Platelets play a vital role in hemostasis. Until recently, there was no means to increase platelet production in order to treat or prevent bleeding.
In this issue of Blood, Jenkins and colleagues describe the results of a phase 1 clinical trial in healthy adults with eltrombopag, a first-in-class, orally bioavailable, small-molecule, nonpeptide thrombopoietin (TPO) receptor agonist. This study demonstrates that eltrombopag causes a dose-dependent increase in platelet count unaccompanied by adverse events, induction or priming of platelet activation, or rebound thrombocytopenia in healthy volunteers. Trials of eltrombopag and another TPO receptor agonist, the peptibody AMG531, have been extended to patients with severe idiopathic thrombocytopenic purpura (ITP), many of whom were refractory to other therapies. A high proportion of such patients treated with both agents has shown significant responses with acceptable tolerability and toxicity profiles to date.1–3
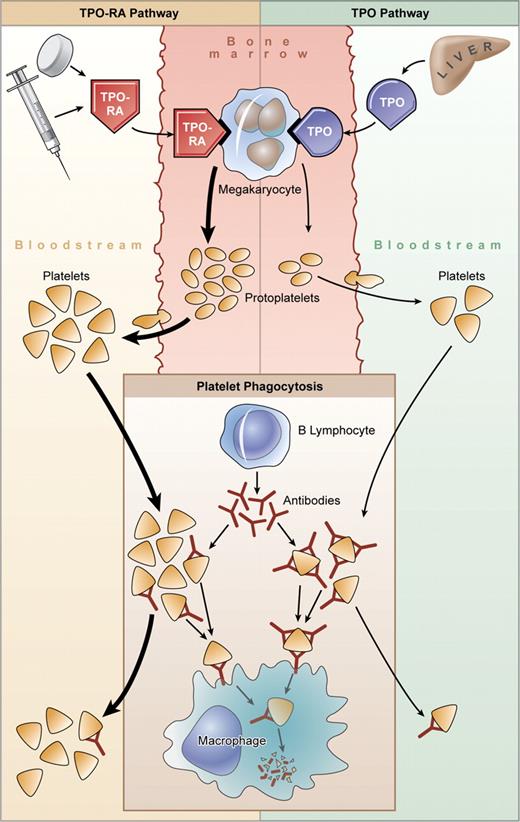
It may seem counterintuitive that TPO receptor agonists would have a significant impact on patients with severe ITP when this disorder has long been thought to be caused by antibodies that accelerate platelet destruction through FcRγ-mediated clearance (see the figure). This view is supported by the outcome of therapies that impede this clearance process (eg, intravenous immunoglobulin [IVIG], anti-D, corticosteroids, splenectomy), which can increase the platelet count rapidly. Failure to respond to splenectomy has been attributed to platelet clearance in the liver and elsewhere. The bone marrow in ITP typically shows normal or increased numbers of megakaryocytes and large, often reticulated (ie, young), platelets are seen in the peripheral blood. Platelet kinetics showing impaired production might be attributable to intramedullary platelet destruction by macrophages (naked megakaryocytes) that is rectified by corti-costeroids.4
Thrombopoietin receptor agonists in ITP. Platelet production in patients with ITP is variable. Megakaryopoiesis may be impaired by antibody, and platelets are destroyed by macrophages within the spleen and elsewhere, perhaps including the bone marrow. Plasma levels of TPO are normal or only slightly increased in patients with ITP, and hepatic production is insufficient to overcome antibody-mediated platelet destruction. Megakaryocytes stimulated with TPO receptor agonists (TPO-RAs) generate platelets in greater numbers, which may also lessen sensitization with antibody and reduce both peripheral clearance and perhaps intramedullary destruction. Illustration by Kenneth Probst.
Thrombopoietin receptor agonists in ITP. Platelet production in patients with ITP is variable. Megakaryopoiesis may be impaired by antibody, and platelets are destroyed by macrophages within the spleen and elsewhere, perhaps including the bone marrow. Plasma levels of TPO are normal or only slightly increased in patients with ITP, and hepatic production is insufficient to overcome antibody-mediated platelet destruction. Megakaryocytes stimulated with TPO receptor agonists (TPO-RAs) generate platelets in greater numbers, which may also lessen sensitization with antibody and reduce both peripheral clearance and perhaps intramedullary destruction. Illustration by Kenneth Probst.
On the other hand, plasma TPO levels are normal or minimally increased even in ITP patients with severe thrombocytopenia, some antibodies impair megakaryocyte proliferation and development in vitro, and megakaryocytes may show features of apoptosis. Thus, the long-held belief that platelet production in ITP is substantially increased but unable to compensate for peripheral destruction is being revisited.
A separate issue is that the side effects and poor tolerability of current therapies have helped fashion recent recommendations to limit treatment to patients with severe thrombocytopenia or bleeding. This, in turn, may affect the quality of life, impedes use of otherwise indicated medications that impair platelet function, necessitates frequent monitoring, and affords little or no margin for the patient in case of trauma or surgical emergencies.
Thrombopoietic agents hold promise for patients with severe or refractory ITP, and for patients prior to planned procedures. As experience accumulates, and assuming long-term safety is documented, extension to other settings can be considered, including the use of these agents in maintenance treatment to avert the side effects of existing treatments. However, not all ITP patients respond to TPO receptor agonists, and predictors of response would be helpful. Furthermore, usage will need to be considered in the context of recent studies suggesting that high doses of dexamethasone on presentation may favorably alter the natural history of the disease5–7 and the fact that some patients achieve a “cure” after splenectomy or a single course of rituximab.
The potential use of TPO receptor agonists is also evident in patients suffering from thrombocytopenia caused by conditions with fewer treatment options, including myelodysplasia, viral hepatitis (to permit use of antiviral [ie, interferon] therapy), and chemotherapy-induced thrombocytopenia (where it can be shown that thrombocytopenia requires transfusion or limits otherwise life-prolonging treatment), among others. The studies by Jenkins et al with eltrombopag in this issue and related studies with this and other TPO receptor agonists portend a potentially exciting new approach to the treatment of patients with ITP and other causes of severe thrombocytopenia.
The author has served as a consultant for Amgen and Glaxo-Smith-Kline. ■