Abstract
The HOXA9 homeoprotein exerts dramatic effects in hematopoiesis. Enforced expression of HOXA9 enhances proliferation of primitive blood cells, expands hematopoietic stem cells (HSCs), and leads to myeloid leukemia. Conversely, loss of HOXA9 inhibits proliferation and impairs HSC function. The pathways by which HOXA9 acts are largely unknown, and although HOXA9 is a transcription factor, few direct target genes have been identified. Our previous study suggested that HOXA9 positively regulates Pim1, an oncogenic kinase. The hematologic phenotypes of Hoxa9- and Pim1-deficient animals are strikingly similar. Here we show that HOXA9 protein binds to the Pim1 promoter and induces Pim1 mRNA and protein in hematopoietic cells. Pim1 protein is diminished in Hoxa9−/− cells, and Hoxa9 and Pim1 mRNA levels track together in early hematopoietic compartments. Induction of Pim1 protein by HOXA9 increases the phosphorylation and inactivation of the proapoptotic BAD protein, a target of Pim1. Hoxa9−/− cells show increased apoptosis and decreased proliferation, defects that are ameliorated by reintroduction of Pim1. Thus Pim1 appears to be a direct transcriptional target of HOXA9 and a mediator of its antiapoptotic and proproliferative effects in early cells. Since HOXA9 is frequently up-regulated in acute myeloid leukemia, Pim1 may be a therapeutic target in human disease.
Introduction
While Hox genes and their homeodomain protein products are widely recognized as fundamental regulators of embryonic development,1 there is now substantial evidence for their role in blood cell formation.2 Among the 39 HOX proteins, HOXA9 appears to have an especially important role in hematopoiesis. Evidence that HOXA9 plays a physiologic role in hematopoiesis comes from studies of Hoxa9-deficient mice. These mutant animals display a variety of lymphoid and myeloid defects, including a very blunted neutrophilic response to G-CSF administration.3,4 In addition to these defects in lineage-committed cells, Hoxa9−/− marrow cells display markedly reduced multilineage repopulating ability in vivo and severely impaired proliferation of primitive pluripotent progenitors in vitro.5 These hematopoietic defects are more striking than in any other single or double Hox mutation reported to date6–8 and even more dramatic than in animals lacking the entire Hoxb cluster of homeobox genes.9 This is likely due to the fact that Hoxa9 is one of the most highly expressed Hox family members in the hematopoietic stem cell (HSC)–enriched fraction of bone marrow.9,10
Enforced expression of Hoxa9 in murine marrow cells results in enhanced proliferation of pluripotent progenitors and a dramatic expansion of the HSC compartment,11 an effect also observed when Hoxb4 is retrovirally overexpressed.12 In the case of Hoxa9, this increase in proliferation and self-renewal leads inevitably to a fatal, transplantable acute myeloid leukemia (AML) in mice.13 These findings correlate well with the clinical observation that HOXA9 is frequently activated in human AML,14,15 particularly in cases involving mutations in the nucleophosmin gene16 and in cases bearing MLL fusion genes.17 Furthermore a recurring translocation in AML results in the fusion of the HOXA9 and NUP98 genes.18 Animal studies indicate that the leukemogenic potential of certain MLL fusion genes requires HOXA9 as a downstream mediator.19 HOXA9 has also emerged as a potential diagnostic feature and prognostic factor in human AML.20
Despite these dramatic effects in normal and leukemic hematopoiesis, the molecular pathways by which HOXA9 acts in primitive blood cells is very poorly understood. While HOXA9 is thought to function as a transcription factor, few authentic gene targets have been identified. Although previous studies have identified a handful of putative HOXA9 target genes in nonhematopoietic cells, including flk-2 and Eph4,21,22 no direct targets have been established in blood cells. In a previous gene-expression profile study, we identified the Pim1 gene as a putative HOXA9 target in hematopoietic cells, as its expression was significantly and rapidly up-regulated in the human myelomonocytic leukemic cell line U937 following transient HOXA9 overexpression.23 Given the role of Pim1, a known oncogene, in blood cell proliferation and survival,24 we sought here to determine if Pim1 could be a mediator of the biologic effects of HOXA9 in hematopoiesis.
Materials and methods
Retroviral constructs and virus production
MSCV-HOXA9-IRES-EGFP and MSCV-HOXA9NS-IRES-EGFP retroviral constructs were generated by subcloning a cDNA encoding a full-length murine HOXA9 or HOXA9NS (HOXA9 DNA-binding mutant containing an Asn→Ser mutation in the homeodomain25 ; a gift from Dr Mark Kamps, University of California San Diego) into the MSCV-IRES-EGFP vector.26 MSCV-Pim1-IRES-EGFP was gift from Dr Andrew Kraft (Hollings Cancer Center, Medical University of South Carolina). High-titer retrovirus was obtained by transfecting Phoenix amphotropic packaging cells using Metafectene transfection reagent (Biontex Laboratories, Munich, Germany). Virus-containing medium was harvested at 48 and 72 hours later after transfection, filtered through a 0.45-μm filter, and used without storing or freezing.
Cell culture and retroviral transduction
U937 and K562 human leukemia cell lines (American Type Culture Collection, Manassas, VA) were cultured in RPMI1640 medium with 10% FCS. Bone marrow cells from wild-type and Hoxa9-deficient mice were harvested 4 days after intraperitoneal injection with 150 mg/kg 5-fluorouracil and cultured in prestimulatory media consisting of Dulbecco modified Eagle medium (DMEM) supplemented with 20% heat-inactivated FCS, 100 ng/mL recombinant murine (rm) SCF, 50 ng/mL rm IL-6, and 10 ng/mL rm IL-3 (StemCell Technologies, Vancouver, BC, Canada) for 48 hours. Human leukemia cell lines or mouse bone marrow cells were infected with retrovirus on 2 consecutive days by spinoculation in the presence of 4 μg/mL polybrene (Sigma, St Louis, MO). Three days after the second spinoculation, the cells were analyzed by fluorescence-activated cell sorting (FACS) analysis for transduction efficiency (GFP+).
Bone marrow cell sorting
Cell staining and enrichment procedures for sorting of multipotent bone marrow (BM) cells (long-term HSCs [LT-HSCs], short-term HSCs [ST-HSCs], and multipotent progenitors [MPPs]) and myeloid progenitors (CMP/GMP) were performed as previously described and as outlined in Figure 4Ai-iv.27 Briefly, cells were finally resuspended in HBSS with 2% heat-inactivated FCS and 1 μg/mL PI and sorted on a FACSVantage (Becton Dickinson, San Jose, CA). Dead cells were gated out by high–propidium iodide staining and forward light scatter.
Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
GFP+ sorted cells from the different MIG-, MIG-HOXA9–, or MIG-HOXA9NS–transduced cells were resuspended in Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was isolated according to the manufacturer's instructions. Total RNA from equivalent numbers of double FACS–sorted mouse bone marrow stem and progenitor populations (1000-8000 cells) was isolated using Trizol reagent. Equal amounts of total RNA were reverse transcribed to cDNA using the Superscript II polymerase (Invitrogen). All PCR reactions were performed in an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) using SYBR green (Applied Biosystems) as described in detail in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). β-actin mRNA was used for normalization. Statistical analyses were performed using Student t test for 2 samples.
Western blot analysis
Cells were lysed in 50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, aprotinin at 10 μg/mL, leupeptin at 10 μg/mL, and pepstatin at 10 μg/mL. Lysates were clarified by centrifugation at 20 800g for 20 minutes at 4°C. Supernatants were collected and Western-blotting assay was performed as described previously.28 Proteins were detected with antibodies against HOXA9 (Upstate, Lake Placid, NY), BAD, and Ser112-BAD (Cell Signaling Technology, Charlottesville, VA) and α-tubulin and β-actin from Santa Cruz Biotechnology (Santa Cruz, CA).
ChIP
Chromatin immunoprecipitation (ChIP) analysis was performed using the ChIP-IT Enzymatic kit, according to the manufacturer's instructions (Active Motif, Carlsbad, CA). Briefly, 5 × 107 HOXA9-immortalized marrow cells were harvested and cross-linked in 1% formaldehyde for 10 minutes at 37°C. Chromatin samples (average length 500 to 1000 nucleotides [nt]) were prepared and precleared, first, using protein G beads only, and second, using normal rabbit IgG with protein G beads. Precleared chromatin samples were incubated with rabbit anti-HOXA9 antibody (Upstate) or normal rabbit IgG as a negative control. PCR was performed using a primer pair for the distal region of the mouse Pim1 promoter/enhancer region (forward, 5′-CGGTTGGATAAAGGGTGGTT-3′ [−2505 to −2486]; reverse, 5′-GATGCTGCTCGGTTGGATGG-3′ [−2174 to −2155]) and 2 primers pairs for 2 proximal regions of the same promoter (see Document S1).
Propidium iodide cell-cycle and apoptosis analysis
MIG- or MIG-Pim1–transduced mouse marrow cells (1 × 106; each with > 85% GFP+ cells) were used for cell-cycle and apoptosis analysis. Briefly, cells were fixed in cold 70% ethanol on ice for 1 hour and then incubated in freshly made staining solution (50 μg/mL propidium iodide, 1 mg/mL RnaseA, and 0.1% glucose in PBS) at room temperature for 30 minutes. FACS analysis was performed with a Becton Dickinson FACScan using Cellquest software (Becton Dickinson).
Assays for myeloid progenitors and proliferation assays
Colony-forming assays were performed in semisolid medium, as previously described,29 using M3434 media (StemCell Technologies). Colonies were enumerated 7 days after plating. Cell proliferation assays were performed in duplicate by plating cells in 12-well plates at an initial density of 1 × 105/mL in growth media (DME-H21 supplemented with 10% fetal calf serum and 10 ng/mL each of the following murine cytokines: IL-1, IL-3, SCF, IL-11, thrombopoietin, Flt-3, and IL-7).
Results
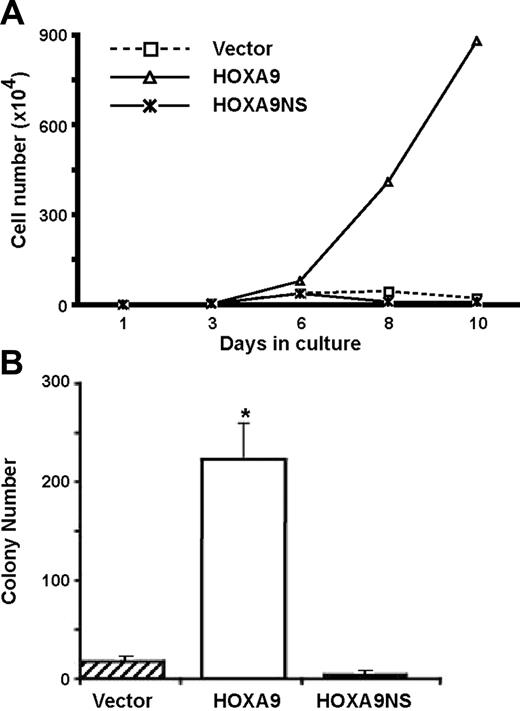
We have previously reported that lineage-negative (Lin−) c-kit+ marrow cells from Hoxa9-deficient mice show severely impaired proliferation and delayed emergence of committed myeloid progenitors in vitro and that both of these defects are completely rescued by the reintroduction of HOXA9.5 Our 2 published gene-expression surveys (Dorsam et al23 and Ferrell et al30 ) indicated that HOXA9 rapidly modulated the mRNA levels of a large number of genes, both in hematopoietic cell lines and in primary marrow cells, suggesting that HOXA9's molecular function is primarily at the level of transcription. However, HOXA9 has also been implicated in the posttranscriptional regulation of gene expression via its interactions with the eIF4E pathway.31 To clarify whether the transcriptional activity of HOXA9 was required for its effects of hematopoietic cell proliferation and self-renewal, we transduced Hoxa9−/− cells with retroviral vectors expressing either GFP alone or GFP together with either wild-type HOXA9 or a DNA-binding mutant of HOXA9 containing an Asn51Ser mutation (HOXA9NS).25 When freshly transduced cells were grown in liquid suspension cultures, the HOXA9-transduced cells showed the expected dramatic increase in cell proliferation, whereas cells transduced with the DNA-binding mutant showed no increase in cell growth over that of control cells transduced with GFP alone (Figure 1A). Similarly when aliquots of transduced cells were plated in clonogenic assays for committed progenitors after 8 days in culture, Hoxa9-transduced cells showed a marked increase in myeloid colonies, whereas cells transduced with the DNA-binding mutant showed no increase over control (Figure 1B). These results indicate that the hematopoietic effects of HOXA9 require its DNA-binding activity.
Overexpression of HOXA9 enhances cell proliferation and emergence of myeloid progenitors in a DNA-binding–dependent manner. (A) Bone marrow cells isolated from 5-FU–treated Hoxa9−/− mice were transduced with retroviral vectors expressing GFP alone (vector), or GFP together with either HOXA9 or the HOXA9NS DNA-binding mutant, and then placed in liquid suspension cultures. Viable cells were counted every 2 to 3 days for up to 10 days. The proliferation rate of cells transduced with wild-type HOXA9 was dramatically enhanced compared with cells transduced with the DNA-binding mutant or GFP alone. (B) After 8 days in culture, aliquots of the transduced cells described in panel A were plated in clonogenic assays to measure the number of committed myeloid progenitors. Wild-type HOXA9 effected a marked increase in colony numbers compared with the vector control and with the DNA-binding mutant (*P < .01). These experiments were repeated 3 times. Error bars indicate standard error.
Overexpression of HOXA9 enhances cell proliferation and emergence of myeloid progenitors in a DNA-binding–dependent manner. (A) Bone marrow cells isolated from 5-FU–treated Hoxa9−/− mice were transduced with retroviral vectors expressing GFP alone (vector), or GFP together with either HOXA9 or the HOXA9NS DNA-binding mutant, and then placed in liquid suspension cultures. Viable cells were counted every 2 to 3 days for up to 10 days. The proliferation rate of cells transduced with wild-type HOXA9 was dramatically enhanced compared with cells transduced with the DNA-binding mutant or GFP alone. (B) After 8 days in culture, aliquots of the transduced cells described in panel A were plated in clonogenic assays to measure the number of committed myeloid progenitors. Wild-type HOXA9 effected a marked increase in colony numbers compared with the vector control and with the DNA-binding mutant (*P < .01). These experiments were repeated 3 times. Error bars indicate standard error.
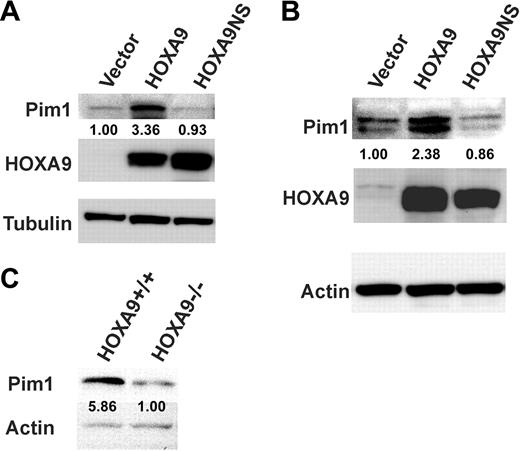
A previous gene-expression profiling survey suggested that the Pim1 oncogene might be a downstream target of HOXA9.23 Since Pim1 encodes a serine-threonine kinase that plays an important role in cell survival and proliferation24 and is expressed in the human HSC-enriched CD34+ cell compartment,32 we decided to determine whether the Pim1 gene could be a direct target and a key mediator of HOXA9 effects in primitive hematopoietic cells. Western-blot analysis of proteins from myeloid cell lines and Hoxa9−/− marrow cells transduced with a Hoxa9 retroviral vector confirmed that wild-type HOXA9 protein increased Pim1 protein over 3-fold in U937 cells (Figure 2A) and nearly 2.5-fold in Hoxa9−/− marrow cells (Figure 2B), whereas the HOXA9 DNA-binding mutant failed to induce Pim1 protein although the levels of mutant and wild-type HOXA9 protein achieved by both viruses were comparable. The changes observed in Pim1 protein correlated well with changes in the levels of Pim1 mRNA from the transduced cells (Figure 3A-B). Consistent with these data, the level of Pim1 protein found in HSC-enriched lin− cells from Hoxa9−/− fetal livers was reduced nearly 6-fold compared with the levels detected in wild-type fetal liver cells (Figure 2C). Together, these results suggest that HOXA9 is required to maintain normal levels of Pim1 in primitive hematopoietic cells.
HOXA9 expression increases Pim1 protein and RNA levels in hematopoietic cells. The human myelomonocytic cell line U937 (A) and bone marrow cells from 5-FU–treated Hoxa9−/− animals (B) were transduced with the same 3 vectors as described in Figure 1. Protein isolated from the cells 4 days after transfection was subjected to Western-blot analysis for Pim1 and HOXA9. While both wild-type and mutant HOXA9 vectors produced equivalent amounts of HOXA9 protein, only the wild-type protein induced Pim1 expression. (C) Pim1 protein levels are reduced in fetal liver cells from Hoxa9−/− mice. Protein isolated from Lin− cells from day-15.5 fetal livers from wild-type and Hoxa9−/− mice was subjected to Western-blot analysis for Pim1. Pim1 protein was reduced almost 6-fold in the Hoxa9−/− fetal liver cells. The number under the Pim1 lanes represents the relative amounts of protein compared with the control lane as measured by densitometric scanning of the Pim1 bands after normalization for loading, using either tubulin (A) or actin (B-C) as a loading control.
HOXA9 expression increases Pim1 protein and RNA levels in hematopoietic cells. The human myelomonocytic cell line U937 (A) and bone marrow cells from 5-FU–treated Hoxa9−/− animals (B) were transduced with the same 3 vectors as described in Figure 1. Protein isolated from the cells 4 days after transfection was subjected to Western-blot analysis for Pim1 and HOXA9. While both wild-type and mutant HOXA9 vectors produced equivalent amounts of HOXA9 protein, only the wild-type protein induced Pim1 expression. (C) Pim1 protein levels are reduced in fetal liver cells from Hoxa9−/− mice. Protein isolated from Lin− cells from day-15.5 fetal livers from wild-type and Hoxa9−/− mice was subjected to Western-blot analysis for Pim1. Pim1 protein was reduced almost 6-fold in the Hoxa9−/− fetal liver cells. The number under the Pim1 lanes represents the relative amounts of protein compared with the control lane as measured by densitometric scanning of the Pim1 bands after normalization for loading, using either tubulin (A) or actin (B-C) as a loading control.
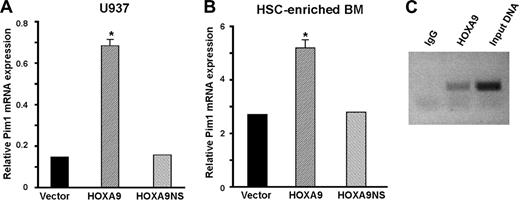
HOXA9 induces Pim1 mRNA expression and binds to the Pim1 promoter region in primary hematopoietic cells. RNA isolated from U937 cells (A) and HSC-enriched Hoxa9−/− marrow cells (B) transduced as described in Figure 1 was subjected to quantitative real-time RT-PCR analysis. Wild-type HOXA9, but not mutant HOXA9, significantly increased Pim1 mRNA levels (*P < .01). The experiments depicted in panels A and B were repeated 3 times. (C) Chromatin immunoprecipitation assays were performed on chromatin isolated from HOXA9-immortalized primary murine cell lines using either a HOXA9 antibody or IgG as a negative control. Using PCR primers to a distal segment of the Pim1 promoter, significant binding of HOXA9 to this region was demonstrated. Primers to 2 more proximal regions of the Pim1 promoter failed to show binding by HOXA9 (data not shown).
HOXA9 induces Pim1 mRNA expression and binds to the Pim1 promoter region in primary hematopoietic cells. RNA isolated from U937 cells (A) and HSC-enriched Hoxa9−/− marrow cells (B) transduced as described in Figure 1 was subjected to quantitative real-time RT-PCR analysis. Wild-type HOXA9, but not mutant HOXA9, significantly increased Pim1 mRNA levels (*P < .01). The experiments depicted in panels A and B were repeated 3 times. (C) Chromatin immunoprecipitation assays were performed on chromatin isolated from HOXA9-immortalized primary murine cell lines using either a HOXA9 antibody or IgG as a negative control. Using PCR primers to a distal segment of the Pim1 promoter, significant binding of HOXA9 to this region was demonstrated. Primers to 2 more proximal regions of the Pim1 promoter failed to show binding by HOXA9 (data not shown).
Chromatin immunoprecipitation (ChIP) assays, using HOXA9-transduced murine hematopoietic cells, were performed to investigate whether the Pim1 gene is a direct target of HOXA9. An antibody to HOXA9 brought down a 2.5-kb fragment upstream of the Pim1 transcriptional start site (Figure 3C), but no enrichment was detected for 2 other fragments from the proximal 1.7-kb 5′ promoter/enhancer region (not shown). Sequence scanning of the murine Pim1 promoter region disclosed a perfect consensus binding site (TGATTTAT)33 for HOXA9 and its DNA-binding partner PBX located at −2464 to −2457 from the transcriptional start site of Pim1 and within the distal promoter fragment that was bound by HOXA9 in the ChIP assay. By comparison, scanning of the human Pim1 gene revealed 2 perfect HOXA9/PBX binding sites located approximately 3600-bp upstream of the transcriptional start site. In addition, both the murine and human Pim1 promoters displayed multiple potential binding sites (TTAT) for HOXA9 alone.
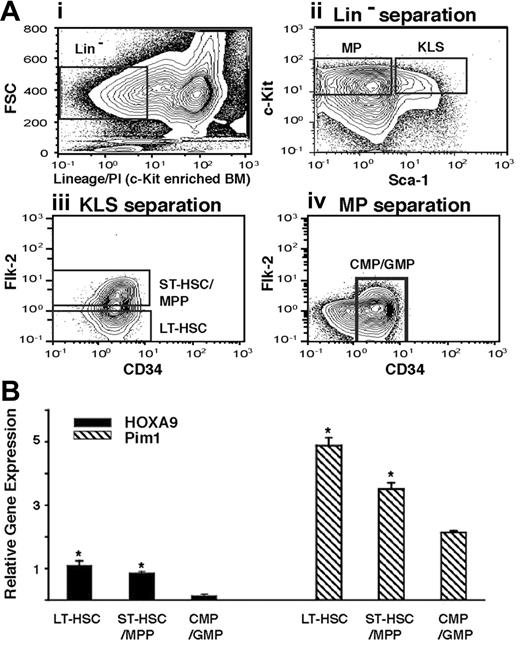
We reasoned that if the Pim1 gene is a true physiologic target of HOXA9, the expression levels of the 2 genes might track together in different primitive hematopoietic compartments. To compare the expression of Hoxa9 and Pim1 in early hematopoietic populations, we measured their mRNA levels in highly purified populations of murine LT-HSC, ST-HSC/MPP, and CMP/GMP using quantitative real-time RT-PCR (Figure 4Ai-iv). Pim1 was readily detected in all 3 early pools, with the highest expression in the LT-HSCs, in a pattern that mimicked the levels of Hoxa9 mRNA in the same populations (Figure 4B).
Expression levels of Hoxa9 and Pim1 track together in early hematopoietic compartments. (A) FACS sorting strategy. Normal c-kit–enriched murine marrow cells (i) were FACS sorted into myeloid progenitor (MP; Lin− c-kit+ Sca-1−) and KLS (Lin− c-kit+Sca-1+) fractions (ii). The KLS fraction was further separated into LT-HSC (flk-2−) and ST-HSC/MPP (flk-2+) fractions (iii), and the MP fraction was further enriched for the CD34+ CMP/GMP populations (iv). (B) mRNA levels of Hoxa9 and Pim1 in primitive hematopoietic compartments. RNA isolated from the 3 fractions described in panel 4Ai-iv was subjected to quantitative real-time PCR (Q RT-PCR) as described in “Assays for myeloid progenitors and proliferation assays,” under “Materials and methods,” and expression levels were normalized to GAPDH mRNA levels. Both Hoxa9 and Pim1 show their highest level of expression in the LT-HSC fraction, with progressively declining levels in more differentiated fractions (*P < .01 comparing LT-HSC or ST-HSC/MPP fractions vs CMP/GMP fractions).
Expression levels of Hoxa9 and Pim1 track together in early hematopoietic compartments. (A) FACS sorting strategy. Normal c-kit–enriched murine marrow cells (i) were FACS sorted into myeloid progenitor (MP; Lin− c-kit+ Sca-1−) and KLS (Lin− c-kit+Sca-1+) fractions (ii). The KLS fraction was further separated into LT-HSC (flk-2−) and ST-HSC/MPP (flk-2+) fractions (iii), and the MP fraction was further enriched for the CD34+ CMP/GMP populations (iv). (B) mRNA levels of Hoxa9 and Pim1 in primitive hematopoietic compartments. RNA isolated from the 3 fractions described in panel 4Ai-iv was subjected to quantitative real-time PCR (Q RT-PCR) as described in “Assays for myeloid progenitors and proliferation assays,” under “Materials and methods,” and expression levels were normalized to GAPDH mRNA levels. Both Hoxa9 and Pim1 show their highest level of expression in the LT-HSC fraction, with progressively declining levels in more differentiated fractions (*P < .01 comparing LT-HSC or ST-HSC/MPP fractions vs CMP/GMP fractions).
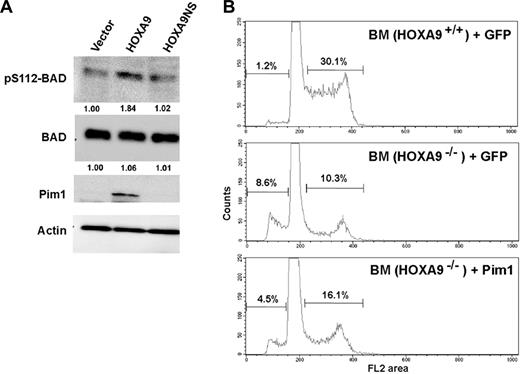
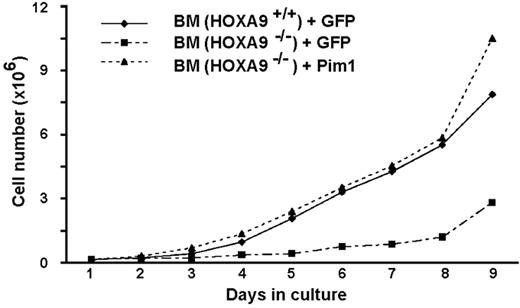
Since a known function of Pim kinases is to phosphorylate and inactivate the proapoptotic BAD protein,34,35 we assessed the phosphorylation status of BAD in myeloid cells transduced with wild-type or DNA-binding mutant Hoxa9. As shown in Figure 5A, HOXA9 expression increased Pim1 protein levels and BAD phosphorylation in a DNA-binding–dependent manner. To determine if Pim1 mediates the hematopoietic effects of Hoxa9, we performed “add-back” experiments with Pim1 to determine whether restoration of Pim1 activity improves the functional defects of Hoxa9-deficient marrow cells. In 2 separate experiments, we observed an increased fraction of apoptotic cells in Hoxa9−/− marrow compared with wild-type cells, as measured by the sub-G0 hypodiploid fraction, and found that transduction of Hoxa9−/− bone marrow with a Pim1 expression vector produced a consistent decrease in the percentage of apoptotic cells (Figure 5B). Hoxa9−/− cells also showed a reduced fraction of cycling cells (S-G2/M) and this reduction was partially reversed by the reintroduction of Pim1. Consistent with the cell-cycle analysis, Pim1 restored the in vitro proliferation of Hoxa9−/− cells to a normal rate (Figure 6). We speculate that the normalization of proliferation rate despite only partial correction of the defects in apoptosis and cell cycling reflects the potent stimulus provided by the cocktail of 7 cytokines included in the proliferation assay (see “Assays for myeloid progenitors and proliferation assays,” under “Materials and methods”).
HOXA9 induces Pim1-associated phosphorylation of the proapoptotic BAD protein, and Pim1 partially rescues the apoptotic defect in Hoxa9−/− primitive cells. (A) Protein isolated from U937 cells transduced with the 3 vectors described in Figure 1 was subjected to Western-blot analysis. As expected, Pim1 protein was induced by wild-type HOXA9 but not by the GFP or HOXA9NS vectors. BAD protein levels were similar in cells transduced with each of the 3 vectors (second row). However wild-type HOXA9 induced increased phosphorylation of BAD protein at serine 112 as shown by a pS112-specific antibody (top row). Actin levels were measured to ensure equal loading. (B) Wild-type and Hoxa9−/− marrow cells from 5-FU–treated animals were transduced with vectors expressing either GFP alone or GFP together with Pim1. FACS analysis of cell cycle revealed that Hoxa9−/− cells transduced with the MIG control vector showed significantly increased percentages of apoptotic sub-G0 cells compared with wild-type control cells (8.6% vs 1.2%). This apoptotic defect was reduced approximately 2-fold by the introduction of the Pim1 expression vector. Hoxa9−/− cells also showed a significantly reduced fraction of cells in the S and G2/M phases of the cell cycle compared with wild-type cells (10.3% vs 30.1%) and this fraction was increased (16.1% vs 10.3%) by the expression of Pim1.
HOXA9 induces Pim1-associated phosphorylation of the proapoptotic BAD protein, and Pim1 partially rescues the apoptotic defect in Hoxa9−/− primitive cells. (A) Protein isolated from U937 cells transduced with the 3 vectors described in Figure 1 was subjected to Western-blot analysis. As expected, Pim1 protein was induced by wild-type HOXA9 but not by the GFP or HOXA9NS vectors. BAD protein levels were similar in cells transduced with each of the 3 vectors (second row). However wild-type HOXA9 induced increased phosphorylation of BAD protein at serine 112 as shown by a pS112-specific antibody (top row). Actin levels were measured to ensure equal loading. (B) Wild-type and Hoxa9−/− marrow cells from 5-FU–treated animals were transduced with vectors expressing either GFP alone or GFP together with Pim1. FACS analysis of cell cycle revealed that Hoxa9−/− cells transduced with the MIG control vector showed significantly increased percentages of apoptotic sub-G0 cells compared with wild-type control cells (8.6% vs 1.2%). This apoptotic defect was reduced approximately 2-fold by the introduction of the Pim1 expression vector. Hoxa9−/− cells also showed a significantly reduced fraction of cells in the S and G2/M phases of the cell cycle compared with wild-type cells (10.3% vs 30.1%) and this fraction was increased (16.1% vs 10.3%) by the expression of Pim1.
Reintroduction of Pim1 normalizes the in vitro proliferation of primitive Hoxa9−/− marrow cells. Marrow cells from 5-FU–treated animals were transduced with vectors expressing either GFP or GFP together with Pim1. Freshly transduced cells were placed in liquid cultures, and viable cell numbers were counted daily. Wild-type marrow cells transduced with a GFP expression vector were used as a normal control. This experiment was performed 2 times.
Reintroduction of Pim1 normalizes the in vitro proliferation of primitive Hoxa9−/− marrow cells. Marrow cells from 5-FU–treated animals were transduced with vectors expressing either GFP or GFP together with Pim1. Freshly transduced cells were placed in liquid cultures, and viable cell numbers were counted daily. Wild-type marrow cells transduced with a GFP expression vector were used as a normal control. This experiment was performed 2 times.
Discussion
Our data demonstrate that HOXA9 binds to a distal region of the Pim1 promoter in hematopoietic cells and that Pim1 is transcriptionally up-regulated by the overexpression of HOXA9, resulting in increased levels of Pim1 protein in hematopoietic progenitor cells. The known biologic effects of the Pim1 kinase make it an attractive candidate as a mediator of HOXA9's effects on hematopoiesis. The Pim1 gene was one of the first oncogenes identified as a common site of proviral integration in Moloney murine leukemia virus (Mu-LV)–induced T-cell lymphomas in mice.36 Mice bearing a Pim1 transgene under the control of an Eμ enhancer develop T-cell lymphomas with a low penetrance and a long latency,37 which can be accelerated by exposure to mutagenic agents.38 The Pim1 oncogene was subsequently shown to be 1 of a 3-member family (Pim1, Pim2, and Pim3) of genes encoding serine-threonine kinases with important effects on cell proliferation and survival.
Although the hematologic abnormalities reported for mice lacking either Pim1 by itself or all 3 Pim family members are fairly subtle, they bear striking similarities to our previously reported findings in Hoxa9−/− animals,3–5 most notably a blunted proliferative response and reduced myeloid colony formation in response to cytokines, abnormal IL-7–driven pre-B-cell growth, and impaired T-cell proliferation.39,40 In addition, mast cells derived from Pim1−/− marrow cells proliferate poorly in response to IL-3.41 These abnormalities appear to be largely due to the loss of Pim1 but were exacerbated to some degree by the loss of Pim2 and Pim3.40 Another unexplained abnormality in Pim-deficient mice is a reduced red-cell size,40,42 an anomaly that we also observe in Hoxa9−/− animals (see Document S1).
A notable biologic function of Pim1 in hematopoietic cells appears to be as a mediator of cytokine-induced cell growth. Pim1 expression is transcriptionally up-regulated by a variety of growth factors, including IL-3 and GM-CSF,43,44 through the JAK-STAT signaling pathway. STAT proteins are believed to bind the proximal Pim1 promoter through an IFNγ-activation site (GAS) located at −923 to −915.45 The mechanisms by which Pim1 enhances cell proliferation appear to involve acceleration of cell-cycle progression both at the G1/S and G2/M transitions by phosphorylating and activating the phosphatases Cdc25A and Cdc25C, respectively.46,47 It is noteworthy that our experiments show that Hoxa9−/− blood cells have a reduction in the fraction of cycling cells, a defect that is improved by the introduction of Pim1.
A large body of literature supports the notion that Pim kinases play an even greater role in cell survival and prevention of apoptosis (see White48 for review). Overexpression of wild-type Pim1 in hematopoietic cell lines significantly blocks apoptosis triggered by cytokine withdrawal and by exposure to cytotoxic agents such as doxorubicin,49–51 whereas introduction of kinase-deficient Pim mutants reduces cell survival.50 One well-established molecular pathway by which Pim proteins exert their antiapoptotic effects is by phosphorylating and inhibiting the proapoptotic BH3 protein BAD.34,35 Unphosphorylated BAD normally binds to and inactivates antiapoptotic Bcl proteins such as Bcl-XL and Bcl-2. Phosphorylation of BAD at Ser112 blocks the binding of BAD to Bcl proteins. Our data show that overexpression of HOXA9 does increase BAD phosphorylation, presumably by increasing Pim1 levels, and that reintroduction of Pim1 reduces the apoptotic rate of Hoxa9-deficient marrow cells.
Pim1 is expressed in a variety of hematopoietic organs including thymus, spleen, bone marrow, and fetal liver as well as a number of nonhematopoietic tissues such as prostate, brain, and epithelium.52,53 Although prior studies show that Pim1 is expressed in human CD34+ blood cells,32 its expression levels in different primitive hematopoietic compartments have not been previously reported. Our studies confirm its expression in the early pluripotent hematopoietic progenitors, with its highest expression in the LT-HSC compartment, in a pattern that mimics Hoxa9. This pattern suggests that Pim1 could play important functions at the stem-cell level, a hypothesis that is yet to be rigorously assessed.
The data presented here indicate that the induction of Pim1 by HOXA9 requires an intact HOXA9 DNA-binding domain and therefore is likely to be at the transcriptional level. However, the control of Pim1 expression is complex and includes regulation of Pim1 mRNA45 and protein stability.54 A particularly interesting pathway for posttranscriptional control involves the translation initiation factor eIF4E (see Richter and Sonenberg55 for review). eIF4E enhances the nuclear-to-cytoplasmic export of certain mRNA species.56 It is also required for the regulation of cap-dependent translation by binding to the 7-methylguanosine residue that caps the 5′ end of eukaryotic mRNAs, a process that is antagonized by a number of inhibitory proteins that bind and sequester eIF4E.55 Pim1 protein expression has been shown to be regulated by its guanine-cytosine (GC)–rich 5′ untranslated region and is markedly increased by the overexpression of eIF4E.57 A recent study made the intriguing finding that HOXA9 is able to bind eIF4E via a binding site in the N-terminal region of the HOXA9 protein and enhances the nuclear export and translational efficiency of certain mRNAs.31 Mutation of a tyrosine in the eIF4E-binding site on HOXA9 ablates the interaction between the 2 proteins and prevents HOXA9 from enhancing nuclear export and translational efficiency. We have noted relatively small reductions in Pim1 protein in hematopoietic cells transduced with an eIF4E-binding mutant of HOXA9 compared with cells transduced with a wild-type HOXA9 vector (data not shown; Y.-L.H., C.L., H.J.L., manuscript in preparation), suggesting that while HOXA9 may effect Pim1 expression in part through the eIF4E pathway, its predominant effect, at least in primary hematopoietic cells, is at the level of Pim1 gene transcription.
It seems likely that Pim1 can be activated by other HOX proteins, since overexpression of several other individual Hox genes results in similar increases in hematopoietic cell proliferation and self-renewal12,29 and can lead to AML.29,58,59 Moreover, the similar consensus binding sites for different HOX proteins make it probable that several different homeoproteins could bind to the Pim1 promoter.33 Given the high-level expression of HOXA9 in normal hematopoietic stem cells,9,10 it seems likely that HOXA9 is the dominant HOX protein in terms of activating Pim1 during normal blood-cell development.
While the current data suggest that Pim1 is a key target gene for HOXA9, it seems certain that this homeoprotein influences other pathways as well. Our previous microarray studies showed that many genes were modulated by overexpression of HOXA9,23,30 and in the current study the correction of the hematopoietic abnormalities in Hoxa9−/− marrow cells by the introduction of Pim1 was incomplete. While no other well-validated direct gene targets for HOXA9 have been reported for blood cells, a few direct targets have been suggested from studies of nonhematopoietic tissues. Rossig et al21 showed direct binding of HOXA9 protein to the promoter regions of the endothelial nitric oxide synthase (eNOS) and VEGF-R2 genes in endothelial cells. Whether HOXA9 binds to and modulates these 2 genes in hematopoietic cells is currently unknown, but our previous studies suggest that HOXA9's target genes and its transcriptional effects on those genes are highly cell-context dependent,23 perhaps reflecting differences in heterochromatin patterns or in the availability of DNA-binding cofactors, such as MEIS and PBX proteins, in different tissues. A few previous studies have identified targets of other HOX proteins in hematopoietic cells. The HOXA10 homeodomain protein binds to cis elements of 2 genes encoding respiratory burst oxidase proteins that are expressed in differentiating myeloid cells.60,61
Recent data have implicated an increasingly important role for Pim kinases in AML. Pim1 is highly expressed in cells expressing the internal tandem duplication of the Flt3 gene, a common mutation in human AML.62 Treatment of these cells with a specific FLT3 inhibitor markedly down-regulates Pim1 and induces cell death, whereas enforced expression of Pim1 renders the cells resistant to the cytotoxicity of the inhibitor. Since FLT3 appears to activate Pim1 through the STAT5 pathway, we speculate that HOXA9 overexpression may be an alternate pathway to up-regulate Pim1 in AML cells. Pim kinases also appear to be critical in conferring resistance to the selective kinase inhibitor rapamycin, which has been undergoing clinical testing in leukemias. Rapamycin appears to work by inhibiting the function of mammalian target of rapamycin (mTOR) and thereby blocking the Akt pathway.63,64 mTOR in turn plays a role in resisting cytotoxic stress from antileukemic agents such as cytosine arabinoside and etoposide.65,66 Given the frequent overexpression of HOXA9 in human AML14 and growing evidence that Pim1 activation enhances cell resistance to antileukemic therapy, the development of specific Pim1 kinase inhibitors could be a valuable tool in the clinical treatment of HOXA9-associated leukemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by grants from the Research Service of the Department of Veterans Affairs (VA; H.J.L., C.L.) and the National Institutes of Health (DK48642 and CA91245, H.J.L.; CA08009, C.L.). H.J.L. is a VA Career Development Award Recipient and C.L. is a VA Career Scientist.
We wish to thank Dr Nancy Magnuson of Washington State University for many helpful discussions on the transcriptional regulation of Pim1.
Authorship
Contribution: Y.-L.H. did the bulk of the experimental work, including the Western blot analyses, QRT-PCR, ChIP assays, phosphorylation studies, and retroviral transfections; E.P. performed the FACS sorting studies shown in Figure 2 and assisted in the writing of the paper; S.F. assisted with the cell-culture work, including the proliferation and colony assays and FACS analysis for apoptosis; C.L. provided expertise on the design of the molecular biologic aspects of the project, prepared the final figures for publication, and assisted in the writing of the manuscript; and H.J.L. oversaw the entire project, designed the overall experimental strategy, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Jeffrey Lawrence, Hematology Research (151H), UCSF VA Medical Center, 4150 Clement St, San Francisco, CA 94121; e-mail: jeffl@medicine.ucsf.edu.