PD-1 up-regulation is correlated with the exhaustion of virus-specific CD8 T cells in people chronically infected with HIV-1. Zhang and colleagues report that PD-1 is up-regulated on HIV-specific CD8 T cells in typical disease progressors (TPs) but not in long-term nonprogressor (LTNP) patients, and PD-1 expression is down-regulated in HIV-1 patients with successful response to HAART therapy.
Zhang and colleagues report that PD-1 is up-regulated on human immunodeficiency virus (HIV)–specific CD8 T cells in typical disease progressors (TPs) but not in long-term nonprogressor (LTNP) patients. PD-1 up-regulation is associated with reduced expression of CD8 effectors perforin and IFN-γ, and decreased T-cell proliferation. Of interest, as blockade of PD-1 with anti–PD-L1 mAb in vitro restores effector functions of CD8 T cells, HIV-1 patients with successful response to highly active antiretroviral therapy (HAART) appear to also restore HIV-specific CD8 T cells in vivo. These findings suggest that sustained PD-1 expression on HIV-specific CD8 T cells depends on active HIV-1 viremia, and reducing viremia with HAART can reverse the defects associated with PD-1 expression in HIV-specific CD8 T cells.
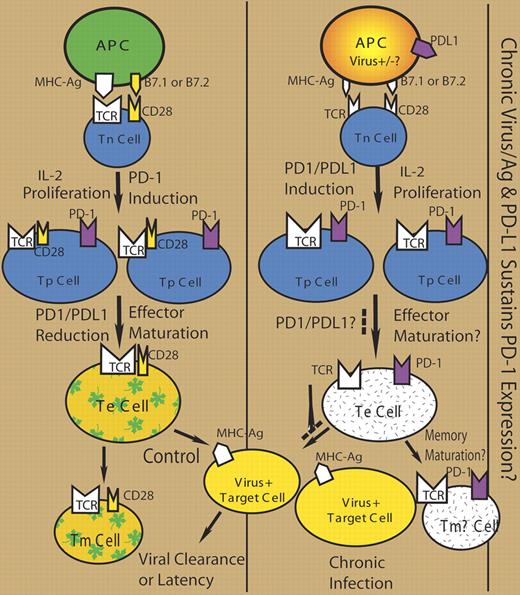
In the seminal report on the role of PD-1 in the exhaustion of virus-specific CD8 T cells,1 PD-1 is up-regulated on “exhausted” CD8 T cells in mice chronically infected with lymphocytic choriomeningitis virus (LCMV). Of importance, blockade of PD-1 with anti–PD-L1 mAb in chronically infected mice enhances proliferation and effector functions of the exhausted T cells, correlated with reduced LCMV infection. Several groups have since reported similar up-regulation of PD-1 on HIV-specific T cells in HIV-1–infected people.2–4 The level of PD-1 on HIV-specific T cells is correlated with HIV-1 viral loads and inversely with CD4 T-cell counts. Treatment of PD-1+ “exhausted” HIV-specific T cells with anti–PD-L1 in vitro also increases their effector functions.2,4 Of interest, PD-1+ HIV-specific CD8+ T cells show a poorly differentiated phenotype (CD27hiCD28loCD57loCD127loCCR7−CD45RA− or CD27+CD45RO+) that is correlated with dysfunctional CD8+ T cells.3,4 In the current report, PD-1 up-regulation is also associated with reduced expression of CD8 effectors perforin and IFN-γ. Therefore, “exhausted” HIV-specific CD8 T cells are also impaired in phenotypic and functional maturation, correlated with sustained expression of PD-1 (see the figure).
The inhibitory receptor PD-1 is rapidly up-regulated on activated T cells (reviewed in Sharpe et al5 ). Its expression is decreased on effector T cells and diminished on memory T cells after antigen clearance. Upon antigen restimulation, effector T cells also up-regulate expression of PD-1. Therefore, sustained stimulation of naive and/or effector T cells during chronic virus infection will lead to accumulation of PD-1+ T cells. Antigen-presenting cells (APCs) in HIV-1–infected patients express high levels of PD-L1 or B7-H1.6 It is likely that impaired APCs with PD-L1 may help sustain PD-1 expression on T cells and impair their effector maturation, and allow chronic viral infection (see figure).
The current report further demonstrates that PD-1 up-regulation and the associated defects in HIV-specific CD8 T cells are correlated with HIV-1 disease progression, and the defects are reversible because inhibition of HIV-1 viremia by HAART restores virus-specific CD8 T cells with reduced PD-1 expression. This highlights PD-1/PD-L1 interaction as a potential therapeutic target for chronic HIV-1 infection. It is possible to recover HIV-specific T-cell functions either by reducing viremia or by blockading PD-1/PD-L1 interaction. However, it is important to point out that blockading PD-1 and PD-L1 interaction may have serious autoimmune implications, as PD-L1 mutant mice succumb quickly to chronic LCMV infection with severe tissue damage.1 It also highlights the importance to elucidate the mechanism of PD-1 in virus-specific T-cell exhaustion and in impairing T-effector maturation.
The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal