (not shown). Interestingly, the CD27low cells observed in animals that received tumor and anti-CD40 mAb were absent in animals that were injected with tumor alone (Figure 1B). Furthermore, by day 5 after treatment the CD27low cells were present at a lower frequency relative to the CD27high CD8 T cells.
The expression of 4-1BB and CD27 by CD8 T cells during the response to anti-CD40. Mice were inoculated with 5 × 107 BCL1 cells intravenously and treated with anti-CD40 mAb (1 mg) 4 days later. On the days indicated, the expression of 4-1BB and CD27 on splenic CD8 T cells was monitored by flow cytometry. (A) The dot plots show the expression of 4-1BB (top) and CD27 (bottom) on CD8 T cells in mice 3 days after anti-CD40 treatment compared with untreated controls. (B-C) The histograms show the changes in 4-1BB and CD27 expression on CD8 cells on days 3 to 6 following anti-CD40 and in untreated controls (left); gray lines show the expression level in naive mice. Results represent 1 of 3 similar experiments.
The expression of 4-1BB and CD27 by CD8 T cells during the response to anti-CD40. Mice were inoculated with 5 × 107 BCL1 cells intravenously and treated with anti-CD40 mAb (1 mg) 4 days later. On the days indicated, the expression of 4-1BB and CD27 on splenic CD8 T cells was monitored by flow cytometry. (A) The dot plots show the expression of 4-1BB (top) and CD27 (bottom) on CD8 T cells in mice 3 days after anti-CD40 treatment compared with untreated controls. (B-C) The histograms show the changes in 4-1BB and CD27 expression on CD8 cells on days 3 to 6 following anti-CD40 and in untreated controls (left); gray lines show the expression level in naive mice. Results represent 1 of 3 similar experiments.
Effect of blocking costimulation via 4-1BB and CD27 on the therapeutic response to anti-CD40 mAb
Having shown that the expression of the costimulatory receptors 4-1BB and CD27 on splenic CD8 T cells is altered during the treatment of tumor with anti-CD40 mAb, we next investigated the importance of these coreceptors to the therapeutic response. To address this question we used neutralizing mAbs specific for 4-1BBL and CD70, the respective ligands for 4-1BB and CD27. That these mAbs were able to block 4-1BBL/4-1BB and CD70/CD27 interactions was confirmed by showing that they blocked binding of soluble 4-1BBL–Fc and CD70-Fc to activated T cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Furthermore, both mAbs bound to their target antigens with similar affinity as determined by BIACore analysis. The KD values for the anti–4-1BBL and anti-CD70 mAbs were 3.8 × 10−9 M and 3.0 × 10−9 M, respectively. Blocking efficiency was also confirmed using CD40 mAb-dependent expansion of ovalbumin-specific CD8 T cells (Figure S1).
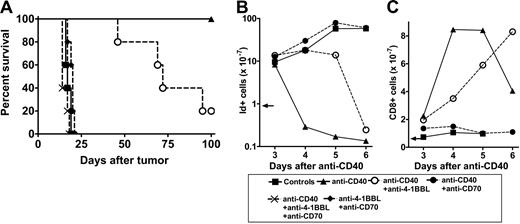
We then determined the effect of the anti-CD70 and anti–4-1BBL mAbs on the therapeutic response of BCL1 cells to anti-CD40 mAb. As expected, administration of anti-CD40 mAb provides long-term protection for BCL1-bearing mice.9,10 Remarkably, the anti-CD70 mAb completely blocked this therapeutic activity (Figure 2A). Figure 2B shows that expansion of the BCL1 tumor in these mice paralleled that in the control group and without the sharp decline in tumor seen after anti-CD40 mAb alone. In contrast, mice receiving the blocking anti–4-1BBL mAb survived for a median of 73 days, suggesting some impairment in the development of long-term immunity in the absence of 4-1BB costimulation (Figure 2A). We consistently found that anti-CD40 mAb-treated mice receiving both anti–4-1BBL and anti-CD70 succumbed to the tumor slightly earlier than untreated animals (median survival, 14 days compared with 17 days), suggesting that this combination might also block the weak spontaneous response to the tumor.
Effect of anti–4-1BBL and anti-CD70 on the therapeutic activity of anti-CD40 in BCL1 cells. (A) Groups of mice (n = 5) received 1 × 107 BCL1 cells intravenously on day 0 and then anti-CD40 mAb on days 4, 5, 6, and 7 (250 μg/d). Where shown, mice also received anti–4-1BBL and/or anti-CD70 intraperitoneally on days 4, 7, 9, and 11 (500 μg/d). Mice were monitored for tumor development. Survival to the humane end point was plotted using the Kaplan-Meier method and analyzed for significance using the log-rank test. Control versus anti-CD40 treated, P < .002; control versus CD40 + anti–4-1BBL, P < .002; anti-CD40–treated versus anti-CD40 in the presence of anti–4-1BBL, P < .02. Representative result from 3 experiments. (B-C) Mice received 5 × 107 BCL1 cells intravenously on day 0 and then anti-CD40 mAb intravenously on day 4, and, where shown, anti–4-1BBL or anti-CD70 mAb intraperitoneally on days 4, 5, and 6 (500 μg/ day). Splenic tumor (idiotype+) and CD8 T cells were monitored by flow cytometry. (B) The total number of tumor cells (B) and the total number of CD8 cells (C) are shown. The arrows indicate the level of tumor and CD8 cells at the time of treatment. Data points are mean of 2 animals. The results represent 1 of 4 similar experiments.
Effect of anti–4-1BBL and anti-CD70 on the therapeutic activity of anti-CD40 in BCL1 cells. (A) Groups of mice (n = 5) received 1 × 107 BCL1 cells intravenously on day 0 and then anti-CD40 mAb on days 4, 5, 6, and 7 (250 μg/d). Where shown, mice also received anti–4-1BBL and/or anti-CD70 intraperitoneally on days 4, 7, 9, and 11 (500 μg/d). Mice were monitored for tumor development. Survival to the humane end point was plotted using the Kaplan-Meier method and analyzed for significance using the log-rank test. Control versus anti-CD40 treated, P < .002; control versus CD40 + anti–4-1BBL, P < .002; anti-CD40–treated versus anti-CD40 in the presence of anti–4-1BBL, P < .02. Representative result from 3 experiments. (B-C) Mice received 5 × 107 BCL1 cells intravenously on day 0 and then anti-CD40 mAb intravenously on day 4, and, where shown, anti–4-1BBL or anti-CD70 mAb intraperitoneally on days 4, 5, and 6 (500 μg/ day). Splenic tumor (idiotype+) and CD8 T cells were monitored by flow cytometry. (B) The total number of tumor cells (B) and the total number of CD8 cells (C) are shown. The arrows indicate the level of tumor and CD8 cells at the time of treatment. Data points are mean of 2 animals. The results represent 1 of 4 similar experiments.
We next investigated the effects of blocking with anti–4-1BBL and anti-CD70 mAbs on the kinetics of the CD8 T-cell response to anti-CD40 mAb. Compared with anti-CD40 mAb alone, treatment in the presence of anti–4-1BBL resulted in a delay of about 2 days in the expansion of CD8 T cells and a slower clearance of tumor cells (Figure 2B-C). However, although delayed, the final number of CD8 cells was at least as high as that reached with anti-CD40 alone. In contrast, anti-CD70 mAb completely blocked the anti-CD40–induced eradication of tumor cells. This lack of response was reflected in the failure of the CD8 T-cell population to expand and explains the ability of anti-CD70 mAb to completely block the anti-CD40–mediated therapy (Figure 2A-B). These results demonstrate that blocking costimulation via CD27 has a profound effect on the anti-CD40 mAb-mediated therapeutic response. In contrast, the influence of the 4-1BBL:4-1BB interaction appears to be relatively modest.
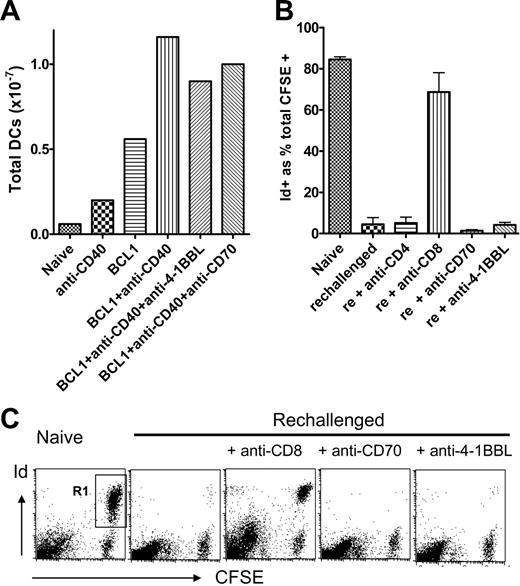
Because both anti–4-1BBL and anti-CD70 had an effect on the anti-CD40 mAb-induced CD8 T-cell response, we assessed whether they may be acting by abrogating the anti-CD40–induced increase in the number of splenic DCs and/or expression of costimulatory molecules in tumor-bearing mice either as a result of direct binding or through an immunoregulatory loop. Neither mAb inhibited either the increase in the number of DCs (Figure 3A) or the increase in expression of B7.1, B7.2, and ICAM-1 on DCs (not shown) after anti-CD40 mAb treatment. Thus, blocking of these coreceptors does not influence DC recruitment and is unlikely to affect their activation. Furthermore, we have previously shown that anti-CD70 mAb does not block CD4+ T-cell priming,19 thus excluding the notion that anti-CD70 mAb exerts direct immunoregulatory effects on DCs.
Anti–4-1BBL and anti-CD70 do not inhibit the anti-CD40–induced expansion of splenic DCs or the effector stage of the response in vivo. (A) Mice received BCL1 cells, anti-CD40, and anti–4-1BBL and anti-CD70 as described in Figure 1B. The total number of DCs recovered from each spleen after collagenase digestion was determined by flow cytometry using PE–anti-CD11c. Data points are the mean of 2 mice, and results are 1 of 2 similar experiments. (B-C) Mice received 2 × 107 BCL1 cells intraperitoneally on day 0 and then anti-CD40 mAb (1 mg) on day 1. On day 8, 1 mg of the appropriate blocking mAb was given intraperitoneally and then 5 hours later 2 × 107 CFSE-labeled splenocytes from BCL1 tumor-bearing mice (> 70% BCL1 tumor). Peritoneal cavity cells were harvested 24 hours later, stained with PE-labeled anti-BCL1 Id, and evaluated by flow cytometry. (B) CFSE+/Id+ cells surviving as a percentage of total CFSE+ cells are shown. Error bars represent mean × SD for 3 mice; rechallenged, and rechallenged + anti-CD4, anti-CD70, and anti–4-1BBL groups were significantly different from naive, P < .005; rechallenged + anti-CD8 was not significantly different from naive. (C) Representative dot plots are shown. The double positive (CFSE+ and Id+) cells (R1) represent the surviving tumor cells.
Anti–4-1BBL and anti-CD70 do not inhibit the anti-CD40–induced expansion of splenic DCs or the effector stage of the response in vivo. (A) Mice received BCL1 cells, anti-CD40, and anti–4-1BBL and anti-CD70 as described in Figure 1B. The total number of DCs recovered from each spleen after collagenase digestion was determined by flow cytometry using PE–anti-CD11c. Data points are the mean of 2 mice, and results are 1 of 2 similar experiments. (B-C) Mice received 2 × 107 BCL1 cells intraperitoneally on day 0 and then anti-CD40 mAb (1 mg) on day 1. On day 8, 1 mg of the appropriate blocking mAb was given intraperitoneally and then 5 hours later 2 × 107 CFSE-labeled splenocytes from BCL1 tumor-bearing mice (> 70% BCL1 tumor). Peritoneal cavity cells were harvested 24 hours later, stained with PE-labeled anti-BCL1 Id, and evaluated by flow cytometry. (B) CFSE+/Id+ cells surviving as a percentage of total CFSE+ cells are shown. Error bars represent mean × SD for 3 mice; rechallenged, and rechallenged + anti-CD4, anti-CD70, and anti–4-1BBL groups were significantly different from naive, P < .005; rechallenged + anti-CD8 was not significantly different from naive. (C) Representative dot plots are shown. The double positive (CFSE+ and Id+) cells (R1) represent the surviving tumor cells.
CD27 and 4-1BB do not operate at the effector stage of the anti-CD40 mAb-mediated antitumor response
Because 4-1BBL and CD70 are expressed by the BCL1 tumor (Figure S2), we investigated whether the anti–4-1BBL and anti-CD70 mAbs blocked the effector stage of the CTL response. We have observed (R.R.F., unpublished observations, May 2000) that BCL1 cells can be treated effectively with anti-CD40 mAbs in the peritoneal cavity of mice. This system was used to investigate the effector stage of CTL-mediated killing in vivo. Mice received intraperitoneally BCL1 and anti-CD40 mAb and then 8 days later (peak of response) were injected intraperitoneally with blocking anti-CD8, anti-CD70, or anti–4-1BBL mAb. Five hours later they were injected intraperitoneally with 2 × 107 CFSE-labeled BCL1 cells as targets. Naive mice were used as controls. Twenty-four hours later, peritoneal cavity cells were harvested and analyzed for surviving CFSE-labeled BCL1 tumor cells. The results are summarized in the histogram in Figure 3B and examples of individual plots shown in Figure 3C. Naive mice showed that 80% of recovered CSFE-labeled cells were idiotype+ tumor (Figure 3C). In contrast, tumor cells were completely eradicated in mice that had undergone anti-CD40 therapy of BCL1, underlining the effectiveness of the treatment. As expected, injection of anti-CD8 mAb almost totally blocked the clearance of CFSE-labeled tumor by CTLs. However, anti–4-1BBL and anti-CD70 had no effect on clearance. This lack of effect was confirmed in an in vitro cytotoxicity assay using purified CTLs taken from BCL1-bearing mice 5 days after anti-CD40 treatment (not shown), where, again, unlike anti-CD8 mAb, neither anti–4-1BBL nor anti-CD70 blocked CTL activity.
Direct immunotherapy via CD27 costimulation
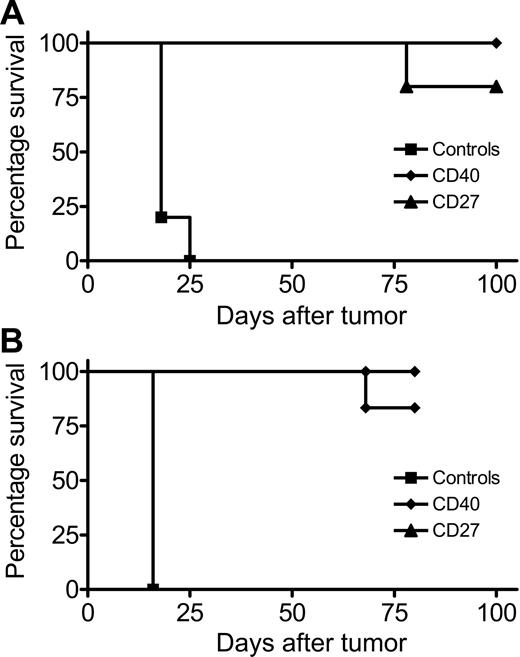
The results under “Effect of blocking costimulation via 4-1BB and CD27 on the therapeutic response to anti-CD40 mAb” suggest that the striking CTL response seen after tumor inoculation and anti-CD40 mAb treatment is completely dependent on c-stimulatory signals delivered following engagement of CD27 by its ligand CD70. Therefore, to determine whether we could shortcut the agonistic activity via CD40 and stimulate CD27 directly, we investigated the effect of administering agonistic anti-CD27 mAb on the growth of the BCL1 lymphoma. The agonistic activity of the anti-CD27 mAb used in the therapy was confirmed by its ability to cause expansion of adoptively transferred OT-I cells (Figure S3). As shown in Figure 4A, the anti-CD27 mAb afforded complete protection against the BCL1 tumor and gave results very similar to those obtained with anti-CD40 mAb. Similar therapeutic activity was obtained with a second lymphoma, A31 (Figure 4B). Furthermore, although CD27 is expressed on the BCL1 tumor cells, anti-CD27 mAb failed to provide any protection in severe combined immunodeficient (SCID) mice (results not shown), demonstrating that its activity is dependent on an intact adaptive immune response and is not due to a direct effect on the lymphoma cells. Thus, these results demonstrate that the delivery of a costimulatory signal via CD27 using anti-CD27 mAb is an effective way to generate cellular immunity against lymphoma.
Therapeutic potency of anti–4-1BBL and anti-CD27 mAb against lymphoma. Mice (5 per group) received injections of (A) 2 × 107 BCL1 cells or (B) 2 × 107A31cells intravenously on day 0 and then on days 4, 5, 6, and 7 treated with control IgG, anti-CD40, or anti-CD27 mAb (250 μg/d; intravenously day 4, intraperitoneally days 5, 6, and 7). Mice were monitored for tumor development. Survival to the humane end point was plotted using the Kaplan-Meier method (right) and analyzed for significance using the log-rank test. Control versus anti-CD40– and anti-CD27–treated groups for both tumors, P < .005. The results represent 1 of 2 similar experiments.
Therapeutic potency of anti–4-1BBL and anti-CD27 mAb against lymphoma. Mice (5 per group) received injections of (A) 2 × 107 BCL1 cells or (B) 2 × 107A31cells intravenously on day 0 and then on days 4, 5, 6, and 7 treated with control IgG, anti-CD40, or anti-CD27 mAb (250 μg/d; intravenously day 4, intraperitoneally days 5, 6, and 7). Mice were monitored for tumor development. Survival to the humane end point was plotted using the Kaplan-Meier method (right) and analyzed for significance using the log-rank test. Control versus anti-CD40– and anti-CD27–treated groups for both tumors, P < .005. The results represent 1 of 2 similar experiments.
Discussion
For some time it has been known that, even as a monotherapy, anti-CD40 mAb has efficacy in a range of vaccine settings, including induction of antitumor immunity in murine models and more recently in patients. For example, recent data from Vonderheide et al13 have shown that a fully human anti-CD40 mAb can deliver partial responses in 27% of patients with melanoma when given at just 0.2 mg/kg. Similar results were obtained using a trimeric CD40L fusion protein that demonstrated therapeutic activity in patients with advanced cancer.35 For the future there is growing evidence that CD40 agonists, although active alone, may prove more effective when used in combination with antigen and other adjuvants, such as TLR ligands and IFNα.36
We have undertaken this study to understand the mechanism by which anti-CD40 mAb triggers CD8 T-cell–dependent antitumor immunity. It has been proposed that anti-CD40 mAb induces DC maturation which renders them more effective in stimulating tumor-specific CD8 T cells. CD40 signaling has been shown to induce expression of costimulatory ligands on DCs, including B7.1, B7.2, CD70, and 4-1BBL14–18 ; however, their relative importance during the generation of antitumor immunity is not fully understood. We demonstrate here that the CD70:CD27 costimulatory pathway is essential for the therapeutic activity of anti-CD40 mAb. These findings support and extend previous studies demonstrating that CD40-licensed DCs promote a CD8 T-cell response to the model antigen ovalbumin in a CD70/CD27-dependent manner.14,15 In support of the critical role of the CD70:CD27 pathway, we also show that antibody-mediated delivery of a costimulatory signal via CD27 alone is sufficient to trigger the therapeutic antitumor response, indicating that direct CD27 engagement can bypass the requirement for CD40-mediated maturation of DCs. Furthermore, it appears that the level of tumor antigen processing and presentation to tumor-specific T cells, and the DC maturation that is occurring without deliberate maturation from anti-CD40 mAb, is sufficient to generate effective CTLs when triggered via CD27. Our observation that anti-CD70 mAb does not inhibit either the anti-CD40–induced increase in splenic DC number or the increase in the expression of costimulatory molecules, taken together with our previous results showing that, although CD70 is required for CD4+ Th cell–dependent and innate receptor-mediated CD8+ T-cell priming, it is dispensable for the response of CD4 T cells,19 suggesting a direct role for CD70 in CD8+ T-cell priming, rather than an effect on antigen presentation and processing by DCs.
Cross-presentation of tumor antigens by DCs is critical not only for priming of T-cell responses but also for induction of tolerance.7 Thus, cross-presentation of tumor antigens under steady state conditions by DCs has been shown to induce T-cell tolerance even when antigen is expressed by B-cell lymphoma which resides in secondary lymphoid organs.37,38 Interestingly, the tolerogenic response resulting from cross-presentation of tumor antigens by immature DCs appears to dominate over the ability of the malignant B cells to stimulate T-cell responses.38 Consistent with this notion we found that, although the BCL1 lymphoma used in our study expressed B7.1, B7.2, CD70, and 4-1BBL (Figure S2; data not shown), it failed to trigger effective T-cell immunity, thus corroborating the essential role of DC maturation in T-cell priming. A role for DC maturation by agonistic anti-CD40 mAb is also inferred from previous studies showing that anti-CD40 mAb therapy is effective against CD40− tumors.8–10 Interestingly, however, engagement of CD27 by agonistic anti-CD27 mAb appears to be sufficient to override the tolerogenic response that might be triggered by DCs in the absence of CD40 signaling.
The comparison of the role of CD27 with that of 4-1BB in the antilymphoma CD8 T-cell response generated by anti-CD40 mAb clearly shows that these costimulatory receptors influence CD8 T-cell expansion and survival in different ways. Thus, although blockade of costimulation via 4-1BB prevents the induction of an optimal antitumor CD8 T-cell response, blockade of CD27 costimulation completely abrogates the therapeutic response. A similar hierarchy of CD27 and 4-1BB contribution to the primary CD8 T-cell response elicited by influenza virus has recently been observed.27 The different functional outcomes of 4-1BB versus CD27 engagement may reflect differences in their activation kinetics, with CD27 likely to be engaged earlier because of its constitutive expression on naive T cells. Alternatively, CD27 may initiate qualitatively and/or quantitatively different signaling programs that lead to different cellular responses. Interestingly, the effect of blocking the CD27 costimulation pathway is similar to that observed following CD28:B7.1/2 blockade which also completely abrogated the therapeutic response of anti-CD40 mAb.10
Taken together these data strongly support enhanced T-cell costimulatory signaling as the major mechanism responsible for the therapeutic activity of agonistic anti-CD40 mAb. From an immunotherapeutic point of view direct stimulation of CD27 on T cells could prove beneficial over activation of CD40 because the expression of CD27 is restricted to T cells, natural killer (NK) cells, and a subset of B cells, which contrasts with the more widely distributed CD40.1,39 As such, the therapeutic usefulness of agonistic anti-CD40 mAb may be limited because of toxicity resulting from the induction of proinflammatory cytokines.40,41 Our study predicts that activation of the CD27 costimulatory pathway using agonistic mAbs could provide a highly effective strategy to boost T-cell responses elicited by cancer vaccines in humans.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the Tenovus laboratory who provided expert technical support and valuable discussion.
This work was supported by Tenovus of Cardiff, Cancer Research UK, and the Leukaemia Research Fund.
Authorship
Contribution: R.R.F. designed experiments, performed research, and wrote the paper; V.Y.T. performed research; G.R.C. made some of the original observations; T.F.R. produced new reagents; J.C.G. performed research; P.W.J. planned research; A.L.T. produced new reagents; A.A.-S. planned research and wrote the paper; and M.J.G. planned research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.A.-S. and M.J.G. contributed equally to this study.
Correspondence: Martin J. Glennie, Tenovus Research Laboratory, Cancer Sciences Division, University of Southampton School of Medicine, Tremona Rd, Southampton, SO16 6YD United Kingdom; e-mail: mjg@soton.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal