Abstract
The treatment of children affected by severe congenital neutropenia (SCN) with G-CSF strongly reduces the risk of sepsis by reversing neutropenia. However, SCN patients who respond to the treatment with the growth factor still have an elevated risk of succumbing to sepsis. Because the disease is usually caused by heterozygous mutations of ELA2, a gene encoding for neutrophil elastase (NE), we have investigated in G-CSF–responder and nonresponder patients affected by SCN the expression of polypeptides that constitute the antimicrobial machinery of these cells. In peripheral blood–derived neutrophils of patients with heterozygous mutations of ELA2 who were treated with G-CSF, NE was nearly absent as detected by immunofluorescence and immunoblotting, suggesting that production of the mutant protein interferes with normal gene expression. This defect was associated with abnormal expression of other granule-associated proteins such as myeloperoxidase, lactoferrin, cathepsin G, and human-neutrophil-peptide. Moreover, in one patient with partial response to G-CSF, we observed an impairment of neutrophil antimicrobial activity against Candida albicans, and, to a lower extent against Escherichia coli. Thereby, we propose that the treatment with G-CSF is not sufficient to correct all of the functional deficiency of neutrophils, and this might account for the consistent risk of infections observed in SCN patients.
Introduction
Neutrophils are essential components of the innate immune system because they constitute the first line of defense against bacterial and fungal pathogens. An efficient response against these microorganisms requires that neutrophils carry a fully operational machinery, including proteases, antimicrobial peptides, and reactive oxygen.1,2 In contrast, a reduction of neutrophil blood counts or a defect in their antimicrobial apparatus exposes the host to threats from many pathogens as observed in chronic granulomatous disease and in other functional defects of phagocytes.3–6
Severe congenital neutropenia (SCN) is an uncommon hematologic disorder characterized by reduction of absolute neutrophil counts (ANCs; usually < 0.2 × 109 cells/L), due to maturation arrest of neutrophil precursors in the bone marrow at the promyelocyte stage. If left untreated, the large majority of children affected by SCN dies in the first years of life from invasive infections.7,8 However, the empiric use of the polypeptide granulocyte colony-stimulating factor (G-CSF) for the treatment of SCN has drastically changed the clinical outcome of this condition by increasing absolute neutrophil count values and reducing the episodes of infection in the vast majority of patients.7–10 Nonetheless, SCN patients who are receiving G-CSF are at high risk of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). In particular, a higher risk of MDS/AML is observed in SCN children who display a poor response to the treatment with G-CSF and/or receive large doses of the growth factor (above 8 μg/kg per day). In addition, even if the introduction of G-CSF in the treatment of SCN patients has dramatically improved survival and reduced morbidity from infections, infectious complications in treated patients are still observed at a rate of 0.9% per year.10,11
In the several years, the identification of the genetic basis of SCN has underlined the key role of neutrophil elastase (NE) in myelopoiesis and focused the attention of many researchers on the strict link between granule formation and neutrophil differentiation. Indeed, heterozygous mutations of the gene that encodes for neutrophil elastase (ELA2) constitute the molecular basis of this autosomal dominant inherited disease.12,13 Molecular screening of ELA2 in patients with congenital neutropenia has shown that patients with specific mutations (eg, G185R) are inclined to present a more severe clinical phenotype, requiring higher doses of G-CSF and, possibly, are at higher risk of developing MDS/AML.14,15 In this context, a primary question related to the use of this growth factor in this genetic disease is if G-CSF is capable of restoring normal neutrophil functions besides correcting neutropenia.
In order to address this question, we have investigated in children affected by SCN receiving G-CSF whether the growth factor is able to correct neutropenia and/or to reconstitute the entire antimicrobial machinery of fully-matured neutrophils. Because granule protein synthesis and packaging of primary granules, and thereafter of specific granules, go along with promyelocytic differentiation up to mature neutrophils,16–18 we analyzed expression of NE, myeloperoxidase (MPO), lactoferrin, cathepsin G, human neutrophil peptide (HNP), lysozyme, and NADPH oxidase components in cells isolated from SCN receiving G-CSF. We observed that ELA2 genotype strongly influenced the ability of G-CSF to correct neutropenia, but the expression and activity deficiency of many granule-associated proteins was evident despite the correction of neutropenia by treatment with the growth factor.
Patients, materials, and methods
Approval for these studies was obtained from the Spedali Civili of Brescia institutional review board. Informed consent was provided in accordance with the Declaration of Helsinki for the 5 subjects with SCN, 2 subjects with idiopathic neutropenia, and the healthy control subjects.
Patients and clinical details
Five SCN-affected children (P1, P2, P3, P4, P5) and 2 with idiopathic neutropenia (C3 and C4) were recruited from the hematologic unit of Ospedale dei Bambini in Brescia. All subjects were undergoing treatment with G-CSF (5-40 μg/kg) at the time the study was initiated. Diagnosis of all patients was made by experts on the basis of clinical and hematologic data, valuating conventional criteria of persistent severe neutropenia, severe bacterial and fungal infection, and bone marrow maturation arrest at the myelocyte-promyelocyte stage. Patients P1 and P2 have been subjected to bone marrow transplantation, while the other patients are currently receiving G-CSF treatment. However, patient P2 has recently lost the engraftment of donor's bone marrow and is currently receiving G-CSF. For each experiment performed, at least one healthy subject and/or an age-matched child were included for comparison. The control group was used upon informed consent and consisted of healthy adult donors, and 2 children with idiopathic neutropenia or age-matched children who were admitted to the hospital for minor head trauma.
Mutational analysis
DNA was extracted from peripheral blood leukocytes using standard techniques. All 5 exons of ELA2 and at least 15 bases of flanking regions were amplified by polymerase chain reaction (PCR; primers and amplification conditions available upon request). PCR products were purified and bidirectionally sequenced as previously described using Big Dye Terminator Chemistry (Applied Biosystems, Foster City, CA) on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). For each new mutation found, at least 50 healthy controls were screened using PCR amplification and direct sequencing.
Neutrophils
Peripheral neutrophil cells were purified by Ficoll-Hypaque separation density-gradient centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden) from 5 to 10 mL heparinized blood, followed by red cell lysis (NH4Cl 0.829%, EDTA 0.125 mM, NaH2CO3 0.1%) and washing with PBS. Cell recovery varied from 1.5 × 106 cells (patients P1 and P2) up to 4 × 106 cells (P3). Neutrophil purity was assessed on the basis of May-Grünwald Giemsa stain, and of G-CSF receptor/CD15 expression at flow cytometry analysis (neutrophils > 97%).
O−2 release was estimated by cytochrome c reduction. Briefly, neutrophils (2 × 105) were resuspended in HBSS/Ca2+/Mg2+ (pH 7.4) containing 80 μM ferricytochrome C type III (Sigma, St Louis, MO) and stimulated with 100 nM fMLP, 20 ng/mL PMA, or 200 μg/mL opsonized zymosan from S cerevisiae (Sigma) for 60 minutes. When required, cells were preincubated with 50 ng/mL G-CSF (Lenograstim; Chugai Pharmaceutical, Tokyo, Japan) or 50 ng/mL GM-CSF (Schering-Plough, Kenilworth, NJ) for 30 minutes before fMLP addition. Cells were placed in clear-bottomed 96-well plates, and cytochrome c reduction was evaluated at 550 nm.
Lactoferrin production by neutrophilic cells or lactoferrin plasma concentration was detected by sandwich enzyme-linked immunosorbent assay (ELISA) system, using BIOXYTECH Lactof-EIA assay provided by OXIS International (Foster City, CA), with a detection limit of 2 pg/mL. When indicated, neutrophils were stimulated with fMLP (10 nM), CXCL8 (10 ng/mL), or medium alone for 15 minutes. In the collected supernatants or plasma samples, lactoferrin concentration was measured by ELISA and expressed as average ± SD of 2 distinct experiments.
Immunocytochemistry and immunofluorescence
Peripheral blood neutrophils were purified by Ficoll separation medium gradient centrifugation, followed by red cell lysis and washing with PBS. Cells were counted and used for cytospin preparations, and then slides were air-dried and used for immunocytochemistry and immunofluorescence staining. Neutrophil elastase expression was analyzed using rabbit polyclonal antibody to human neutrophil elastase (1:300) from Calbiochem (San Diego, CA), revealed with FITC-conjugated antirabbit mAb (1:25; DAKO Cytomation, Glostrup, Denmark); nuclei were counterstained for 1 minute in 0.5 mg/mL 4,6-diamidino-2-phenylindole. Myeloperoxidase expression was analyzed using rabbit polyclonal antibody to human myeloperoxidase (1:3000; DAKO Cytomation) followed by streptavidin-biotin complex immunoperoxidase technique, with diaminobenzidine as chromogen; nuclei were counterstained with Mayer hematoxylin. For antigen retrieval, the slides were immersed in citrate buffer and microwaved for 5 minutes at 750 W. Cells were photographed using an Olympus BX60 fluorescence microscope and objectives with numerical apertures of 0.40 (10 ×), 0.70 (20 ×), 0.85 (40 ×), and 0.90 (60 ×), equipped with a DP-70 Olympus digital camera (Olympus, Melville, NY). Images were acquired using analySIS Image Processing software (Soft Imaging System, Münster, Germany). At least 5 fields of magnification, 20 × and 60 × for each sample, were randomly selected and analyzed.
Immunoblotting
Cells were suspended in HBSS (pH 7.4) containing 1 mM di-isopropylfluorophosphate (DFP; Sigma). After 5 minutes this solution was discarded, and the cells were lysed with electrophoresis sample buffer (60 mM Tris/HCl, 20% [vol/vol] glycerol, 4% [wt/vol] SDS, 2% [vol/vol] 2-mercaptoethanol, pH 6.8) and boiled for 5 minutes. Total cell lysates were subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) on 12% or 14% gels and then transferred to nitrocellulose membranes (Amersham). The blots were then rinsed in TBS-T (50 mM Tris, 170 mM NaCl, 0.2% [vol/vol] Tween 20; pH 7.5) and incubated for 60 minutes in TBS-T containing 5% BSA (pH 7.5) (blocking buffer), before overnight incubation (4°C) with the following rabbit antibodies: anti-gp91phox, anti-p47phox, anti-p67phox, anti-p40phox, anti-p22phox (kindly provided by Dr F. B. Wientjes, Department of Medicine, University College, London, United Kingdom); anti–human neutrophil elastase (Calbiochem); anti–human neutrophil myeloperoxidase (Assay Design, Ann Arbor, MI); anti–human lactoferrin (Sigma); anti–cathepsin G (BIODESIGN Int, Saco, ME) or anti–β-actin (Sigma); mouse anti–human lysozyme (BIODESIGN Int); and goat anti-HNP (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:500 in TBS-T containing 1 mg/mL BSA. The blots were incubated with horseradish peroxidase–conjugated anti–rabbit, anti–mouse IgG (Amersham), or anti–goat IgG (Santa Cruz Biotechnology). Bound antibodies were detected by enhanced chemiluminescence Western blotting detection reagents (Amersham).
MPO activity assay
The EnzChek Myeloperoxidase (MPO) Activity Assay Kit (E33856; Molecular Probes, Leiden, The Netherlands) was used for rapid and sensitive determination of MPO chlorination activity in neutrophil lysates. Fluorescence was measured with a fluorescence microplate reader using fluorescence excitation and emission at 485 and 530 nm, respectively. The background fluorescence measured for each zero-MPO control reaction was subtracted from each fluorescence measurement before plotting.
Phagocytosis assay
Neutrophils from patient 2 (P2) and from a healthy subject (CTR) were incubated for 40 minutes at 37°C with serum-opsonized zymosan (10 particles/cell) or serum-opsonized C albicans (4 particles/cell), washed twice with PBS, transferred onto glass slides, and stained with May-Grünwald Giemsa before examination under light microscopy.
Killing assay
Neutrophils were resuspended in HBSS/Ca2+/Mg2+ (pH 7.4) and prewarmed for 10 minutes at 37°C in a shaking water bath. Opsonized microorganisms were added at a ratio of 3 bacteria/neutrophil (E coli ATCC 25922) or 2 blastospores/neutrophil (C albicans ATCC 24433), with a final neutrophil concentration of 3 × 105 cells/100 μL. Microorganisms were also incubated in the absence of neutrophils to account for growth during the assay. The tubes were incubated at 37°C in a shaking water bath. After 90 minutes, samples were diluted in 1 mL pyrogen-free distilled water brought to pH 11.00 with NaOH just before use19 ; all the samples were then inverted twice. After standing for 5 minutes at room temperature and vortexing vigorously for 5 seconds, samples were diluted in PBS solution to give a bacterial or fungal concentration of 2 × 103/mL. Triplicates of 100-μL aliquots of each dilution were plated on LB or Sabouroud agar Petri dishes. The colony-forming units (CFUs) were counted after an overnight incubation at 37°C (E coli) or at 30°C (C albicans). The percent killing was calculated as follows: (1 − [CFU after incubation with cells/CFU control culture]) × 100.
Statistical analysis
Comparison of values between healthy donors and patients was performed where indicated by ANOVA for unpaired data. For single comparison among groups, Bonferroni correction was applied. Differences were defined as significant for P values less than .05.
Results
We have recently identified 5 SCN patients (ANC < 0.1 × 109cells/L) with various heterozygous mutations of ELA2 (Table 1). In order to identify the biologic correlates of the response to G-CSF in SCN patients, we evaluated the expression of neutrophil elastase and of other granule-associated protein in children with mutations of ELA2 during the treatment with the G-CSF (Table 1). We found that patients P3 (P176fsX182) and P4 (N34-H41del) showed a satisfactory increase of neutrophil counts (3.6 and 1.9 × 109 cells/L), while the remaining 3 either presented a blunted response to the treatment (P2, G185R; P5 A98D [0.8 × 109 cells/L]) or were not responsive (P1, G185R) even at high doses of G-CSF (up to 40 μg/kg).
Absolute neutrophil counts in SCN children receiving G-CSF
| Patient ID . | Age at diagnosis . | ELA2 . | NE amino acid . | G-CSF, μg/kg . | ANC, × 109 cells/L . | |
|---|---|---|---|---|---|---|
| At diagnosis . | After therapy . | |||||
| P1 | 2 mo | 4924G>A | G185R | 40 | 0.03 | 0.03 |
| P2 | 10 d | 4924G>A | G185R | 20 | 0.1 | 0.84 |
| P3 | 8 d | 4899del | P176fs × 182 | 5 | <0.01 | 3.6 |
| P4 | 4 mo | 1903-1926del | N34-H41del | 5 | 0.07 | 1.9 |
| P5 | 3 mo | 4498C>A | A98D | 5 | <0.01 | 0.8 |
| Patient ID . | Age at diagnosis . | ELA2 . | NE amino acid . | G-CSF, μg/kg . | ANC, × 109 cells/L . | |
|---|---|---|---|---|---|---|
| At diagnosis . | After therapy . | |||||
| P1 | 2 mo | 4924G>A | G185R | 40 | 0.03 | 0.03 |
| P2 | 10 d | 4924G>A | G185R | 20 | 0.1 | 0.84 |
| P3 | 8 d | 4899del | P176fs × 182 | 5 | <0.01 | 3.6 |
| P4 | 4 mo | 1903-1926del | N34-H41del | 5 | 0.07 | 1.9 |
| P5 | 3 mo | 4498C>A | A98D | 5 | <0.01 | 0.8 |
Nucleotide position is given according to sequence GenBank Y00477. Amino acid numbers begin from the first residue after the presignal peptide cleavage.
del indicates deletion; fs, frameshift.
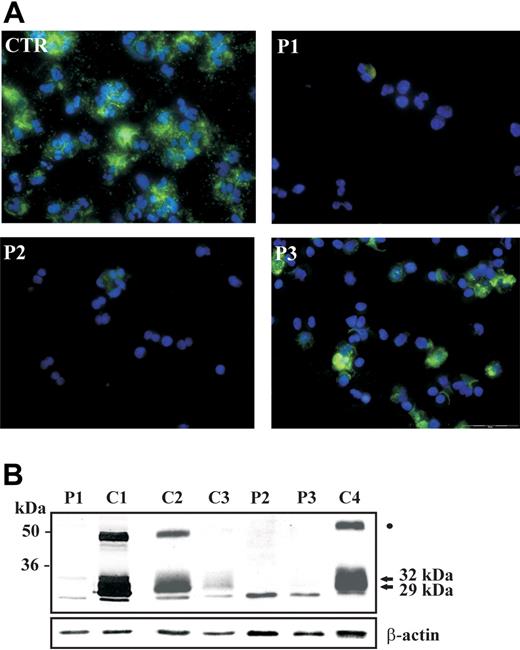
Then we asked what the effect was of G-CSF on the expression of neutrophil elastase and myeloperoxidase, which are major components of primary granules and are synthesized at the promyelocytic stage of maturation.20 To this aim, we separated neutrophils from patients P1, P2, and P3 while they were receiving G-CSF and analyzed the expression of neutrophil elastase and of other granule-associated proteins. Immunofluorescence staining with anti–neutrophil elastase rabbit polyclonal antibody showed that expression of the protein was detectable only in a minority of cells derived from SCN patients, while it was abundantly present in cytoplasm of neutrophils from control subjects (Figure 1A). Neutrophil elastase is a monomeric polypeptide that is synthesized in endoplasmic reticulum as 32 kDa, and subjected to glycosylation in Golgi apparatus. Subsequent processing with removal of amino-terminal and carboxy-terminal peptides takes place during its transport to primary granules where it is stored as mature polypeptide of about 27 to 29 kDa.20,21 Analysis of neutrophil elastase expression by immunoblotting in SCN patients showed that the extent of protein expression was severely reduced in neutrophils of G-CSF–treated SCN patients, compared with neutrophils of control subjects, in spite of G-CSF treatment (Figure 1B). Moreover, the relative amount of the different proteolytic polypeptides, of sizes ranging from the unprocessed 32-kDa form to the 29-kDa matured polypeptide, was dramatically altered, suggesting that normal neutrophil elastase processing is not restored in SCN patients during G-CSF treatment. Of note, analysis of NE expression in 2 patients with idiopathic neutropenia (C3 and C4), but normal ELA2, showed normal processing of the protein (Figure 1B). An analysis of NE expression in the other 5 patients with severe neutropenia due to other causes in comparison to an additional SCN patient (P4) showed that the abnormal protein processing was not related to the treatment with G-CSF, but was strictly associated with ELA2 mutations (data not shown). Next, we evaluated ELA2 expression at the mRNA level in hematopoietic cells isolated from bone marrow of a SCN patient (P3). Analysis of ELA2 mRNA by real-time PCR showed that the extent of expression in the cells derived from the patient was comparable to the control subject (data not shown). This is in accord with previous observations showing that ELA2 mutations lead to abnormal processing and intracellular targeting of the protein.15,20
Abnormal expression of neutrophil elastase in neutrophils of SCN patients during treatment with G-CSF. (A) Immunofluorescence staining of NE in circulating neutrophils from SCN patients. Polymorphonuclear lymphocytes (PMNs) were obtained from peripheral blood, cytocentrifugated, and stained with anti-NE antibody; nuclei were counterstained with DAPI. The majority of cells from a control subject (CTR) show a strong cytoplasmatic expression of NE, whereas NE is detectable in a minority of cells obtained from patients 1, 2, and 3. Experiment shown is representative of 2 performed. (B) Cell lysates of neutrophils purified from 3 patients (P1, P2, and P3), 2 healthy control subjects (C1, C2), and 2 idiopathic neutropenia patients (C3 and C4) were subjected to Western blot analysis with anti–neutrophil elastase antibody. β-Actin was used to normalize protein levels. The 29- and 32-kDa polypeptides are indicated by arrows. The band indicated by a dot could correspond to the previously identified elastase-alpha1 antitrypsin complex.21
Abnormal expression of neutrophil elastase in neutrophils of SCN patients during treatment with G-CSF. (A) Immunofluorescence staining of NE in circulating neutrophils from SCN patients. Polymorphonuclear lymphocytes (PMNs) were obtained from peripheral blood, cytocentrifugated, and stained with anti-NE antibody; nuclei were counterstained with DAPI. The majority of cells from a control subject (CTR) show a strong cytoplasmatic expression of NE, whereas NE is detectable in a minority of cells obtained from patients 1, 2, and 3. Experiment shown is representative of 2 performed. (B) Cell lysates of neutrophils purified from 3 patients (P1, P2, and P3), 2 healthy control subjects (C1, C2), and 2 idiopathic neutropenia patients (C3 and C4) were subjected to Western blot analysis with anti–neutrophil elastase antibody. β-Actin was used to normalize protein levels. The 29- and 32-kDa polypeptides are indicated by arrows. The band indicated by a dot could correspond to the previously identified elastase-alpha1 antitrypsin complex.21
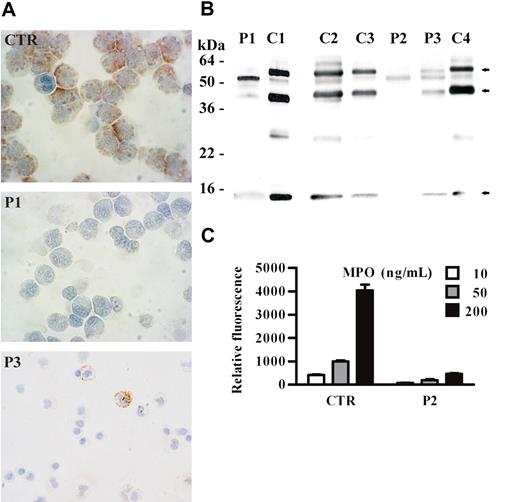
Next, we investigated whether MPO, the other major constituent of primary granules, is expressed and normally processed as tetrameric glycoprotein, consisting of 2 heavy (60 kDa) and 2 light (15 kDa) chains, in neutrophils of SCN patients receiving G-CSF.22 Immunocytochemical analysis of neutrophils obtained from SCN patients showed that MPO is absent or poorly expressed in cells, even while patients were receiving G-CSF therapy (Figure 2A). In addition, Western blot analysis showed that the electrophoretic mobility of the light chain was similar both in healthy and in neutropenic patients, while the heavy chain had an abnormal size, suggesting an altered cleavage and/or glycosylation of MPO subunits (Figure 2B). Similarly to the 60-kDa heavy chain, a band of 40 kDa, most likely representing a degradation product of the heavy chain,23 showed an altered electrophoretic mobility (Figure 2B). We then investigated whether abnormal MPO expressed by SCN patients was functional. To this end, we analyzed the MPO-dependent chlorination activity in lysates of neutrophils isolated from patient 2, and we found that this activity was greatly reduced (Figure 2C).
Abnormal expression of myeloperoxidase in neutrophils of SCN patients during treatment with G-CSF. (A) Immunocytochemical staining of MPO in circulating neutrophils from SCN patients. PMNs were obtained from peripheral blood, cytocentrifugated, and stained with anti-MPO antibody; nuclei were counterstained with Mayer hematoxylin. In cells from a control subject (CTR), a strong granular expression of MPO is observed in the cytoplasm. In contrast, MPO is detectable in a minority of cells obtained from patient 3 (P3) and undetectable in cells from patient 1 (P1). (B) The Western blot shown in Figure 1B has been reprobed with specific antimyeloperoxidase antibody. The 3 myeloperoxidase bands are indicated by arrows. (C) MPO chlorination activity in neutrophil cell lysates (3 dilutions of each sample) from a healthy subject (CTR) and patient 2 (P2) was analyzed by EnzChek Myeloperoxidase (MPO) Activity Assay Kit (“Patients, materials, and methods”). Results are expressed as relative fluorescence and are shown as mean values ± SD of 3 experiments. Statistical analysis demonstrates that the chlorination activity in neutrophil cell lysates is lower in SCN patients compared with healthy subjects (P < .05).
Abnormal expression of myeloperoxidase in neutrophils of SCN patients during treatment with G-CSF. (A) Immunocytochemical staining of MPO in circulating neutrophils from SCN patients. PMNs were obtained from peripheral blood, cytocentrifugated, and stained with anti-MPO antibody; nuclei were counterstained with Mayer hematoxylin. In cells from a control subject (CTR), a strong granular expression of MPO is observed in the cytoplasm. In contrast, MPO is detectable in a minority of cells obtained from patient 3 (P3) and undetectable in cells from patient 1 (P1). (B) The Western blot shown in Figure 1B has been reprobed with specific antimyeloperoxidase antibody. The 3 myeloperoxidase bands are indicated by arrows. (C) MPO chlorination activity in neutrophil cell lysates (3 dilutions of each sample) from a healthy subject (CTR) and patient 2 (P2) was analyzed by EnzChek Myeloperoxidase (MPO) Activity Assay Kit (“Patients, materials, and methods”). Results are expressed as relative fluorescence and are shown as mean values ± SD of 3 experiments. Statistical analysis demonstrates that the chlorination activity in neutrophil cell lysates is lower in SCN patients compared with healthy subjects (P < .05).
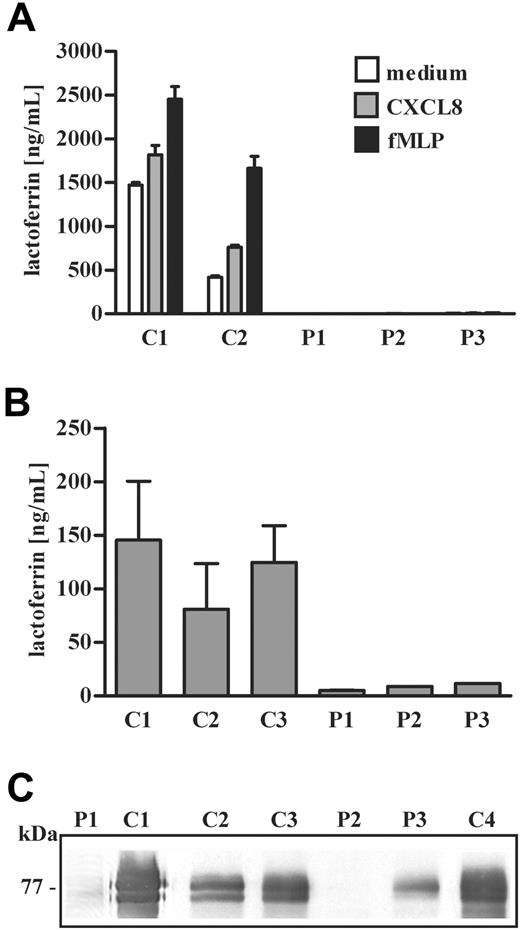
We then evaluated the presence of other antimicrobial proteins in neutrophils and blood serum of G-CSF–treated SCN patients. First we looked at lactoferrin, a constituent of specific granules, that is also detectable in large amounts in blood circulation.24 Specific granules are rapidly mobilized to cell surface in neutrophils activated by various stimuli, including chemoattractants such as the formyl-MLP peptide (fMLP) and CXCL8, thereby leading to lactoferrin secretion.25 Analysis of lactoferrin secretion in neutrophils from SCN patients showed that the antimicrobial protein was undetectable in supernatants obtained after stimulation with fMLP or IL-8, suggesting a reduced cellular content of the protein (Figure 3A). Indeed, blood lactoferrin concentration was severely reduced in all SCN patients (Figure 3B). In order to investigate intracellular expression of lactoferrin in neutrophils of SCN patients undergoing treatment with G-CSF, we performed an immunoblot analysis of the protein. We found that lactoferrin was undetectable in neutrophil lysates from SCN patients bearing the G185R mutation (P1 and P2), while it was expressed at reduced levels in neutrophils of patient 3 (P3), compared with levels detected in control subjects (Figure 3C).
Lactoferrin expression and release in neutrophils of SCN patients undergoing treatment with G-CSF. (A) Neutrophils isolated from patients 1, 2, and 3 (P1, P2, and P3) were stimulated with fMLP (10 nM), CXCL8 (10 ng/mL), or medium alone for 15 minutes. In the collected supernatants, lactoferrin concentration was determined by ELISA and expressed as average + SD of 2 distinct experiments. Statistical analysis demonstrates that the amount of lactoferrin released in supernatants derived from SCN patients is significantly lower than lactoferrin detected in supernatants from control subjects (P < .05). (B) Lactoferrin concentration in plasma of SCN patients (P1, P2, and P3) was determined in at least 3 samples. Average + SD of 3 distinct determinations is presented on y-axis. Statistical analysis demonstrates that the amount of plasma lactoferrin is lower in SCN patients compared with healthy subjects (P < .05). (C) The Western blot shown in Figures 1B and 2B has been reprobed with a specific anti–human lactoferrin antibody as described in “Patients, materials, and methods.”
Lactoferrin expression and release in neutrophils of SCN patients undergoing treatment with G-CSF. (A) Neutrophils isolated from patients 1, 2, and 3 (P1, P2, and P3) were stimulated with fMLP (10 nM), CXCL8 (10 ng/mL), or medium alone for 15 minutes. In the collected supernatants, lactoferrin concentration was determined by ELISA and expressed as average + SD of 2 distinct experiments. Statistical analysis demonstrates that the amount of lactoferrin released in supernatants derived from SCN patients is significantly lower than lactoferrin detected in supernatants from control subjects (P < .05). (B) Lactoferrin concentration in plasma of SCN patients (P1, P2, and P3) was determined in at least 3 samples. Average + SD of 3 distinct determinations is presented on y-axis. Statistical analysis demonstrates that the amount of plasma lactoferrin is lower in SCN patients compared with healthy subjects (P < .05). (C) The Western blot shown in Figures 1B and 2B has been reprobed with a specific anti–human lactoferrin antibody as described in “Patients, materials, and methods.”
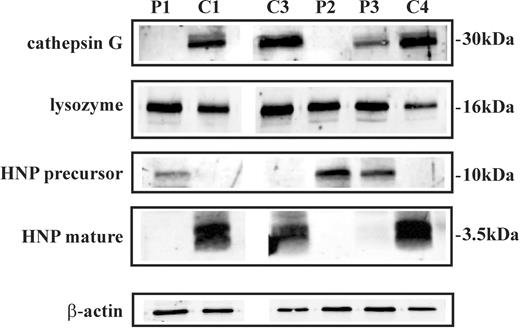
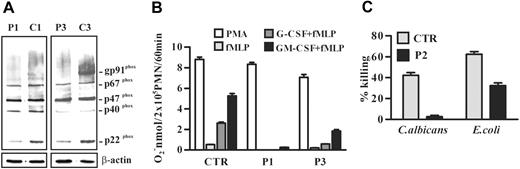
We then analyzed the expression of other defensive proteins contained in primary and/or secondary granules of neutrophils by Western blot analysis. We found that cathepsin G and mature HNP were undetectable, whereas HNP precursor was present in SCN neutrophil lysates (Figure 4). Moreover, lysozyme was normally expressed in neutrophils of the patients (Figure 4). Thereafter, we investigated the expression and function of NADPH oxidase, the multicomponent enzyme that plays a central role in host defense by catalyzing superoxide anion production. For its activity, the assembly of the cytosolic subunits p47phox, p67phox, p40 phox, and Rac with the membrane-associated heterodimer of gp91phox and p22phox is required.26,27 An immunoblot analysis showed that the NADPH oxidase cytosolic components p47phox, p67phox, and p40 phox were expressed at similar levels in control and SCN neutrophils, whereas the membrane-associated subunits gp91phox and p22phox were reduced in neutrophils of patients (Figure 5A). In order to determine whether this alteration of NADPH oxidase subunit expression could affect the enzymatic activity of the complex, we evaluated superoxide anion release in neutrophils from SCN patients undergoing treatment with G-CSF. We found that superoxide anion production was normal after SCN neutrophil challenge with an optimal concentration of the phorbol myristate acetate (PMA), which activates NADPH oxidase by direct interaction with protein kinase C, while it was significantly reduced in SCN neutrophils stimulated with fMLP, a receptor-dependent activator of the enzyme (Figure 5B). Flow cytometry analysis of CXCR1 and fMLP receptor expression showed that the 2 G-protein–coupled receptors were detectable in neutrophils of patient P3 as well as in cells of healthy subjects (data not shown), suggesting that the impairment of fMLP-mediated oxidative burst is probably related to a defective signaling machinery.
Expression of cathepsin G, lysozyme, and HNP in neutrophils of SCN patients during treatment with G-CSF. Cell lysates of neutrophils purified from patients 1, 2, and 3 (P1, P2, and P3), a healthy control subject (C1), and 2 idiopathic neutropenia patients (C3 and C4) were subjected to Western blot analysis with antibodies raised against neutrophil cathepsin G, lysozyme, HNP precursor, and HNP mature protein. β-Actin was used to normalize protein levels.
Expression of cathepsin G, lysozyme, and HNP in neutrophils of SCN patients during treatment with G-CSF. Cell lysates of neutrophils purified from patients 1, 2, and 3 (P1, P2, and P3), a healthy control subject (C1), and 2 idiopathic neutropenia patients (C3 and C4) were subjected to Western blot analysis with antibodies raised against neutrophil cathepsin G, lysozyme, HNP precursor, and HNP mature protein. β-Actin was used to normalize protein levels.
NADPH oxidase expression and activity in neutrophils from SCN patients. (A) Cell lysates of neutrophils purified from 2 patients (P1 and P3) and 2 healthy subjects (C1 and C3) were subjected to Western blot analysis with anti-gp91phox–, p47phox-, p67phox-, p40phox-, or p22phox-specific antibodies. The gp91phox appears as a broad smear because it is highly glycosylated. β-Actin was used to compare protein levels. (B) Neutrophils (2 × 105) from patients 1 (P1) and 3 (P3) and from a healthy subject (CTR) were stimulated with 100 nM fMLP or 20 ng/mL PMA for 60 minutes. When required, cells were preincubated with 50 ng/mL G-CSF or GM-CSF for 30 minutes before fMLP addition. O2− release was measured by cytochrome c reduction. Results shown are expressed as the mean value + SD of 3 independent experiments. (C) Neutrophils from patient 2 (P2) and a healthy subject (CTR) were incubated with opsonized C albicans blastospores or E coli for 90 minutes. Neutrophils were then lysed, diluted, and plated on Sabouroud or LB agar Petri dishes. The CFUs were counted after an overnight incubation, and the percentage of killing was evaluated as in “Patients, materials, and methods.” Results shown are expressed as the mean value ± SD of 2 experiments. Statistical analysis demonstrates that antimicrobial activities of neutrophils against C albicans and E coli are reduced in SCN patient compared with a healthy subject (P < .05).
NADPH oxidase expression and activity in neutrophils from SCN patients. (A) Cell lysates of neutrophils purified from 2 patients (P1 and P3) and 2 healthy subjects (C1 and C3) were subjected to Western blot analysis with anti-gp91phox–, p47phox-, p67phox-, p40phox-, or p22phox-specific antibodies. The gp91phox appears as a broad smear because it is highly glycosylated. β-Actin was used to compare protein levels. (B) Neutrophils (2 × 105) from patients 1 (P1) and 3 (P3) and from a healthy subject (CTR) were stimulated with 100 nM fMLP or 20 ng/mL PMA for 60 minutes. When required, cells were preincubated with 50 ng/mL G-CSF or GM-CSF for 30 minutes before fMLP addition. O2− release was measured by cytochrome c reduction. Results shown are expressed as the mean value + SD of 3 independent experiments. (C) Neutrophils from patient 2 (P2) and a healthy subject (CTR) were incubated with opsonized C albicans blastospores or E coli for 90 minutes. Neutrophils were then lysed, diluted, and plated on Sabouroud or LB agar Petri dishes. The CFUs were counted after an overnight incubation, and the percentage of killing was evaluated as in “Patients, materials, and methods.” Results shown are expressed as the mean value ± SD of 2 experiments. Statistical analysis demonstrates that antimicrobial activities of neutrophils against C albicans and E coli are reduced in SCN patient compared with a healthy subject (P < .05).
Phagocytosis experiments showed that SCN neutrophils normally internalized both opsonized Candida albicans and zymosan, and zymosan ingestion induced superoxide anion production (Table 2), suggesting that the mechanisms involved in pathogen uptake and in phagocytosis-dependent oxygen radical production are conserved in these cells.
Phagocytosis and O2− production in SCN
| . | CTR . | P2 . |
|---|---|---|
| Phagocytic index | ||
| ops-zymosan | 8.54 ± 1.89 | 11.87 ± 1.76 |
| ops-Candida | 4.06 ± 1.44 | 4.44 ± 1.59 |
| O2−, nmol/2 × 105 PMN/60 min | ||
| ops-zymosan | 3.56 ± 0.94 | 4.16 ± 0.82 |
| . | CTR . | P2 . |
|---|---|---|
| Phagocytic index | ||
| ops-zymosan | 8.54 ± 1.89 | 11.87 ± 1.76 |
| ops-Candida | 4.06 ± 1.44 | 4.44 ± 1.59 |
| O2−, nmol/2 × 105 PMN/60 min | ||
| ops-zymosan | 3.56 ± 0.94 | 4.16 ± 0.82 |
Phagocytosis was assayed in neutrophils from patient 2 (P2) and from a healthy subject (CTR) after 40-minute incubation with opsonized Candida yeasts or zymosan (“Patients, materials, and methods”). Phagocytic index is shown as mean value ± SD of ingested particles per 100 cells. In parallel, neutrophils (2 × 105) were stimulated with 200 μg/mL opsonized zymosan for 60 minutes, and O2− release was measured by cytochrome c reduction. Results are expressed as the mean value ± SD of 2 experiments.
We finally investigated whether the reduction and/or abnormality of defensive proteins observed in SCN neutrophils results in a decreased antimicrobial activity. To this aim, we analyzed the ability of neutrophils from one SNC patient to kill C albicans and E coli. We found that these cells were unable to inhibit Candida growth, whereas their capacity for bactericidal activity against E coli was significantly reduced (P < .05, Figure 5C), suggesting a defect of neutrophils from SCN patients to control infections from these microorganisms.
Discussion
Because of the ability of G-CSF to restore the neutrophil counts in many conditions characterized by neutropenia, this highly effective growth factor of myeloid cells has been empirically tested in a large series of disorders of myelopoiesis including SCN. Recent studies on the pathogenesis of this inherited disease have focused our attention on the effect of G-CSF on granule formation and expression of antimicrobial polypeptides in children affected by SCN who were treated with the growth factor. Analysis of NE in granulocytes from SCN patients undergoing treatment with G-CSF has shown that the protein was detectable at low amounts as assessed by immunofluorescence, suggesting that the expression of the mutant interferes with the production of the mature protein, even from the normal allele. Moreover, immunoblot analysis has revealed that NE was undetectable in neutrophils of SCN children, suggesting that NE processing and possibly intracellular trafficking were abnormal despite the treatment with G-CSF. Because the adaptor protein 3 complex mediates intracellular trafficking of neutrophil elastase from trans-Golgi network to primary granules,20 this finding is in accord with the observation that the absence of β3A subunit results in abnormal delivery of NE to plasma membrane and in neutropenia, as observed in Hermansky-Pudlak 2 patients and in gray collie dogs with AP-3 deficiency.20,28,29 Moreover, expression of the G185R mutation in retrovirally transduced HL-60 promyelocytes further demonstrated that the NE is mislocalized to plasma membrane.15 Mislocalization of NE at plasma membrane will probably account for the abnormal myelopoiesis because the enzyme might inappropriately interact with regulatory proteins of hematopoiesis including CXCR4, CXCL12, G-CSF, G-CSF receptors, and Notch proteins as potential substrates of this serine protease.30–34
We have shown by immunofluorescence staining that abnormal expression and cellular targeting of NE in neutrophils of SCN patients will affect the generation of primary granules. This is also supported by the concurrent severe reduction of MPO expression that was observed by immunohistochemistry analysis of SCN neutrophils. Immunoblotting analysis of MPO demonstrated that the protein was still expressed, but its posttranslational processing was not completed as suggested by the atypical pattern of the MPO subunits detected in cell lysates of SCN patients. While the light MPO subunit (15 kDa) was still detectable, but at reduced levels, the high-molecular-weight peptides corresponding to the heavy MPO subunit (60 kDa) as well as its degradation product (40 kDa) were absent or significantly reduced, whereas an intermediate-molecular-weight peptide was detectable in neutrophils of SCN patients. This observation suggests that in the absence of normal NE expression and/or trafficking, even MPO cannot completely mature in neutrophils of SCN patients undergoing treatment with G-CSF. Moreover, our results account for previous findings reporting that mRNA expression of NE and MPO is impaired in bone marrow–derived myeloid precursors of severe congenital neutropenia patients.35,36 In contrast to these findings, we were unable to detect differences of ELA2 mRNA levels in total hematopoietic cells isolated from bone marrow of P3; however, the effect of ELA2 mutations on mRNA expression might not be detectable in total hematopoietic cells from bone marrow but only in selected myeloid precursors.
Investigation of NE, MPO, and lactoferrin expression in neutrophils of the 2 SCN patients with the G185R mutation has shown that the defect of the abnormal processing of these polypeptides is more striking in these patients, suggesting that the corresponding mutant has a profound effect on the early stages of neutrophil differentiation. It is interesting that transfection studies of the G185R mutant into RBL cell line have shown that this protein cannot be revealed by immunofluorescence, while it is still detectable by immunoblotting.21 Moreover, we found that children with a refractory response to G-CSF and the G185R genotype expressed an extremely low amount of lactoferrin, while the antimicrobial protein was still detected in cell extracts of patients with other ELA2 mutations or with other causes of neutropenia undergoing treatment with G-CSF,36,37 suggesting that an early death of promyelocytes during their differentiation prevented the formation of specific granules.15,38
This succession of events is not fully restored by treatment with G-CSF, although we observed a great degree of variability in the severity of the maturation defect among children with different types of ELA2 mutations. Therefore, the incomplete maturation of neutrophils in SCN may explain why a significant number of patients die from sepsis (8% of cumulative incidence over 10 years) even if they had shown a satisfactory response to the treatment with G-CSF.11 In fact, while the observed risk of dying of sepsis was higher in the patients less responsive to the growth factor (14%) compared with children with good response (11%), the use of G-CSF was not sufficient to prevent lethal infections in these children. The increased susceptibility to infections might be related to the abnormal expression in neutrophils of SCN patients of antimicrobial polypeptides, such as NE, lactoferrin, MPO, cathepsin G, and HNP, in spite of the treatment of the patients with G-CSF. It is conceivable that the decreased expression of these peptides by neutrophils of SCN patients could lead to an impairment of their antimicrobial functions. In fact, we report that these cells were unable to kill C albicans and showed a decreased killing activity against E coli. Therefore, it is likely that the antimicrobial activities that are dependent on MPO, lactoferrin, cathepsin G, and HNP, and other unknown abnormal proteins, are not correctly functioning in neutrophils of SCN patients, whereas the defense mechanisms that require polypeptides such as lysozyme, which are correctly expressed in cells of SNC patients, are still effective against some microorganisms. Notably, we found that phagocytosis is apparently normal in SCN neutrophils, suggesting that the reduced killing ability of these cells does not rely on defects of microbial internalization. Moreover, although the expression of membrane-associated NADPH oxidase subunits is decreased, oxygen radical production in response to PMA and zymosan particles is conserved in SCN neutrophils, whereas it is defective upon fMLP stimulation. Therefore, activation mechanisms of oxygen-dependent killing machinery appear to be only partially conserved in SCN neutrophils, in spite of the treatment of patients with G-CSF.
Clearly, further studies are needed to determine whether the defect of granule formation can be generalized to all SCN patients treated with G-CSF, but these new results and previous evidence that G-CSF therapy accelerates leukemogenesis in SCN call into question the overall efficacy of treatment with G-CSF and raise the issue of whether it should even be used for the treatment of this condition. This is particularly evident in SCN children with unsatisfactory response to the therapy, whose elevated risk of dying of sepsis or developing MDS/AML makes them the best candidates for bone marrow transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from PRIN 2005 (R.B.); Fondazione Cariplo Network Operativo per le Biomedicina di Eccellenza in Lombardia (NOBEL) grant (R.B. and Luigi D. Notarangelo); Ministero dell'Università e della Ricerca (MIUR) Programma di Ricerca di Rilevante Interesse Nazionale (PRIN) 2004, MIUR (Centro per l'Innovazione Diagnostica e Terapeutica, IDET); Fondazione Berlucchi (L.D.N.); Fondazione Cariverona “Bando 2003” and “Bando 2004–Integrazione tra tecnologia e sviluppo di settore” (S.D.); European Union QLR-2000-01395 and European Union FP6, LSHB-CT-2004-503319-Allostem (F.F.); grant no. 40F.37 “Programma nazionale di Ricerca sull'AIDS” (Istituto Superiore di Sanità, 2004); and Fondazione Beretta (Brescia, Italy).
We are grateful to Silvia Costa for help with Western blot experiments, Tiziana Musso for critical discussion, and Caterina Coppola for secretarial work.
Authorship
Contribution: M.D. performed Western blot analysis and neutrophil functional studies, and contributed to writing the paper; S.F. isolated neutrophils, performed neutrophil functional studies, and contributed to writing the paper; G.S. performed genetic analysis of ELA2 in SCN patients; W.V. performed immunofluorescence staining of elastase and myeloperoxidase in neutrophils; L.T. isolated neutrophils and performed immunoblotting; F.G. performed immunofluorescence staining of elastase and myeloperoxidase in neutrophils of SCN patients; E.Z. performed killing experiments; D.F. isolated neutrophils and performed neutrophil functional studies; Lucia D. N. and F.P. took care of the patients; F.F. critically evaluated immunocytochemistry experiments; Luigi D. N. contributed to writing the paper; S.D. studied Western blot analysis and neutrophil functional studies in SCN patients; and R.B. designed the experiments and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
M.D. and S.F. contributed equally to this work.
Correspondence: Raffaele Badolato, Istituto di Medicina Molecolare “Angelo Nocivelli,” Università di Brescia, c/o Spedali Civili, 25123 Brescia, Italy; e-mail: badolato@med.unibs.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal